Abstract
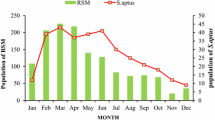
The ladybird beetle, Stethorus gilvifrons, is a major predator of the red spider mite, Oligonychus coffeae, infesting tea. Biology, life table and predatory efficiency of S. gilvifrons were studied under laboratory conditions. Its average developmental period from egg to adult emergence was 19.2 days. After a mean pre-oviposition period of 5.3 days, each female laid an average of 149.3 eggs. Adult females lived for 117.3 days and males for 41.5 days. The life table of the beetle was characterized by an intrinsic rate of increase (r) of 0.066 day−1, net reproductive rate (R 0) of 72.2 eggs/female, gross reproduction rate (Σm x ) of 82.3 eggs/female, generation time (T) of 64.9 days, doubling time of 10.5 days and finite rate of increase (λ) of 1.07 day−1. Population dynamics of S. gilvifrons and its prey, O. coffeae, was monitored by sampling 25 tea leaves from each experimental block grown under the prevailing field conditions. Populations of S. gilvifrons reached a peak during January to March and had low incidence during June to November. Peaks in the populations of S. gilvifrons coincided with the abundance of O. coffeae in tea fields. Weather factors such as low temperature, high humidity and heavy rainfall adversely affected the populations of S. gilvifrons. The predatory efficiency of S. gilvifrons increased during the growth of larval instars. An adult female consumed 205.0 eggs, 92.2 larvae, 81.8 nymphs and 52.4 adult mites per day.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ladybird beetles belonging to the genus Stethorus (Coleoptera: Coccinellidae) are obligate predators of tetranychid mites (Putman 1955). They frequent a wide range of crop plants where tetranychid mites are abundant (McMurtry et al. 1970). Several species have been reported to be effective biological control agents. They are known to be voracious predators with all the motile life stages feeding on all prey stages. They have high host-finding ability, high dispersal potential and long-living adults (Roy et al. 2005).
Spider mites (Acari: Tetranychidae) infesting commercially cultivated tea plants, Camellia sinensis L. (O. Kuntze) are typical colonizing species characterized by very high rate of population increase and high densities (Muraleedharan et al. 2005). Among them, the red spider mite (RSM), Oligonychus coffeae (Nietner), normally infests the upper surface of mature tea leaves and when the severity of infestation increases they move even to the lower surface of older leaves as well as tender tea shoots. Due to feeding, the maintenance foliage turns ruddy bronze, making RSM-infested fields distinct even from a distance. Severe infestation ultimately leads to defoliation (Selvasundaram and Muraleedharan 2003). The control of RSM is mainly achieved by the use of synthetic acaricides. Extensive use of these chemicals leads to pesticide residue problems in the made tea (Muraleedharan 1995).
These complex problems of environmental contamination and food safety require the development of alternative control methods. In this context, many studies had been conducted on the natural enemies of spider mites to evaluate their potential as biological control agents. Muraleedharan (1988) had reported the lady beetle, Stethorus gilvifrons Mulsant as an important predator of this tea mite in south India. Published information on S. gilvifrons is limited and more information is needed to explore the possibility of using this species as a potential control agent against RSM. Therefore, bioecological studies of S. gilvifrons were undertaken to document the population dynamics and its predatory efficiency against RSM infesting tea. Data relating to the biological and life table parameters will help to include S. gilvifrons as a potential predator in IPM strategies against RSM.
Materials and methods
Study area and sampling
The study area was located in the Anamallais (Coimbatore District, TamilNadu state) at an altitude of 1,065 m above the mean sea level. The experimental block consisted of 500 tea bushes (mixed tea clones) planted at a spacing of 1.2 × 1.2 m and last pruned in 2005 at a height of 60 cm above the ground. The experimental block was divided into five plots (A–E) each consisting of 100 tea bushes to take into consideration the influence of microclimate where the tea plants are grown under shade trees. The field was kept free from pesticide application since pruning. Leaves (25) were collected at random from each plot at fortnightly interval for a period of 2 years from January 2006 to December 2007. Leaves were individually placed in plastic bags with holes for proper air circulation and brought to the laboratory where the number of different life stages of S. gilvifrons and the red spider mites was counted under stereomicroscope (Olympus No. 1220) using 10× magnification. Weather data were obtained from the metrological observatory of UPASI Tea Research Institute, Valparai.
Stock culture of Stethorus gilvifrons and red spider mites
Stethorus gilvifrons and its prey, O. coffeae were collected from tea fields of UPASI Experimental Farm. A stock colony of S. gilvifrons had been maintained in the insectary at 25 ± 1°C, 75 ± 5% RH and 16L:8D photoperiod in a large screened box (40 × 25 × 10 cm) containing tea leaves infested with RSM. After field collection, spider mites were immediately transferred onto 1-year-old potted tea plants grown under greenhouse conditions and used as stock culture. From the stock, RSM adults were transferred onto fresh tea leaf (6 × 6 cm) placed on moistened cotton pads (ca. 1.5 cm thick) in plastic trays (42 × 30 × 6.5 cm). Rearing trays were kept under controlled conditions of 25 ± 1°C, 75 ± 5% RH and 16L:8D photoperiod. Withered and drying leaves were regularly replaced.
Life history and life table studies
Adults of S. gilvifrons were collected with a clean pipette aspirator from the RSM colony and introduced into a Plexiglas box (8 cm diameter, 7 cm depth) containing mite-infested tea leaf placed on moist cotton. Adults were allowed to lay eggs for 24 h and egg laden tea leaf was placed in a Petri dish (9.5 cm diameter) containing cotton wool soaked with water and the number of eggs laid by each beetle was counted. All the eggs were observed at 8 h intervals for their development till hatching. Newly hatched larvae were individually transferred onto mite-infested tea leaf (6 × 6 cm) for development and moulting until pupation. Pupae were placed in small clear plastic Petri dishes (9 cm in diameter, 1.5 cm in depth). As soon as adults emerged, they were sexed and a pair of male and female was introduced into each Plexiglas box with mite-infested tea leaf and the duration of copulation was observed. In order to document fecundity and longevity of adults they were transferred to another tea leaf infested with RSM at every 24 h till the female beetle died. Number of eggs laid on each leaf was counted daily under a stereomicroscope. All experiments were conducted with 10 replicates in an insectary at 25 ± 1°C, 75 ± 5% RH and a photoperiod of 16L:8D. For morphometric study, semi-permanent slides were prepared and measurements were taken using ocular and stage micrometer in a research microscope (ZEISS-Jeneval GF-PA).
Predatory efficiency of Stethorus gilvifrons
Stethorus gilvifrons and its prey, O. coffeae were collected as described above. All life stages of O. coffeae (300 in total) were transferred individually onto tea leaf (4 × 4 cm) placed in Plexiglas boxes (8 cm diameter, 7 cm depth) and then a single S. gilvifrons larva/adult was released. The box was covered with fine muslin net to provide sufficient ventilation and placed in an incubator at 25 ± 1°C, 75 ± 5% RH and 16L:8D photoperiod. Daily consumption of S. gilvifrons was examined under stereomicroscope at every 24 h. Stethorus gilvifrons was transferred to another Plexiglas box with pre-determined number of RSM and this procedure was repeated until their pupation. Each experiment was replicated five times.
Statistical analysis
The multiple regression analysis among the populations of S. gilvifrons and certain biotic (RSM population) and abiotic factors (sunshine, relative humidity, rainfall and temperature) was carried out using ‘SigmaStat 3.5’ software, to develop a model to predict the population dynamics of S. gilvifrons. Predation of S. gilvifrons on RSM was determined by analysis of variance (ANOVA) and means were separated by Duncan’s multiple range test (DMRT). The data on the duration of developmental stages of S. gilvifrons were used to calculate life table parameters which included age-specific survival rate (l x ), age-specific fecundity (m x ), intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R 0), mean generation time (T = lnR 0)/r) and gross reproductive rate (GRR = Σm x ) (Brich 1948; Southwood 1978). Doubling time (DT = (ln2)/r) was calculated as described by Mackauer (1983).
Results
Population dynamics of Stethorus gilvifrons and its prey
Incidence of S. gilvifrons coincided with cyclic oscillations of RSM population during the study period (Fig. 1). Populations of S. gilvifrons showed an increasing trend from December onwards and reached a peak between January and March. Red spider mite population was extremely low until mid-September, increased to a maximum from mid-November to March and again declined after a few weeks. The multiple regression equation fitted with certain biotic and abiotic factors to predict the population of S. gilvifrons was (Table 1): Y = −42.51 + 0.05 RSM − 0.65 T min + 3.05 T max − 1.04 RHmin + 0.95 RHmax − 0.0056 rainfall − 5.68 sunshine (R 2 = 0.851, F 7,21 = 17.143, P < 0.001). Low temperature, low RH, heavy rainfall and low sunshine hours had a negative influence on the predator population, whereas high temperature and high humidity had significant positive effects.
Life history and life table parameters
Newly laid eggs of S. gilvifrons are shiny, yellow and elliptical (0.36 ± 0.003 mm long, 0.18 ± 0.004 mm wide). They were deposited individually on midribs and veins, firmly glued to the leaves. Mean duration of hatching was 5.9 days. Fertilized eggs appeared granular in the first 2 days, and two red eyespots developed 1 day before hatching. The eggs became transparent on the day of hatching and the developing embryo could clearly be seen through the chorion of the egg. Unfertilized eggs were slightly smaller and didn’t show any colour changes as they gradually shriveled and died.
Four instars were recognized. Larvae moulted 3× with instars closely resembling each other; the presence of a shed exoskeleton and head capsule size was used to differentiate between instars. All instars had numerous dark brown setae over the tergites and pleurites with dark brown pigmentation at the bases of the dorsal setae. Before each moult, a sticky substance was secreted at the end of the abdomen, gluing the larva to the leaf surface. Mean instar period lasted from 1.5 (1st) to 2.9 days (2nd), and total larval period (1st–4th instar) was 8.7 days (Table 2).
After the last moult, the 4th instar firmly attached itself to the substratum by means of a sticky fluid. The quiescent prepupa stage at the end of the 4th instar lasted several hours, during which it changed color from red to black. The pupal stage of S. gilvifrons lasted an average of 4.8 days. Newly emerged adults are light reddish and gradually changed to black. Mean length and width of males and females were 1.20 × 0.86 and 1.30 × 0.90 mm, respectively (Table 3). Multiple matings were observed and copulation lasted for 15–30 min. Overall egg-to-adult period was 19.2 days (Table 4). Out of the 40 S. gilvifrons eggs, only 28 adults emerged of which 15 were females (53.6%) and 13 were males (46.4%) indicating a 1: 0.86 female:male sex ratio.
Life table studies
Adults of S. gilvifrons lived an average of 117 days and females lived longer than males (Table 5). Adults mated 24 h after emergence and mated females began laying eggs after an average preovipositon period of 5.3 days. Females oviposited for 97.9 days and average life time egg production was 149. Average daily oviposition was 4.4 eggs/day. Age-specific survival rate (l x ) and age-specific fertility (m x ) are presented in Fig. 2. Intrinsic rate of natural population increase (r) was 0.066 day−1, while net reproductive rate (R o) was estimated as 72.2 eggs/female. Gross reproduction rate (Σm x ) was 82.3 eggs/female, generation time (T) 64.9 days, doubling time (DT) 10.5 days and finite rate of increase (λ) 1.1 day−1.
Predatory efficiency of Stethorus gilvifrons
Number of mites consumed by S. gilvifrons larvae increased from the 1st to the 4th instar. Adult female beetles consumed significantly more RSM eggs (F 4,24 = 252.09, P < 0.001), larvae (F 4,24 = 161.38, P < 0.001), nymphs (F 4,24 = 99.23, P < 0.001) and adults (F 4,24 = 101.86, P < 0.001) than the males and larval instars (Table 6).
Discussion
Peaks in population density of S. gilvifrons coincided with those of O. coffeae. Kishimoto (2002) reported the seasonal occurrence of spider mites and their predators in three Japanese pear orchards where predacious beetles such as Stethorus japonicus Kamiya (Coccinellidae) and Oligota spp. (Staphylinidae) and predatory thrips, Scolothirps takahashii Priesner (Thripidae) were abundant and their population trend was closely associated with that of the prey. Roy et al. (2005) reported that seasonal activity of the predators Stethorus punctillum Weise (Coccinellidae) and Neoseiulus fallacis (Garman) (Acari: Phytoseiidae) also coincided with that of their prey, Tetranychus mcdanieli McGregor in raspberry. Stethorus gilvifrons completely disappeared from tea fields in June when RSM density became very low.
The pre-oviposition and oviposition periods and the duration of developmental stages of S. gilvifrons were similar to those of other species, such as Stethorus siphonulus Kapur on Tetranychus urticae Koch in Papaw (Raros and Haramoto 1974), Stethorus picipes Casey on Oligonychus punicae Hirst (Tanigoshi and McMurtry 1977), Stethorus vagans (Blackburn) on T. urticae (Ullah 2000), and S. japonicus on T. urticae (Mori et al. 2005). Total fecundity at 25 ± 1°C in S. gilvifrons (149 eggs) was less than that measured at or around the same temperature for S. japonicus (501 eggs; Mori et al. 2005), Stethorus madecassus Chazeau (184; Chazeau 1974), S. picipes (221; Tanigoshi and McMurtry 1977) and S. punctillum (279; Roy et al. 2003), suggesting that S. gilvifrons has lower total fecundity at this temperature in comparison with above mentioned species. The sex ratio obtained for S. gilvifrons was within the range of values reported for S. japonicus, Stethorus punctum (LeConte), S. siphonulus, S. vagans and Stethorus vinsoni Kapur (Putman 1955; Raros and Haramoto 1974; Tanigoshi and McMurtry 1977; Ullah 2000; Mori et al. 2005). Longevity of S. gilvifrons was also similar to that of other Stethorus species (Mathur 1969; Puttaswamy and Channabasavanna 1977; Richardson 1977).
The intrinsic rate of natural increase (r) is a key demographic parameter useful for predicting the population growth potential of an animal under given environmental conditions (Ricklefs and Miller 2000). The r value of S. gilvifrons (0.066) was less than that measured for Stethorus loxtoni Britton & Lee (0.152; Richardson 1977), S. madecassus (0.155; Chazeau 1974), S. picipes F 4,24 = 252.09, P < 0.001 (0.121; Tanigoshi and McMurtry 1977), Stethorus loi Sasaji. (0.160; Shih et al.1991), S. japonicus (0.156; Mori et al. 2005) and S. gilvifrons (0.133; Taghizadec et al. 2008). Theoretically, a predator that has a population growth rate equal to or greater than its prey should efficiently regulate the population of its prey (Sabelis 1992). In biological control practice, r value is increasingly used as a means for selecting promising biocontrol candidates on the basis of their reproductive potential and to predict the outcome of pest-natural enemy interactions (Jervis and Copland 1996). The r value of S. gilvifrons was lower than that of its prey, O. coffeae on tea at 25 ± 2°C (Muraleedharan et al. 2005). Nevertheless, the voracious S. gilvifrons consistently suppressed populations of O. coffeae. A similar situation was observed by McMurtry et al. (1974) on S. picipes (r m = 0.12 day−1 at 25°C) preying on O. punicae on avocados. It is possible that under favorable conditions, S. gilvifrons can eliminate prey more rapidly than they can reproduce.
Adults and larvae of S. gilvifrons fed on all stages of RSM, and adult females consumed more RSM than larvae. Similar results were reported by Ahmed and Ahmed (1989), Afshari (1999) and Fiaboe et al. (2007). The high rate of RSM consumption by S. gilvifrons adults suggested that this effective winged predator has certain advantages as a potential control agent due to its high longevity and ability to rapidly locate RSM ‘hot spots’. Therefore, conservation and augmentation of this predator in the tea ecosystem may prove to be an essential component in the IPM strategy against red spider mite.
References
Afshari GA (1999) A survey on the ladybirds belong to genus Stethorus and study on the biology, prey consumption and population dynamics of Stethorus gilvifrons in sugarcane farms in Khuzestan, Iran. M.Sc.Thesis, Ahvaz, Iran
Ahmed ZI, Ahmed RF (1989) Biological studies of predator Stethorus gilvifrons Mulsant (Coleoptera: Coccinellidae) on the strawberry spider mite, Tetranychus turkestani Ugarov and Nikolski (Acari: Tetranychidae). J Biol Sci Res 20:20–23
Brich LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Chazeau J (1974) Developpement et fecondite de Stethorus madecassus (Coleopteres: Coccinellidae), eleve en conditions exterieures dans le sud-ouest de Madagascar. Cah. ORSTOM ser Biol 25:27–33
Fiaboe KKM, Gondim MGC Jr, de Moraes GJ, Ogol CKPO, Knapp M (2007) Bionomics of the acarophagous ladybird beetle Stethorus tridens fed Tetranychus evansi. J Appl Entomol 131(5):355–361
Jervis MA, Copland MJW (1996) The life cycle. In: Jervis M, Kidd B (eds) Insect natural enemies: practical approaches to their study and evaluation. Chapman and Hall, London, pp 63–161
Kishimoto H (2002) Species composition and seasonal occurrence of spider mites (Acari: Tetranychidae) and their predators in Japanese pear orchards with different agrochemical spraying programs. Appl Entomol Zool 37:603–615
Mackauer M (1983) Quantitative assessment of Aphidius smithi (Hymenoptera: Aphidiidae): fecundity, intrinsic rate of increase, and functional response. Can Entomol 115:399–415
Mathur LML (1969) Bionomics of Stethorus gilvifrons (Coleoptera: Coccinellidae). Madras Agric J 56:7–11
McMurtry JA, Huffaker CB, Van de Vrie M (1970) Ecology of tetranychid mites and their natural enemies: a review. I. Tetranychid enemies: their biological characters and the impact of spray practices. Hilgardia 40:331–390
McMurtry JA, Scriven GT, Malone RS (1974) Factors affecting oviposition of Stethorus picipes (Coleoptera: Cocinellidae) with special reference to photoperiod. Environ Entomol 3:123–127
Mori K, Nozawa M, Arai K, Gotoh T (2005) Life history traits of the acarophagous lady beetle, Stethorus japonicus at three constant temperatures. Biocontrol 50:35–51
Muraleedharan N (1988) Final technical Report of the CISR project “Studies on the distribution pattern and biology of the important insect and mite predators and parasites of caterpillars, aphids, thrips and mites infesting tea in South India”. UPASI Tea Research Institute, Valparai, p 80 (Mimeographed)
Muraleedharan N (1995) Biological control of tea pests. In: Ananthakrishnan TN (ed) Biological control of social forest and plantation crops insects. Oxford & IBH Publishing, New Delhi, pp 97–108
Muraleedharan N, Sudarmani DNP, Selvasundaram R (2005) Bioecology and management of the red spider mite infesting tea in south India. In: Proceedings of International symposium on Innovation in tea science and sustainable development in tea industry. China Tea Science Society, Hangzhou, China. pp 756–766
Putman WL (1955) Bionomics of Stethorus punctillum Weise (Coleoptera: Coccinellidae) in Ontario. Can Entomol 87:9–33
Puttaswamy, Channabasavanna GP (1977) Biology of Stethorus pauperculus Weise (Coleoptera: Coccinellidae) a predator of mites. Mysore J Agr Sci 11(1):81–89
Raros ES, Haramoto FH (1974) Biology of Stethorus siphonulus Kapur (Coccinellidae: Coleoptera), a predator of spider mites, in Hawaii. P Hawaiian Entomol Soc 21:457–465
Richardson NL (1977) The biology of Stethorus loxtoni Britton & Lee (Coleoptera: Coccinellidae) and its potential as a predator of Tetranychus urticae Koch (Acarina: Tetranychidae) in California. Ph.D. Thesis. University of California, Berkeley, California, pp 184
Ricklefs RE, Miller GL (2000) Ecology, 3rd edn. Freeman and Company, New York
Roy M, Brodeur J, Cloutier C (2003) Effect of temperature on intrinsic rates of natural increase (rm) of a coccinellid and its spider mites prey. Biocontrol 48:57–72
Roy M, Brodeur J, Cloutier C (2005) Seasonal activity of the spider mite predators Stethorus punctillum (Coleoptera: Coccinellidae) and Neoseiulus fallacis (Acarina: Phytoseiidae) in raspberry, two predators of Tetranychus mcdanieli (Acarina: Tetranychidae). Biol Control 34:47–57
Sabelis MW (1992) Predatory arthropods. In: Crawley MJ (ed) Natural enemies. The population biology of predators, parasites and disease. Blackwell, Oxford, pp 225–264
Selvasundaram R, Muraleedharan N (2003) Red spider mite-biology and control. Hand book of tea culture, Sect. 18. UPASI Tea Research Foundation, Valparai, p 4
Shih CIT, Lin PJ, Chang TW (1991) Biology, predation, life table and intrinsic rate of increase of Stethorus loi Sasaji. Plant Prot Bull Taipei 33:290–300
Southwood TRE (1978) Ecological methods with particular reference to the study of insect population. Chapman and Hall, London, pp 543–562
Taghizadec R, Fathipour Y, Kamali K (2008) Influence of temperature on life table parameters of Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae) fed on Tetranychus urticae Koch. J App Entomol 132:638–645
Tanigoshi LK, McMurtry JA (1977) The dynamics of predation of Stethorus picipes (Coleoptera: Coccinellidae) and Typhlodromus floridanus on the prey Oligonychus punicae (Acarina: Phytoseiidae, Tetranychidae). I. Comparative life history and life table studies. Hilgardia 45:237–261
Ullah I (2000) Aspects of the biology of the ladybird beetle Stethorus vagans (Blackburn) (Coleoptera: Coccinellidae). Ph.D. thesis, University of Western Sydney, Hawkesbury, Richmond, NSW, Available via DIALOG. http://library.uws.edu.au/adt-NUWS/public/adt- NUWS20031103.132342
Acknowledgments
The authors are grateful to the National Tea Research Foundation (NTRF), Kolkata for financial assistance and Dr. P. Mohan Kumar, Director, UPASI Tea Research Foundation, Tea Research Institute, Valparai for his constant encouragement and support during the study period.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perumalsamy, K., Selvasundaram, R., Roobakkumar, A. et al. Life table and predatory efficiency of Stethorus gilvifrons (Coleoptera: Coccinellidae), an important predator of the red spider mite, Oligonychus coffeae (Acari: Tetranychidae), infesting tea. Exp Appl Acarol 50, 141–150 (2010). https://doi.org/10.1007/s10493-009-9290-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-009-9290-y