Abstract
The development and biological characteristics of Haemaphysalis longicornis were investigated under field conditions in Xiaowutai National Natural Reserve Area, North China. Unfed larvae, nymphs and adults were fed on rabbits and exposed to daylight. Three free-living stages were allowed to develop in field plot selected in a tick natural habitat. The host seeking behavior and seasonal occurrence were observed. Haemaphysalis longicornis were active from mid March to mid October. The premoult period of nymphs and preoviposition of females were regulating phases of their life cycle. The developmental durations of eggs, larvae and adults were constant under field conditions regardless when the development started. The oviposition periods in May and June were statistically shorter than those in July and August. The daily oviposition patterns of females engorged in May and June demonstrated unobvious peak, which differed from those engorged in July and August. The daily oviposition peak of the latter occurred on the 4th day of oviposition. Moreover, a positive correlation was found between the mass of the laid egg and the body weight of engorged females (r = 0.62, P < 0.001). The female reproductive efficiency indices were 2.9, 6.1, 10.5 and 9.0 in May, June, July and August, respectively. The mean weight (3.33 mg) of engorged nymphs molting to females was significantly higher than that (2.35 mg) of those molting to males (P < 0.001), but the body weights of both sexes were overlapping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hard tick Haemaphysalis longicornis Neumann is widely distributed in China (Teng and Jiang 1991), New Zealand, Korea, Japan and Australia (Tenquisf and Charleston 2001). It parasitizes a variety of hosts including domestic and wild animals and human beings under natural conditions (Teng and Jiang 1991; Tenquisf and Charleston 2001; Inokuma et al. 2002; Kim et al. 2003; Shimada et al. 2003) and transmits zoonotic pathogens including Theileria sergenti (Teng and Jiang 1991), Babesia ovata (Cho et al. 2002), B. equi (Ikadai et al. 2007), rickettsia (Lee et al. 2003) and Coxiella burneti (Q fever) (Hoogstraal et al. 1968), which can cause severe diseases in human and animals. Therefore, it is one of the most detrimental ticks to the health of human and animals.
Investigation on the natural infestation and annual life cycle of H. longicornis has shown that H. longicornis is abundant in spring and summer and absent during winter. Under standard laboratory conditions, the bisexual strain has a mean duration of 135.8 days (Liu and Jiang 1998). When temperature is colder, its development period is prolonged (Yano et al. 1987). Although the temperature and humidity preference of its eggs and engorged larvae and the development, survival, fecundity and behavior of its parthenogenetic strain in field have been studied (Heath 1979, 1981; Sutherst and Bourne 1991), little is known about the ecological aspects of its bisexual strain in nature. Thus, the present study was aimed to describe the developmental and biological characteristics of this tick under field conditions in North China, hoping to provide basic background for further investigations.
Materials and methods
Study site
The field observations were carried out in Xiaowutai National Natural Reserve Area (39°50′-40°07′N, 114°47′-115°30′E) of Hebei Province, North China. A field plot (39°58′566 N, 115°23′185 E, and altitude 839 m) adjacent to pasture was selected for the investigations of biology and seasonal occurrence. The plot was surrounded by 10 cm × 10 cm gutter which was full of water to avoid tick escape, enclosed by wire net to prevent other animals from entering, and covered by approximately 5 cm thick layer of leaf litter containing scattered cluster of grass species Artemisia sacrorum, Chenopodium serotinum and Setaria viridis.
Fig. 1 lists the climate data obtained by a hygrothermograph (Qingsheng Electronic Technology Ltd., Hebei Province, China) located about 50 cm above the ground in the plot from February 2008 to April 2009. They were illustrated as monthly minimum, maximum and mean ambient air temperatures (Tmin, Tmax and Tmean) as well as minimum, maximum and mean relative humidities (RHmin, RHmax and RHmean).
Ticks and animals
Unfed nymphs of H. longicornis collected from flagging vegetation each month from March to July were used in experiment. Rabbits were obtained from Experimental Animals Center in Hebei Medical University. Two rabbits were placed in a wire cages (30 × 40 × 50 cm3) in the plot as host for the parasitic ticks, which were artificially released into the plot for observation of seasonal occurrence. Additional rabbits were maintained outside the plot to host the ticks required for observation of development and biological characteristics. All rabbits were fed rabbit pellets with water provided ad libitum under natural lighting and climate conditions. The use of rabbits was approved by the Animal Care and Use Committee of Hebei Normal University.
Observations of Haemaphysalis longicornis’ development
Some of unfed nymphs were weighed and fed on the rabbits outside the plot. They were recorded daily to determine their feeding period. Naturally detached, engorged nymphs were collected, weighed and placed individually into cylinders, which were then plugged with gauze netting and put into the plot, and observed daily to record premoult periods. After ecdysis, their sex was recorded. In order to facilitate feeding and collection of engorged ticks after detachment, these ticks were released into cloth bags glued on rabbits’ ears.
300 newly hatched larvae and 50 newly emerged adults were placed on the ear of rabbits. They were examined daily to record prefeeding period as defined as the number of days from the hatching or molting to the beginning of attachment. 300 unfed larvae and 100 unfed adults (50 females and 50 males) were weighed and fed on rabbits as described previously (Liu et al. 2005) and examined daily to record feeding period.
Engorged larvae were weighed, confined in cylinders, placed in the plot and examined daily to determine premoult period. 500 eggs were used to determine incubation period as defined as the length of time until hatching.
Observations on the biology of engorged females
Engorged females were used for the investigation of biological characteristics. In detail, they were weighed, confined individually in cylinders, which were put in the plot subsequently, and examined daily to investigate preovipositional and ovipositional periods. After the onset of oviposition, deposited eggs were carefully separated from females and weighed daily. The number of eggs laid was calculated by their total weight based on the predetermined weight of 100 eggs. The reproductive efficiency index (REI) was calculated as the ratio of egg number to engorged female weight (Drummond and Whetstone 1970).
Observations on host questing and seasonal occurrence of ticks at the non-parasitic stage
Some of unfed nymphs sampled from flagging vegetation were released directly into the plot monthly. These nymphs as well as the adults and larvae originated from these nymphs were free to quest and attach to the host rabbits provided in the plot. The engorged ticks were allowed to fall from their hosts and seek suitable habitats for molting or oviposition without artificial disturbance. Visual inspection was performed daily to observe the host questing behavior and feeding progress of the post-embryonic stages.
To test the survival ability, 100 newly emerged adults and 200 newly hatched nymphs were put on the ground in the plot, respectively, in July and September, and confined individually with wire mesh. Their survival rate in winter was determined in April of the next year by incubation at 27 ± 1°C for 24 h in an incubator (6 L:18 D) supplemented with 70% relative humidity. 500 unfed larvae hatched between August and September were placed into glass tubes, which were then plugged with gauze netting and put on the ground in the plot, and examined daily to record the lifespan of the stage.
Data analysis
Data were analyzed by t-test and analysis of variance (ANOVA) using Statistica 6.0 software.
Results
Developmental periods of Haemaphysalis longicornis
The development duration of nymphs varied remarkably in March, April, May, June and July under field conditions (Table 1). It ranged from the longest 106.2 days (from 64 to137 days) in March to the shortest 27.0 days (from 21 to 49 days) in July, and was 69.4 days (from 49 to 109 days), 42.5 days (from 34 to 52 days) and 30.1 days (from 26 to 38 days) in April, May and June, respectively. These differences were largely due to their different premoult periods, which varied from 102.4 days in March to 23.6 days in July, but not their feeding periods, which did not show significant differences in these 5 months.
The development durations of eggs, larvae and adults were correspondingly similar under field conditions (P > 0.05), irrespective of the month duration which development occurred. In detail, the average development periods were 41.5 days for eggs incubation, 2.9, 4.1 and 27.6 days for larval prefeeding, feeding and premoulting, respectively, and 3.3 and 5.7 days for adults prefeeding and feeding, respectively. Table 2 lists the development durations of ticks at these stages under field conditions.
Biology of engorged females
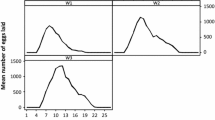
Generally, freshly fallen females would oviposit in suitable sites in leaf litter that had relatively mild microclimate and adequate ventilation. The preoviposition duration of engorged females varied from 1 to 4 weeks depend on their engorgement time (Table 3). Females engorged during May and June, had prolonged preoviposition period, 18.3 and 17.0 days, respectively, compared with those engorged during July and August, 6.6 and 7.4 days, respectively. The commencement of oviposition was occurred consequently between late June and early July. Females engorged in May and June had oviposition period of 10.3 and 11.8 days correspondingly, whereas those engorged in July and August had longer oviposition period of 20.1 and 17.0 days correspondingly. Their reproductive efficiency indices (REI) were 2.9, 6.1, 10.5, and 9.0 in May, June, July and August, respectively.
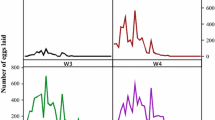
The number of eggs laid daily in May and June showed no visible peak (Fig. 2). In detail, relatively more eggs were laid in the first week, and then gently decreased during the subsequent days. During the whole oviposition period, there were just few small peaks. In contrast, the numbers of eggs laid daily in July and August increased rapidly in the first few days, reached a large peak at the fourth day, then gradually declined during the remaining oviposition period. In addition, about 6 days after the commencement of oviposition, the number of eggs laid daily in July reached another small peak. Although female fecundity was low in May and June, linear regression analysis revealed a highly significant positive correlation between weight of engorged females and the mass of eggs laid (r = 0.6229, P < 0.001) (Fig. 3).
Observations on the host questing behavior and feeding biology in the plot
In the plot, newly hatched larvae would take about 2–5 days of prefeeding periods and then quested for hosts at the bottom of grass. Newly emerged adults would take about 2–4 days for fully development and then climbed up to the tip of grass vegetations and aggregated into clusters under the leaves to quest for hosts. Larvae attached concentratedly on the ears and periocular region, whereas most adults would prefer attaching on the ears of hosts. Ticks’ weight increased slightly in the first few days of feeding (about 2–4 days for larva and nymph and 3–6 days for female before mating), and then rapidly during the last days of engorgement before detachment. The average weights of larva, nymphs and adult before and after feeding are listed in Table 4.
In addition, under field conditions, the weights of female and male nymphs ranged from 1.9 to 5.0 mg and 1.0 to 3.9 mg, respectively. T test revealed that the nymphs molted to females were significantly heavier than those molted to males (P < 0.001). The former weighed 3.33 ± 0.74 mg, whereas the latter weighed only 2.35 ± 0.58 mg. However, their weights in engorgement were overlapped. The relationship between the body weights of H. longicornis nymphs and their resultant sexes is illustrated in Fig. 4.
Observations on the seasonal occurrence and survival ability in the plot
Corresponding to the nymph development, most adults appeared in June with a peak in mid July and were active to August. Larvae were found in late July as the result of various preoviposition, peaked in late August, and reduced in October. The unfed larvae placed in the plot survived about 7–25 days from September to October and none survived into winter.
The nymphs and adults confined by the wire mesh in the plot kept under the leaf litter or small stones without actively seeking for hosts, few even only attached without feeding when placed artificially to the hosts. They all kept quiescence during the subsequent inspections in winter. The survival rate of the nymphs and adults were 91 and 60%, respectively, in April of the following year. Therefore the nymphs and adults of H. longicornis could survive for about 8–10 months through winter until it is adequate to infest.
Discussion
From the observations described above, it is possible to schematize the development and biology of the tick H. longicornis under field conditions. Ticks develop mainly during spring and autumn, and overwinter at the stages of nymph and adult. Only few male ticks attach to hosts during the cold months of late autumn to early spring. Infestation of H. longicornis demonstrated distinct peaks of activity for each of the three parasitic stages in 1 year, showing a 1-year generation pattern.
Liu and Jiang (1998) reported that H. longicornis has a mean life cycle of 135.8 days and its development periods of different stages do not differ significantly in different seasons under laboratory conditions (27 ± 1°C; 75% RH; 6 L:18 D). This is in contrary to our results. Our observation found that under field conditions, the development period of nymphs varies dramatically from 4 weeks to 15 weeks (Table 1) from March to July due to different molting duration. The plausible explanation is that field conditions are more complicated and the complex interplay among photoperiod, temperature, relative humidity as well as some other ecological factors affects the development of the ticks (Fourie and Horak 1994; Padgett and Lane 2001; Labruna et al. 2002). For example, low temperature has been shown to prolong the durations of egg hatching, molting, preoviposition and oviposition of H. longicornis (Yano et al. 1987) possibly due to attenuated metabolic rate at low temperature (Jiang and Bai 1989). Moreover, the appearance in Ixodes rubicundus and Anocentor nitens has been also observed, i.e. variable durations of development can regulate the course of life cycle in different months under natural climate (Fourie and Horak 1994; Borges et al. 1999).
The duration of the preoviposition took about 1 to 4 weeks (Table 3), which together with the molting of nymphs regulated the seasonal occurrence of this tick. Thus, it resultantly synchronized the oviposition during late June and early August. Comparison between the climatic data (Fig. 1) and tick activity showed that the highest ovipositional activity was during the months with higher temperature and relative humidity. Similar patterns have been reported in Amblyomma cajennense (Oliveira et al. 2003) and A. parvum (Nava et al. 2008). Therefore, it can be deduced that the synchronization of oviposition coincided with warm and wet climate is favorable for egg hatching.
As illustrated in Fig. 2, there were no clear peaks of the eggs output in May and June (Fig. 2), while the mean temperatures were 16.1 and 19.1°C (Fig. 1), respectively. Since the fecundity appeared to be almost complete if the temperature was over 20°C (Yano et al. 1987), lower temperature must be a responsible reason for the low fecundity. However, additional studies are required to further detail the development and reproductive preference of H. longicornis. The oviposition curves recorded in July and August were similar to the result obtained under laboratory conditions (Liu and Jiang 1998). Similar patterns were described for other Haemaphysalis spp. species, e.g. H. japonica douglasi (Yao and Chen 1974), H. vietnamensis (Ding and Yin 1996) and H. leporispalustris (Labruna et al. 2000), and some other ixodid ticks, such as A. parvum (Guglielmone et al. 1991), I. rubicundus (Vanderlingen et al. 1999), Rhipicephalus bursa (Yeruham et al. 2000), Dermacentor silvarum (Liu et al. 2005) and Hyalomma asiaticum kozlovi (Chen et al. 2009). Though the REI of engorged females were low in May and June (Table 3), the relationship between the weight of engorged females and the number of eggs laid (Fig. 3) follows a pattern common to other ixodid ticks and videlicet, that is, engorged females with heavier weight lay more eggs (Campbell and Glines 1979; Guglielmone et al. 1991; Solomon and Kaaya 1998; Yeruham et al. 2000; Zhang et al. 2006).
Generally, ixodid nymphs molt to females are heavier than those molt to males (Rechav and Knight 1981; Guglielmone and Moorhouse 1985). Hence, their sex can be predicted based on the weight of engorged nymphs as reported in H. campanulata (Fujisaki et al. 1976), H. formosensis (Fujisaki et al. 1976), H. yeni (Fujisaki et al. 1976), A. inornatum (Gladney et al. 1977), A. parvum (Guglielmone et al. 1991), and I. scapularis (Hu and Rowley 2000). However, the prediction of sex in some ixodid ticks, such as A. variegetum (Centurier and Klima 1979), A. neumanni (Aguirre 1999), D. variabilis (Hu and Rowley 2000) as well as H. longicornis as shown here (Fig. 4) is difficult due to the overlap of the weights in male and female adults. Fujisaki et al. (1976) demonstrated discrete sex differences in engorged-nymph with different weight in the bisexual strain of H. longicornis. The disparity with the result observed here may be explained by the differences in geographic regions and conditions under which ticks were reared.
Pegram and Banda (1990) reported that rainfall and relative humidity exert great influence on the survival periods of unfed stages. In the current study, larval mortality rose rapidly while atmospheric relative humidity fell (all larvea died within 25 days). The lifespan of unfed nymphs and adults were about 8 and 10 months, respectively. But the survival rate of adults was lower than that of nymphs possibly because larger size could cause more water loss and energy consumption in dry and cold winter. In practice, to survive extended periods, sheltered deeply in the leaf litter will be important for ticks, especially during overwintering. Due to the regulation of nymphal molting and preoviposition, only one generation could be observed in 1 year, which is similar to the generation occurrence of the parthenogenetic strains of H. longicornis in southeast Queensland (Sutherst and Bourne 1991) and of A. triste in Uruguay (Venzal et al. 2008). Although observations on the seasonal occurrence of H. longicornis in the confined plot may not indicate precisely the distribution of the ticks, our study provides a starting point for investigation of tick’s dynamic population.
References
Aguirre DH (1999) The life cycle of Amblyomma neumanni Ribaga, 1902 (Acari: Ixodidae) in the laboratory. Exp Appl Acarol 23:159–164
Borges L, Oliveira PR, Ribeiro M (1999) Seasonal dynamics of the free-living phase of Anocentor nitens at Pedro Leopoldo, Minas Gerais, Brazil. Vet Parasitol 87:73–81
Campbell A, Glines MV (1979) Development, survival, and oviposition of the rabbit tick, Haemaphysalis leporispalustris (Packard) (Acari: Ixodidae), at constant temperatures. J Parasitol 65:777–781
Centurier C, Klima R (1979) Ein Beitrag zur Kenntnis der Biologie von Amblyomma variegatum (Fabricius, 1794). Z Angew Entomol 87:131–142
Chen Z, Yu ZJ, Yang XJ, Zheng HY, Liu JZ (2009) The life cycle of Hyalomma asiaticum kozlovi Olenev, 1931 (Acari: Ixodidae) under laboratory conditions. Vet Parasitol 160:134–137
Cho SH, Kim TS, Lee HW, Tsuji M, Ishihara C, Kim JT, Wee SH, Lee CG (2002) Identification of newly isolated Babesia parasites from cattle in Korea by using the Bo-RBC-SCID mice. Korean J Parasitol 40(1):33–40
Ding XC, Yin PY (1996) Study on the biology and seasonal fleuctuations of Haemaphysalis vietnamensis in Nanshan farm, Hunan provice. J China Agric Univ 3(1):109–112
Drummond RO, Whetstone TM (1970) Oviposition of the Gulf Coast tick. J Econ Entomol 63:1548–1551
Fourie LJ, Horak IG (1994) The life cycle of Ixodes rubicundus (Acari: Ixodidae) and its adaptation to a hot, dry environment. Exp Appl Acarol 18:23–35
Fujisaki K, Kitaoka S, Morii T (1976) Comparative observations on some bionomics of Japanese ixodid ticks under laboratory cultural conditions. Natl Inst Anim Health Q 16:122–128
Gladney WJ, Dawkins CC, Price MA (1977) Amblyomma inornatum (Acarina: ixodidae): natural hosts and laboratory biology. J Med Entomol 14:85–88
Guglielmone AA, Moorhouse DE (1985) Differences in nymphs of Amblyomma triguttatum triguttatum Koch moulting to males or females. Acarologia 26:7–11
Guglielmone AA, Mangold AJ, Garcia MD (1991) The life cycle of Amblyomma parvum Aragao, 1908 (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 13:129–136
Heath ACG (1979) The temperature and humidity preferences of Haemaphysalis longicornis, Ixodes holocyclus and Rhipicephalus sanguineus (ixodidae): studies on eggs. Int J Parasitol 9(1):33–39
Heath ACG (1981) The temperature and humidity preferences of Haemaphysalis longicornis, ixodes holocyclus and Rhipicephalus sanguineus (ixodidae): studies on engorged larvae. Int J Parasitol 11(2):169–175
Hoogstraal H, Roberts F, Kohls GM, Vernon JT (1968) Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J Parasitol 54:1197–1213
Hu R, Rowley WA (2000) Relationship between weights of the engorged nymphal stage and resultant sexes in Ixodes scapularis and Dermacentor variabilis (Acari: Ixodidae) ticks. J Med Entomol 37(1):198–200
Ikadai H, Sasaki M, Ishida H, Matsuu A, Igarashi I, Fujisaki K, Oyamada T (2007) Molecular evidence of Babesia equi transmission in Haemaphysalis longicornis. Am J Trop Med Hyg 76(4):694–697
Inokuma H, Fujimoto T, Hosoi E, Tanaka S, Fujisaki K, Okuda M, Onishi T (2002) Tick infestation of sika deer (Cervus nippon) in the western part of Yamaguchi prefecture, Japan. J Vet Med Sci 64(7):615–617
Jiang ZJ, Bai CL (1989) Study on the oviposition in ixdid ticks. J Beijing Normal Univ (Nat Sci) 3:81–85
Kim JE, Park HJ, Lee JY, Cho BK, Lee IY, Lee WK, Koh CJ (2003) Three cases of tick bites by Haemaphysalis longicornis. Korean J Dermatol 41(9):1198–1201
Labruna MB, Leite RC, Faccini JLH, Ferreira F (2000) Life cycle of the tick Haemaphysalis leporis-palustris (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 24:683–694
Labruna MB, Kasai N, Ferreira F, Faccini J, Gennari SM (2002) Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of Sao Paulo, Brazil. Vet Parasitol 105:65–77
Lee JL, Park HS, Jung KD, Jang WJ, Koh SE, Kong SS, Lee IY, Lee WJ, Kim BJ, Kook KH, Park KH, Lee SH (2003) Identification of the spotted fever group Rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol 47(4):301–304
Liu JZ, Jiang ZJ (1998) Studies on the bionomics of Haemaphysalis longicornis Neumann (Acari: Ixodidae) under laboratory conditions. Acta Entomol Sin 41(3):280–283
Liu JZ, Liu ZN, Zhang Y, Yang XL, Gao ZH (2005) Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp Appl Acarol 36:131–138
Nava S, Mangold AJ, Guglielmone AA (2008) Aspects of the life cycle of Amblyomma parvum (Acari: Ixodidae) under natural conditions. Vet Parasitol 156:270–276
Oliveira PR, Borges L, Leite RC, Freitas C (2003) Seasonal dynamics of the Cayenne tick, Amblyomma cajennense on horses in Brazil. Med Vet Entomol 17:412–416
Padgett KA, Lane RS (2001) Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol 38:684–693
Pegram RG, Banda DS (1990) Ecology and phenology of cattle ticks in Zambia: development and survival of free-living stages. Exp Appl Acarol 8:291–301
Rechav Y, Knight MM (1981) Life cycle in the laboratory and seasonal activity of the tick Rhipicephalus glabroscutatum (Acarina: Ixodidae). J Parasitol 67:85–89
Shimada Y, Inokuma H, Beppu T, Okuda M, Onishi T (2003) Survey of ixodid tick species on domestic cats in Japan. Vet Parasitol 111:231–239
Solomon G, Kaaya GP (1998) Development, reproductive capacity and survival of Amblyomma Íariegatum and Boophilus decoloratus in relation to host resistance and climatic factors under field conditions. Vet Parasitol 75:241–253
Sutherst RW, Bourne AS (1991) Development, survival, fecundity and behavior of Haemaphysalis (Kaiseriana) longicornis (Ixodidae) at two locations in southeast Queensland. Int J Parasitol 21:661–672
Teng KF, Jiang ZJ (eds) (1991) Economic insect Fauna of China, Fasc 39, Acari: Ixodidae. Science Press, Beijing
Tenquisf JD, Charleston WAG (2001) A revision of the annotated checklist of ectoparasites of terrestrial mammals in New Zealand. J R Soc N Z 31(3):481–542
Vanderlingen FJ, Fourie LJ, Kok DJ, Vanzyl JM (1999) Biology of Ixodes rubicundus ticks under laboratory conditions: observations on oviposition and egg development. Exp Appl Acarol 23:513–522
Venzal JM, Estrada-Pe a A, Castro O, De Souza CG, Félix ML, Nava S, Guglielmone AA (2008) Amblyomma triste Koch, 1844 (Acari: Ixodidae): hosts and seasonality of the vector of Rickettsia parkeri in Uruguay. Vet Parasitol 155:104–109
Yano Y, Shiraishi S, Uchida TA (1987) Effects of temperature on development and growth in the tick, Haemaphysalis longicornis. Exp Appl Acarol 3:73–78
Yao WB, Chen GD (1974) Study on the life cycle of Haemaphsalis japonica douglasi Null et Warb. Acta Entomol Sin 17(4):500–502
Yeruham I, Hadani A, Galker F (2000) The life cycle of Rhipicephalus bursa Canestrini and Fanzago, 1877 (Acarina: Ixodidae) under laboratory conditions. Vet Parasitol 89:109–116
Zhang JX, Chen Z, Liu JZ, Yang XJ (2006) Study on oviposition and egg development of three species of hard ticks (Acari: Ixodidae) under laboratory conditions. Chin J Pest Contr 22:864–867
Acknowledgments
This project is supported by the National Natural Science Foundation of China (30970406) and Natural Science Foundation of Hebei Province, China (C2007000266).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, H., Yu, Z., Chen, Z. et al. Development and biological characteristics of Haemaphysalis longicornis (Acari: Ixodidae) under field conditions. Exp Appl Acarol 53, 377–388 (2011). https://doi.org/10.1007/s10493-010-9415-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-010-9415-3