Abstract
Acaricidal and sublethal effects of the biopesticide Requiem®EC (containing an essential oil extract of Chenopodium ambrosioides near ambrosioides) on the two-spotted spider mite, Tetranychus urticae Koch, were evaluated in laboratory bioassays. The biopesticide was applied to bean leaves or leaf discs using a Potter spray tower. Acaricidal activity against eggs and immatures was evaluated in successive acute toxicity bioassays. Concentration-mortality data were subjected to probit analysis and the following LC50 values (ml/l) were calculated: 2.47 (eggs), 0.71 (larvae), 1.13 (protonymphs), 2.23 (female deutonymphs), and 6.02 (female teleiochrysalises). In adult bioassay, in which pre-ovipositional females were treated with a series of concentrations (0.31–10 ml/l), a run-off effect ranging 4–80% (after 24 h) and 8–93% (after 72 h) was observed. In two-choice bioassay, T. urticae females preferred the untreated halves of leaves over the halves treated with 1.25–10 ml/l biopesticide and they laid significantly more eggs on the untreated halves in the first 24 h and summed over 72 h. The indices of repellency and oviposition deterence ranged 11.2–77.3 and 14.8–87.9%, respectively. In age-stage two-sex life table bioassay, the females that hatched from eggs treated with 2.5 ml/l biopesticide and reached adulthood on treated leaf surface showed a significantly reduced the intrinsic rate of increase (r = 0.222), compared to the control (r = 0.317). The reduction of population growth was mainly due to a reduced preadult survival rate (0.42 ± 0.04) and extended juvenile developmental time (9.27 ± 0.11 days), compared to the control (0.93 ± 0.03 and 7.70 ± 0.06 days, respectively).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite, Tetranychus urticae Koch, is a highly polyphagous species that attacks more than 1100 host plant species, including a number of agricultural crops and ornamentals, especially in greenhouses where this mite is a major pest (Zhang 2003; Migeon and Dorkeld 2016). Pesticide (acaricide) application has been a predominating method of spider mite control over the past decades. Due to an exceptional natural potential for rapid evolution of resistance and strong selection pressure through chemical control, the resistance of T. urticae to acaricides has become a global phenomenon (Van Leeuwen et al. 2009; Whalon et al. 2016). Ever since the initial reports on resistant populations of tetranychids back in the 1950s, chemical industry has sought to solve the resistance problems by developing and introducing acaricides with novel mechanisms of activity (Knowles 1997; Dekeyser 2005; Marčić 2012; Van Leeuwen et al. 2015). New acaricide active ingredients are currently being introduced under growing legislative restrictions concerning toxicological and ecotoxicological criteria. The resistance problem, relatively limited choices of possible new active ingredients, and public demand for reducing health and environmental risks have shifted the focus of efforts to biopesticides as an alternative to synthetic compounds. Biopesticides, defined as commercial pest control agents manufactured from living organisms or their products, account for a mere 4–5% of the global pesticide market but this percentage is expected to rise to around 20% over the next ten years (Chandler et al. 2011; Villaverde et al. 2014; Isman 2015).
Essential oils, secondary metabolites extracted from aromatic plant species belonging mostly in the families Lamiaceae, Myrtaceae and Rutaceae, are rich source of potentially effective biopesticides (Regnault-Roger et al. 2012; Attia et al. 2013). There have been an increasing number of reports on acaricidal activity of various essential oils and monoterpenes as their major constituents against spider mites (Miresmailli et al. 2006; Badawy et al. 2010; Attia et al. 2011; Araújo et al. 2012; Roh et al. 2013). On the other hand, a few commercial products based on essential oils have been registered for spider mite control in developed countries. The biopesticide Requiem®EC is a commercial formulation manufactured from extracts of American wormseed (or epazote), Chenopodium ambrosioides variety near ambrosioides (Chenopodiaceae), a herbaceous plant species indigenous to Central and South America. The extract is a mixture of monoterpenes (mainly α-terpinene, p-cymene and limonene) formulated as a product labeled for control of insect and mite pests (Chiasson et al. 2004a, b; Regnault-Roger et al. 2012). However, experimental data about its acaricidal properties have remained scarce.
Knowing the biological activity profile of any pesticide obtained in laboratory bioassays is an important precondition for its sustainable use in practice. Besides assessing concentration-mortality responses of different life stages, quantification of effects on the behavior and life history traits of individuals that survived exposure is also necessary for evaluating the overall impact of a pesticide. Moreover, the individual-level effects on life history traits should be integrated and expressed at a hierarchically higher population level, considering that population growth rate is generally recognized as an ecologically more relevant endpoint (Forbes and Calow 2002; Sáenz-de-Cabezôn Irigaray and Zalom 2009; Guedes et al. 2016). One of widely used methods for assessing the response of arthropod populations to pesticides is the use of age-specific life tables with data on female survivorship and production of female progeny (fertility), which provides a basis for calculating the intrinsic rate of increase (Birch 1948; Carey 1993; Stark and Banks 2003). Application of these female-based life tables was criticized by Chi (1988) primarily for disregarding natural variation in juvenile developmental times among females in cohorts, but also for excluding males from the statistics, which may hide significant errors in calculations of population growth parameters. A new approach was therefore proposed based on constructing age-stage two-sex life tables from data for both females and males and on including the variability of juvenile development into a theoretical model (Chi 1988; Huang and Chi 2012a). This approach has recently received a growing acceptance in demographic analyses of effects of various factors on population growth of spider mites (Kavousi et al. 2009; Khanamani et al. 2013; Yin et al. 2013; Maleknia et al. 2016; Tuan et al. 2016), including the effects of acaricides (Wang et al. 2014, 2016; Mohammadi et al. 2016).
Adaptive strategy of the two-spotted spider mite as a colonizing species is based on high reproduction of young adult females as the main dispersers (Sabelis 1985). On the other hand, heterogeneity of spray coverage in the field enables population recovery on poorly covered or uncovered plant surfaces (Sáenz-de-Cabezôn Irigaray and Zalom 2009; Martini et al. 2012), which depends on the reproductive potentials of females that survived or avoided exposure. In that context, the objective of this study was to obtain baseline data regarding acute toxicity of the biopesticide Requiem®EC to different life stages and to evaluate its sublethal effects on behavior and population growth of T. urticae. The results of the study are discussed in terms of population biology of this important mite pest.
Materials and methods
Test organism
A population of T. urticae, set up from samples collected in a ruderal weed habitat on the outskirts of Belgrade, has been reared on bean plants in a climate controlled room (25–30 °C, 16/8 h of light/dark photoperiod) since March 2004.
Biopesticide
The commercial product Requiem®EC (manufactured by Bayer CropScience, Germany), an emulsifiable concentrate formulation containing 25% essential oil extract from Chenopodium ambrosioides near ambrosioides, was obtained from the company representative office in Serbia.
Bioassays
All bioassays were carried out on primary bean leaves or leaf discs (30 mm diameter) positioned upon moistened cotton wads in Petri dishes with the abaxial surface upward. The Petri dishes were kept in a climate chamber at 27 ± 2 °C, under 50–70% RH and 16/8 h of light/dark photoperiod. Leaves and leaf discs were sprayed with the biopesticide suspended in distilled water using a Potter spray tower (2 ml of liquid, 100 kPa air pressure, aqueous deposit 2.7 ± 0.2 mg/cm2).
Acute toxicity bioassays
The acaricidal activity of Requiem®EC against eggs, larvae, protonymphs, female deutonymphs, and female teleiochrysalises of T. urticae was assessed in successive acute toxicity bioassays carried out in five replications by spraying serially diluted concentrations of the biopesticide covering a range of 10–90% mortality. The concentrations were chosen after preliminary tests. Controls were sprayed with distilled water only. The egg bioassay consisted of spraying primary bean leaves that carried 30–50 eggs (24 h old) per leaf. In bioassays with the other stages, leaf discs containing 20 mites per disc were sprayed. Mortality in the bioassays with eggs and motile immature stages was assessed based on the number of treated mites reaching their adult stage. In the bioassay with female teleiochrysalises mortality was assessed from the number of emerged females alive 48 h after spraying. Concentration-mortality data were subjected to probit analysis using the POLO Plus software (LeOra Software, Berkeley, CA, USA). A pairwise comparison of LC50 and LC90 of the life stages was performed using the lethal dose ratio test: if 95% confidence limits for LC ratios included 1 the LCs were not significantly different (Robertson et al. 2007).
The bioassay with preovipositional adult females was set up in five replicates by spraying leaf discs that carried five females per disc (1–4 discs per replication) with the following concentrations of the biopesticide (ml/l): 10 (field recommended rate), 5, 2.5, 1.25, 0.62, and 0.31. Live females, dead females (those not able to move away at least one body length) and run-off females (repelled females that abandoned leaf discs and were trapped in surrounding cotton wads) were counted 24, 48 and 72 h after spraying. Mortality and repellency were calculated as percentage of dead and run-off females, respectively, to the total number of treated females. Fecundity of the treated females, i.e., the number of eggs laid per female at the midpoint of 24-h interval, summed over 72 h (with an assumption that all deaths and run-offs occurred at the midpoint) (Carey 1993) was calculated as well. Mortality, repellency, and fecundity data were analyzed by one-way analysis of variance with the means separated by Fisher’s least significant difference (LSD) test (α = 0.05). Prior to analysis, mortality and repellency data were arcsin√x-transformed, whereas fecundity data were √x-transformed.
Behavioral bioassay
To assess the repellent and oviposition-deterrent effects of Requiem®EC on T. urticae female adults, a two-choice bioassay was carried out in five replications. One half of each leaf was covered with a thin plastic semicircle sheet with its vertical edge running along leaf midrib, and the leaves were sprayed with a series of concentrations (0.31–10 ml/l). After deposits dried, the sheets were removed and 20 adult females (1-day old) were placed on each leaf midrib. Live and dead females, and the eggs they laid, were counted for each 24-h interval over 72 h both on the treated and untreated (covered) halves of each leaf. The run-off females were counted as well.
Repellency and oviposition deterrence data were analyzed using Student’s t test (α = 0.05). Cumulative indices of repellency/oviposition deterrence (%) were calculated as:
where C is the number of mites/eggs on the untreated half, and T is the number of mites/eggs on the treated half, assessed for each 24-h interval and summed over 72 h (Sundaram and Sloane 1995; Takakura 2009; Marčić and Međo 2015). Cumulative run-off effect was calculated as percentage of run-off females to the total number of females.
Demographic toxicity bioassay
To assess the effects of Requiem®EC on demographic parameters of T. urticae, life tables were constructed for two cohorts of mites treated at the egg stage: (1) with distilled water (control cohort; n = 72 eggs) and (2) with the biopesticide applied at 2.5 ml/l, i.e., the concentration within 95% confidence limits of the LC50 estimate for this stage (treatment cohort; n = 144 eggs). The cohorts were formed by placing a single fertilized adult female on each leaf disc and removing it along with all eggs laid, except one, after 24 h. The mites that hatched from those eggs completed their juvenile development on the same treated leaf surfaces. Development and survival were observed daily. Newly emerged preovipositional adult females were transferred individually to untreated leaf discs and paired with adult males. Because more females than males emerged, the missing number of males was transferred from two additional cohorts formed from 20 unfertilized females each (4 discs × 5 females) that laid eggs for 24 h and were treated either with distilled water or the biopesticide. The pairs were kept together and their survival and the number of eggs laid were recorded daily until all individuals were dead.
The raw life history data for females and males (except those obtained from additional cohorts), as well as individuals of unknown sex that died before reaching the adult stage, were analyzed in terms of age-stage two-sex life table theory (Chi and Liu 1985; Chi 1988; Huang and Chi 2012a). The age-specific survival rate (l x ) was calculated as:
where x = age, j = stage, k = the number of stages, and s xj = the age-stage specific survival rate, i.e., the probability that a newborn egg will survive to age x and stage j. The age-specific fecundity (m x ) was calculated as:
where x = age, j = stage, k = the number of stages, s xj = the age-stage specific survival rate, and f xj = the female age-stage-specific fecundity, i.e., the mean fecundity of individuals of age x and stage j. Weighting fecundity by survivorship (l x × m x ) gives the age-specific net maternity. These two age-specific functions were used for estimating several demographic parameters (Kavousi et al. 2009; Khanamani et al. 2013; Tuan et al. 2016). The gross reproductive rate (GRR) was calculated as:
The net reproductive rate (R 0), which expresses a multiplication factor of a population in one generation, i.e., generation growth rate, was calculated as:
The intrinsic rate of increase (r) was estimated using the iterative bisection method from the Euler–Lotka equation:
with age indexed from 0 (Goodman 1982). This parameter is defined as the rate of natural increase in a closed population with constant age-specific mortality and fertility schedules and a stable age distribution. The finite rate of increase (λ), which is a multiplication factor of a population at each time unit, i.e., daily growth rate, was calculated as:
The mean generation time (T), defined as the length of time that is required for a population to increase R 0-fold of its size, was calculated as:
TWOSEX-MSChart software (Chi 2015) was used for data analysis and calculation of parameters. Standard errors were estimated by using the bootstrap technique (Efron and Tibshirani 1993; Huang and Chi 2012b). To obtain stable estimates 100,000 bootstrap replicates were used. Differences between control and treatment were compared by using the paired bootstrap test based on the confidence interval of difference (Tuan et al. 2016).
Results
Acaricidal activity against eggs and immatures
The Chenopodium-based biopesticide Requiem®EC showed various levels of acaricidal activity against different life stages of T. urticae (Table 1). With the lowest LC50 and LC90, larvae were the most susceptible of the life stages. As juvenile development progressed, the biopesticide’s acute toxicity decreased, so that protonymphs were significantly less susceptible than larvae, and deutonymphs less so than protonymphs, whereas teleiochrysalises were the least sensitive pre-adult stage. The biopesticide showed no toxic (ovicidal) action when a concentration series from 0.84 to 3.5 ml/l was applied against eggs. Almost all (≥97%) of the eggs hatched but larval emergence was delayed and juvenile development extended for approximately 1–3 days, depending on concentration. The acaricidal effect was actually the result of a residual action on larvae that emerged from the treated eggs; those larvae were 3.5× and 4× less susceptible than larvae sprayed directly, as indicated by their respective LC50 and LC90.
Biological effects on preovipositional females
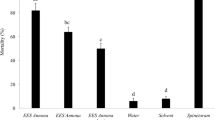
Repellency (run-off) was the major effect of treatments of T. urticae preovipositional adult females, whereas mortality was a minor effect. Figure 1 shows cumulative repellency and mortality effects 72 h after treatment of females. Repellency was most prominent 24 h after treatment, when the percentage of repelled (run-off) females ranged from 4 to 80%, whereas the range was 4–88% 48 h after treatment and 8–93% 72 h after treatment. A bell-shaped profile of repellency was observed: increasing effect at 0.62–5 ml/l was followed by a decrease at 10 ml/l.
Repellency and mortality in Tetranychus urticae females treated with Requiem®EC (ml/l) at preovipositional period (the numbers of run-off and dead females summed over 72 h; mean ± SE, different capital and lower case letters indicate significant differences; ANOVA, followed by Fisher LSD test, P < 0.05)
Mortality of the treated females was 2–28% (after 24 h), 2–40% (after 48 h) and 4–44% (after 72 h). A statistically significant difference in cumulative mortality between control and treatment was found only in females treated with the highest concentration (F 6,28 = 8.54, P < 0.001). All treatments except the one with the lowest concentration caused repellent effects significantly higher than effects in the control. (F 6,28 = 42.88, P < 0.001).
Treated T. urticae females that remained on leaf discs were able to lay eggs. The fecundity of treated females decreased as concentrations increased (Fig. 2). Treatments with concentrations of 10 and 5 ml/l reduced the number of eggs laid almost to none, and fecundity was also reduced significantly by concentrations of 2.5 and 1.25 ml/l. Fecundity decreased also after treatments with the two lowest concentrations but the differences from control data were not significant (F 6,28 = 39.53, P < 0.001).
Behavioral bioassay: repellent and oviposition-deterrent effects
The behavior of T. urticae females was shown in the choice test to be strongly affected by the biopesticide. They preferred the untreated halves of leaves rather than treated halves and this repellent effect was significant for each 24-h interval, and for the cumulative effect, in treatments with 10, 5, and 2.5 ml/l concentrations. The cumulative repellent effect was also significant in treatments with 1.25 and 0.62 ml/l (Fig. 3).
Females also preferred to lay eggs on the untreated rather than treated halves of leaves (Fig. 4). This oviposition-deterrent effect was significant cumulatively in all treatments. When counted for each 24-h interval, the number of eggs laid on the untreated halves was significantly greater in the treatments with 10, 5, 2.5, and 1.25 ml/l. The following indices of repellency and oviposition deterrence were calculated: 13.4 ± 10.1 and 14.8 ± 3.8 (0.31 ml/l), 11.2 ± 5.1 and 27.2 ± 1.7 (0.62 ml/l), 31.5 ± 4.8 and 42.0 ± 5.1 (1.25 ml/l), 50.5 ± 9.8 and 55.2 ± 7.5 (2.5 ml/l), 51.4 ± 5.2 and 68.4 ± 4.7 (5 ml/l), 77.3 ± 7.0 and 87.9 ± 3.8 (10 ml/l).
Cumulative mortality of females was low (≤10%). On the other hand, a run-off effect was observed: some of the females left the leaves even though they had a choice of their treated and untreated halves. The following percentages of cumulative run-off effect were calculated: 4.0 ± 1.9 (0.31 ml/l), 20.0 ± 4.2 (0.62 ml/l), 43.0 ± 6.4 (1.25 ml/l), 10.0 ± 3.5 (2.5 ml/l), 17.0 ± 2.6 (5 ml/l), and 46.0 ± 8.3 (10 ml/l); 76% of all run-off females were found along the margins of treated surfaces.
Demographic bioassay: effects on life-table parameters
Exposure to the biopesticide Requiem®EC affected the survivorship of T. urticae. Figure 5 shows that immatures that hatched from the treated eggs and were exposed to residual action of the biopesticide had considerably lower age-stage-specific survival rates (s xj ) than those in the control. The second important effect which is evident on that graph is a prolongated developmental time and extended overlapping between immature and adult stages in exposed individuals (Fig. 5). The preadult survival rate was 0.42 ± 0.04 and 0.93 ± 0.03, in treatment and control, respectively, whereas juvenile development lasted 9.27 ± 0.11 days and 7.70 ± 0.06 days, respectively; these differences between treatment and control were significant for both parameters (the paired bootstrap test).
The age-specific survival rates of total cohorts (l x ), presented in Fig. 6a, show with more clarity the considerable reduction in survivorship of the treated mites, compared to the control. On the other hand, age-specific fecundity (m x ) in the treated cohort was higher at the peak of reproduction and over most of the ovipositional period. However, reproduction began later in the treated females (ca. 1.6 days) due to their prolonged development (Fig. 6b). Total preovipositional periods lasted 10.56 ± 0.14 days in treated mites and 8.92 ± 0.04 days in control mites, and this difference was significant (the paired bootstrap test). The age-specific net maternity (l x × m x ) which weights fecundity data by survivorship (Fig. 6c) was considerably higher in the treated cohort over the entire oviposition period, especially at the peak of oviposition.
Demographic parameters of T. urticae in the control and treated cohorts are shown in Table 2. The paired bootstrap test revealed significant differences between the cohorts in all parameters, except gross reproductive rate (GRR). Considering that GRR is the sum of age-specific fecundity data over the lifetime of a cohort, this finding was expected. On the other hand, R 0 as the sum of net reproductive schedule was found 2.4-fold higher in the control than in the treated cohort. A significantly higher r was found in the control cohort due both to higher R 0 and shorter developmental time; consequently, the value of λ was significantly higher as well. Mean generation time was significantly extended (for almost 2 days) in the treated cohort.
Discussion
Biological profile showing the toxicity of Requiem®EC to different life stages of T. urticae revealed a lack of ovicidal effect, followed by a considerable residual toxicity to hatched larvae, as well as a higher toxicity to directly treated younger immature stages (larvae, protonymphs) than to older ones (deutonymphs, teleiochrysalises). Direct treatment of female adults had run-off as its major effect. To the best of our knowledge, the only available research data about the acaricidal properties of this biopesticide were reported by Chiasson et al. (2004a) who tested its toxicity to eggs and female adults of the two-spotted spider mite. These authors reported 86% hatch of the eggs sprayed on bean leaf discs with the biopesticide applied at 20 ml/l (2% v/v), which is comparable to our study in which all eggs hatched after spraying with 3.5 ml/l. The authors assessed contact toxicity to female adults by spraying mites that were mounted dorsally on pieces of double-coated tape glued on glass microscope slides and observed 95% mortality 48 h after spraying the slides with 20 ml/l. This result is not comparable to our findings because this technique, substantially different from the one we used, prevented mites from responding behaviorally and probably overestimated mortality. Chiasson et al. (2004a) also assessed the residual toxicity to females placed on bean leaf discs after spraying with 20 ml/l and found mortality not to be significantly different from the control after 48 h exposure; the authors, however, did not specify if possible behavioral response of treated females was assessed.
Repellent or run-off effect has been observed concomitantly with various levels of mortality in acute toxicity bioassays in which female adults of spider mites were exposed to botanical biopesticides such as various azadirachtin-based products (Mansour et al. 1997; Sundaram and Sloane 1995; Međo et al. 2015), essential oils and other plant extracts (Mansour et al. 1986, 2004; Manjunatha Reddy et al. 2014), as well as pyrethroids, synthetic compounds modeled on natural products of plant origin (Fisher and Wrensch 1986; Riedl and Shearer 1991; Holland and Chapman 1994, 1995). A bell-shaped pattern of repellent effect was observed in our bioassay with T. urticae females: an increase of the effect with increasing concentrations of Requiem®EC was eventually followed by a significant decrease at the highest concentration tested. This pattern indicated that a point was reached when a substantial number of treated females became too intoxicated to disperse from leaf discs, as observed previously in bioassays with pyrethroids (McKee et al. 1987; Holland and Chapman 1994) and azadirachtin (Mansour et al. 1997).
In our choice test, a part of T. urticae females ran away from the leaves (even though they had a choice of treated and untreated surface) whereas most of the others preferred to settle and lay eggs on the halves of leaves not treated with Requiem®EC. Repellent and oviposition deterrent effects on spider mites have often been the result of choice tests with essential oils and other botanicals (Dimetry et al. 1993; Sundaram and Sloane 1995; Kumral et al. 2010; Araújo et al. 2012; Roh et al. 2013). Run-off effects in addition to repellency and oviposition deterrence have been reported in choice bioassays with azadirachtin (Sundaram and Sloane 1995; Marčić and Međo 2015). This effect indicates that some females responding behaviorally may not be able to reach untreated surface. Repellent and oviposition deterrent effects of Requiem®EC have also been observed in a bioassay with potato psyllid Bactericera cockerelli (Hemiptera: Psyllidae) (Yang et al. 2010) and pepper weevil Anthonomus eugenii (Coleoptera: Curculionidae) (Addesso et al. 2014).
Our demographic toxicity bioassay showed that treatment of eggs with Requiem®EC and its residual toxic activity against hatched individuals during their juvenile development on treated surface, significantly reduced population growth rates of T. urticae. The reduction was a consequence of lower survivorship of hatched mites in the treated cohort and extended juvenile development and preovipositional period of those mites that survived residual exposure and reached the adult stage. The age of first reproduction determines the value of r in such a way that the earlier an egg is laid the greater is its contribution to population growth rate; the higher the r, the more sensitive it is to decreases in developmental time (Snell 1978; Sabelis 1985). It is therefore of crucial importance to determine precisely the duration of preovipositional period, which is possible using the age-stage two-sex life tables, that take into account the stage overlapping and variation in developmental time among individuals. On the other hand, traditional female age-specific life tables are based on the mean of developmental time, assuming that all females have the same preadult duration and emerged at the same age, which may cause errors in calculating r and other demographic parameters (Huang and Chi 2012a; Khanamani et al. 2013). Few reports are available currently that assess the effects of pesticides on spider mites by employing the age-stage two-sex life table theory. Wang et al. (2014) treated T. urticae eggs with the pyrethroid bifenthrin at LC10 and LC25, and transferred hatched larvae onto untreated leaf surface. They found that the juvenile developmental time and preovipositional period were significantly extended, and R 0, r and λ significantly reduced. Mohammadi et al. (2016) assessed sublethal effects of a citronellol-based biopesticide on T. turkestani: adult females were exposed to the biopesticide applied at LC25 and removed after 6 h, whereas eggs laid by the females were kept and development and reproduction of hatched mites took place on treated leaf surface. They observed an (unexpected) increase in population growth rates in treatment as a consequence of significantly decreased developmental time and increased fecundity.
Our bioassays with T. urticae female adults revealed a strong behavior-modifying action of the biopesticide Requiem®EC at application rates ranging from the field relevant concentration of 10 ml/l, to 1/16 or 1/32 of that concentration. Most of the females treated directly ran off the treated surface, and a considerable part of the females having a choice between treated and untreated surface also walked away from the leaves, whereas most of the others preferred the untreated over treated leaf surface. The authors of several studies dealing with acute toxicity of pyrethroids and some other acaricides to spider mites (Fisher and Wrensch 1986; Riedl and Shearer 1991) considered the run-off mites as a part of total mortality (or biological effectiveness) together with the dead mites found on treated plant surface. They argued that mites leaving such treated surface would otherwise either be killed after reaching another treated plant (leaf) surface or die of starvation away from their host plant. However, spray coverage is spatially and temporally heterogenous, often leaving refugia, i.e., poorly covered, uncovered or newly expanded areas on leaves (Sáenz-de-Cabezôn Irigaray and Zalom 2009; Martini et al. 2012) that could be colonized by spider mites leaving or avoiding the treated surface. In these circumstances, the concept of biological effectiveness should yield to the concept of population recovery which depends on the reproductive potential of mites reaching refugia.
Considering the stable age distribution of T. urticae in which eggs, immatures, and adults account for around 66, 26, and 8%, respectively (Carey 1982), it could be predicted from our results that the concentration of 10 ml/l (i.e., 27 nl/cm2, assuming aqueous deposit 2.7 μl/cm2) would eliminate the largest part of population (virtually all immatures), while most of the surviving females would respond behaviorally. At lower applied concentrations, such behavioral response would be still evident, and a part of immature spider mites would also survive. The concentration that killed around 50% of the mites that hatched from treated eggs (and large numbers of other immatures) would reduce population growth significantly, but survivors would still retain a considerable potential for recovery. Besides assessing the concentration-dependent mortality across life stages, our study also represents the first report on sublethal effects of the biopesticide Requiem®EC on T. urticae and spider mites. Further research focusing on sublethal effects to behaviorally responding females is needed to evaluate the overall impact of Requiem®EC on two-spotted spider mites and to obtain additional data that could improve the use of this biopesticide product.
References
Addesso K, Stansly P, Kostyk B, Mcauslane H (2014) Organic treatments for control of pepper weevil (Coleoptera: Curculionidae). Fla Entomol 97:1148–1156
Araújo MJC, Câmara CAG, Born FS, Moraes MM, Badji CA (2012) Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp Appl Acarol 57:139–155
Attia S, Grissa KL, Lognay G, Heuskin S, Mailleux AC, Hance T (2011) Chemical composition and acaricidal properties of Deverra scoparia essential oil (Araliales: Apiaceae) and blends of its major constituents against Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 104:1220–1228
Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC (2013) A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae), with special reference to natural pesticides. J Pest Sci 86:361–386
Badawy MEI, El-Arami SAA, Abdelgaleil SAM (2010) Acaricidal and quantitative structure activity relationship of monoterpenes against the twospotted spider mite, Tetranychus urticae. Exp Appl Acarol 52:261–274
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Carey JR (1982) Demography of the twospotted spider mite Tetranychus urticae Koch. Oecologia 52:389–395
Carey JR (1993) Applied demography for biologists, with special emphasis on insects. Oxford University Press, New York
Chandler D, Bailey AS, Tatchell GM, Davidson G, Greaves J, Grant WP (2011) The development, regulation and use of biopesticides for integrated pest management. Philos Trans R Soc Part B 366:1987–1998
Chi H (1988) Life table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34
Chi H (2015) TWOSEX-MSChart: a computer program for population projection based on age-stage two-sex life table analysis, Version20150606. http://140.120.197.173/Ecology/
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Acad Sin Bull Inst Zool 24:225–240
Chiasson H, Bostanian NJ, Vincent C (2004a) Acaricidal properties of a Chenopodium-based botanical. J Econ Entomol 97:1373–1377
Chiasson H, Bostanian NJ, Vincent C (2004b) Insecticidal properties of a Chenopodium-based botanical. J Econ Entomol 97:1378–1383
Dekeyser MA (2005) Acaricide mode of action. Pest Manag Sci 61:103–110
Dimetry NZ, Amer SAA, Reda AS (1993) Biological activity of two neem seed kernel extracts against the two-spotted spider mite Tetranychus urticae Koch. J Appl Entomol 116:308–312
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, New York
Fisher SW, Wrensch DL (1986) Quantification of biological effectiveness for pesticides against Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 79:1472–1476
Forbes VE, Calow P (2002) Populaton growth rate as a basis for ecological risk assessment of toxic chemicals. Philos Trans R Soc Lond B 357:1299–1306
Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 119:803–823
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:43–62
Holland JM, Chapman RB (1994) A comparison of the toxic and sub-lethal effects of fluvalinate and esfenvalerate on the twospotted spider mite (Acari: Tetranychidae). Exp Appl Acarol 18:3–22
Holland JM, Chapman RB (1995) Comparative toxic and sublethal effects of fluvalinate on two-spotted spider mite and European red mite. Exp Appl Acarol 19:549–570
Huang YB, Chi H (2012a) Age-stage two-stage life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci 19:263–273
Huang YB, Chi H (2012b) Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: a case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J Agric Fore 61:37–45
Isman MB (2015) A renaissance for botanical insecticides? Pest Manag Sci 71:1587–1590
Kavousi A, Chi H, Talebi K, Bandani A, Ashouri A, Naveh V (2009) Demographic traits of Tetranychus urticae Koch (Acari: Tetranychidae) on leaf discs and whole leaves. J Econ Entomol 102:595–601
Khanamani M, Fathipour Y, Hajiqanbar H (2013) Population growth response of Tetranychus urticae to eggplant quality: application of female age-specific and age-stage two-sex life tables. Int J Acarol 39:638–648
Knowles CO (1997) Mechamisms of resistance to acaricides. In: Sjut V, Butters JA (eds) Molecular mechanisms of resistance to agrochemicals. Springer, Berlin, pp 58–78
Kumral NA, Çobanoğlu S, Yalcin C (2010) Acaricidal, repellent and oviposition deterrent activities of Datura stramonium L. against adult Tetranychus urticae (Koch). J Pest Sci 83:173–180
Maleknia B, Fathipour Y, Soufbaf M (2016) How greenhouse cucumber cultivars affect population growth and two-sex life table parameters of Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 42:70–78
Manjunatha Reddy GV, Srinivasa N, Muralidhara MS (2014) Potentiality of Cinnamomum extracts to two spotted spider mite, Tetranychus urticae Koch and its predator Neoseiulus longispinosus (Evans). J Biopest 7:11–14
Mansour F, Ravid U, Putievsky E (1986) Studies of the effects of essential oils isolated from 14 species of Labiatae on the carmine spider mite, Tetranychus cinnabarinus. Phytoparasitica 14:137–142
Mansour FA, Ascher KRS, Abo-Moch F (1997) Effects of Neemgard on phytophagous and predacious mites and on spiders. Phytoparasitica 25:333–336
Mansour FA, Azaizeh H, Saad B, Tadmor Y, Abo-Moch F, Said O (2004) The potential of Middle Eastern flora as a source of new safe bio-acaricides to control Tetranychus cinnabarinus, the carmine spider mite. Phytoparasitica 32:66–72
Marčić D (2012) Acaricides in modern management of plant-feeding mites. J Pest Sci 85:395–408
Marčić D, Međo I (2015) Sublethal effects of azadirachtin-A (NeemAzal-T/S) on Tetranychus urticae (Acari: Tetranychidae). Syst Appl Acarol 20:25–38
Martini X, Kincy N, Nansen C (2012) Quantitative impact assessment of spray coverage and pest behavior on contact pesticide performance. Pest Manag Sci 68:1471–1477
McKee MJ, Ibrahim YB, Knowles CO (1987) Relationship between dispersal and fecundity of Tetranychus urticae Koch (Acari: Tetranychidae) exposed to flucythrinate. Exp Appl Acarol 3:1–10
Međo I, Marčić D, Milenković S (2015) Acaricidal and behavioral effects of azadirachtin on two-spotted spider mites (Acari: Tetranychidae). In: Marčić D, Glavendekić M, Nicot P (eds) Proceedings of the VII congress on plant protection. Plant Protection Society of Serbia. IOBC-EPRS, IOBC-WPRS, Belgrade, pp 181–186
Migeon A, Dorkeld F (2016) Spider Mites Web—a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 15 Sept 2016
Miresmailli S, Bradbury R, Isman MB (2006) Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag Sci 62:366–371
Mohammadi S, Ziaee M, Seraj AA (2016) Sublethal effects on the population growth and life table parameters of Tetranychus turkestani Ugarov and Nikolskii on three cucumber cultivars. Syst Appl Acarol 21:218–226
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Riedl H, Shearer PW (1991) Double-leaf-disk residue assay for assessing the toxicity of repellent acaricides to spider mites (Acari: Tetranychidae). Exp Appl Acarol 11:149–157
Robertson JL, Russell RM, Preisler HK, Savin NE (2007) Bioassays with arthropods, 2nd edn. CRC Press, Taylor & Francis Group, Boca Raton
Roh HS, Lee BH, Park CG (2013) Acaricidal and repellent effects of myrtacean essential oils and their major constituents against Tetranychus urticae (Tetranychidae). J Asia-Pac Entomol 16:245–249
Sabelis MW (1985) Reproductive strategies. In: Helle W, Sabelis MW (eds) Spider mites, their biology, natural enemies and control. Elsevier, Amsterdam, pp 265–278
Sáenz-de-Cabezôn Irigaray FJ, Zalom FG (2009) Comparative repellent effects of different acaricide residues on predatory and spider mites. Is there a need for including behavior into standardized testing methods? IOBC/wprs Bull 50:95–98
Snell TW (1978) Fecundity, developmental time and population growth rate. Oecologia 32:119–125
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519
Sundaram KMS, Sloane L (1995) Effects of pure and formulated azadirachtin, a neem-based biopesticide, on the phytophagous spider mite, Tetranychus urticae Koch. J Environ Sci Health B 30:801–814
Takakura K (2009) Reconsiderations on evaluating methodology of repellent effects: validation of indices and statistical analysis. J Econ Entomol 102:1977–1984
Tuan SJ, Lin YH, Yang CM, Atlihan R, Saska P, Chi H (2016) Survival and reproductive strategies in twospotted spider mites: demographic analysis of arrhenotokous parthenogenesis of Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 109:502–509
Van Leeuwen T, Vontas J, Tsagkarakou A, Tirry L (2009) Mechanisms of acaricide resistance in the two-spotted spider mite Tetranychus urticae. In: Ishaaya I, Horowitz AR (eds) Biorational control of arthropod pests. Springer, Dordrecht, pp 347–393
Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W (2015) The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic Biochem Physiol 121:12–21
Villaverde JJ, Sevilla-Moran B, Sandin-Espana P, Lopez-Goti C, Alonso-Prados JL (2014) Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag Sci 70:2–5
Wang S, Tang X, Wang L, Zhang Y, Wu Q, Xie W (2014) Effects of sublethal concentrations of bifenthrin on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Syst Appl Acarol 19:481–490
Wang L, Zhang Y, Xie W, Wu Q, Wang S (2016) Sublethal effects of spinetoram on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Pestic Biochem Physiol 132:102–107
Whalon ME, Mota-Sanchez D, Hollingworth RM, Duynslager L (2016) Arthropod pesticide resistance database. www.pesticideresistance.com. Accessed 15 Sept 2016
Yang XB, Zhang YM, Hua L, Peng LN, Munyaneza JE, Trumble JT, Liu TX (2010) Repellency of selected biorational insecticides to potato psyllid, Bactericera cockerelli (Hemiptera: Psyllidae). Crop Prot 29:1320–1324
Yin WD, Qiu GS, Yan WT, Sun LN, Zhang HJ, Ma CS, Adaobi UP (2013) Age-stage two-sex life tables of Panonychus ulmi (Acari: Tetranychidae) on different apple varieties. J Econ Entomol 106:2118–2125
Zhang ZQ (2003) Mites of greenhouses-identification, biology and control. CABI Publishing, CAB International, Wallingford
Acknowledgements
This research was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant TR31043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musa, A., Međo, I., Marić, I. et al. Acaricidal and sublethal effects of a Chenopodium-based biopesticide on the two-spotted spider mite (Acari: Tetranychidae). Exp Appl Acarol 71, 211–226 (2017). https://doi.org/10.1007/s10493-017-0118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0118-x