Abstract
Cold-adapted yeasts were isolated from soil samples collected in Tibet and evaluated as potential biocontrol agents against blue mold (Penicillium expansum) of pear fruit in cold storage. YC1, an isolate identified as Rhodotorula mucilaginosa, was found to exhibit the greatest biocontrol activity among the different isolates that were screened. A washed cell suspension of YC1 exhibited the best biocontrol activity among three different preparations that were used in the current study. A concentration of 108 cells/ml reduced the incidence of decay to 35 %, compared to the control where decay incidence was 100 %. A higher intracellular level of trehalose and a higher proportion of polyunsaturated acids present in YC1, was associated with increased the tolerance of this strain to low temperatures, relative to the other strains that were evaluated. The increased tolerance to low temperature allowed the YC1 strain of yeast to more effectively compete for nutrients and space in wounded pear fruit that had been inoculated with spores of P. expansum and placed in cold storage. The present study demonstrated the ability to select cold-adapted yeasts from cold climates and use them as biocontrol agents of postharvest diseases of fruit placed in cold storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blue mould, caused by Penicillium expansum, is one of the main postharvest, fungal pathogens of pear fruits and causes significant economic losses (Manso and Nunes 2011; Yu et al. 2013; Zhang et al. 2008). Although pear is often stored at low temperature (0–5 °C) to extend its shelf life, this does not prevent spoilage by blue mold. The use of chemical, synthetic fungicides is still the principal method used to control the postharvest decay of fruit crops (Lennox et al. 2004; Sugar and Basile 2011). Concerns about food safety, environmental pollution, and the development of resistance in fungal pathogens have created an interest in exploring alternative approaches for managing postharvest diseases (Fravel 2005; Janisiewicz and Korsten 2002; Mari et al. 2003; Romanazzi et al. 2012; Xu et al. 2011). Antagonistic microorganisms, natural plant-derived compounds, food additives and other generally-regarded-as-safe (GRAS) compounds, and a variety of physical methods have all been investigated (Romanazzi et al. 2012; Tripathi and Dubey 2004). Among these alternatives, the use of microbial antagonists to control postharvest decay is regarded as a promising alternative (Nunes 2011; Sharma et al. 2009; Spadaro and Gullino 2004).
Among different microbial antagonists, yeasts have been of special interest due to their general lack of antibiotic production (Nally et al. 2015; Pantelides et al. 2015; Parafati et al. 2015), rapid colonization of the fruit surface and wound sites (Castoria et al. 2005), abiotic stress tolerance (Liu et al. 2011; Wang et al. 2010a), and compatibility with fungicides and other chemicals (Lima et al. 2011; Lu et al. 2014). Therefore, antagonistic yeasts are considered to have significant potential for reducing or replacing the use of chemical fungicides (Cao et al. 2013; El Ghaouth et al. 2003; Janisiewicz et al. 2014; Lu et al. 2014; Zhu et al. 2015).
Yeasts, however, also have several problems that decrease their widespread applicability, such as their instability in some environments and a short shelf life for formulated products (Droby et al. 2009; Haïssam 2011). These problems are mainly due to their exposure to several different abiotic stresses, including oxidative stress, osmotic stress, heat stress, and cold stress (Liu et al. 2013; Satyanarayana and Kunze 2009). Since many kinds of fruits are stored and transported at low temperature after harvest, the effect of cold stress on the viability of antagonistic yeast is especially relevant (Buzzini and Margesin 2014). One method of addressing this concern is the selection of cold-adapted yeast from extremely cold environments, such as Antarctica, or polar sea water (Lutz et al. 2012; Vero et al. 2013; Wang et al. 2010b). Microorganisms isolated from plant and soil samples at high-elevations in Tibet potentially have unique characteristics, including the ability to grow at low temperature. Unfortunately little research has been conducted to identify and evaluate potential biocontrol agents from this environment. Zhao et al. (2012), however, did report the identification of a Streptomyces sp. from Tibet that had the ability to inhibit gummy stem blight.
The present study focused on selecting yeast species from Tibet which are cold-adapted and also have potential to be used as biocontrol agents to manage postharvest diseases at low temperatures. The evaluation of yeast isolated from Tibet soil samples was based on their ability to prevent blue mold of pear fruits during cold storage. Among the species isolated and evaluated, the YC1 strain of Rhodotorula mucilaginosa was identified as a good biocontrol agent. The biocontrol mechanism of YC1 at low temperature was also investigated.
Method and materials
Fruit
Pear fruits (Pyrus pyrifolia Nak., ‘Shuijing’), of uniform ripeness and size, and without any apparent damage or infection, were harvested at commercial maturity in Hangzhou, Zhejiang Province, China. The fruits were rinsed with tap water after being disinfected with a solution of 0.1 % (v/v) sodium hypochlorite for 1 min and then air-dried at room temperature (20 °C) prior to their use in experiments.
Microorganisms
The postharvest, fungal pathogen P. expansum, was originally obtained from the China General Microbiological Culture Collection Center, No. 3.3703. It was cultured on potato dextrose agar (PDA), consisting of 200 ml boiled potato extract, 20 g glucose, and 20 g agar in 1 L of distilled water, for 7 days at 28 °C. Spore suspensions were prepared by rubbing the surface of the culture with a loop and suspending the obtained material in sterile distilled water. The concentration of the spore suspension was adjusted to 1 × 104 spores/ml using a hemocytometer.
Yeast strains were isolated from 121 soil samples gathered from the Lhasa district of Autonomous Region of China (91°11′E, 29°97′N, attitude ≥3500 m). Soil samples were collected when air temperatures were between 0 and 10 °C, and kept at 4 °C for isolation. One gram from each soil sample was placed in 100 ml sterile water. Ten-fold serial dilutions were prepared with sterile distilled water (10−2, 10−3 and 10−4), and three replicates of 100 µl of each dilution were spread on Rose Bengal Agar (Hangzhou Baisi Biotechnology Co., Ltd.). After 3–5 days of incubation at 28 °C, single yeast colonies varying in morphological appearance were selected, replated and purified, and then stored on nutrient yeast dextrose agar (NYDA), consisting of 8 g nutrient broth, 5 g yeast extract, 10 g glucose and 20 g agar in 1 L of distilled water, at 4 °C for until further evaluation.
Evaluation of biocontrol activity on pear fruit
A total of 46 yeast isolates were obtained and evaluated for biocontrol activity. The yeast strains were grown on NYDA for two generations and then inoculated in 50 ml NYD broth (NYDB) and cultured at 28 °C on a rotary shaker (QYC 2102, Shanghai FUMA) at 200 r/min for 24 h. The ability of the different isolates to prevent blue mold on pear fruit, caused by P. expansum, was used to evaluate the level of biocontrol activity of the yeast isolates. Based on the method reported by Zhang et al. (2010), pear fruits were uniformly wounded with a sterile cork-borer (5 mm diameter × 3 mm deep) on the equator of each pear fruit. Fifty microliter of yeast suspension (1 × 108 cells/ml) was pipetted into each wound. The same volume of sterile distilled water was pipetted into wounds of pears used as controls. After the wounds had dried, spores of the pathogen (1 × 104 spores/ml) were pipetted into each wound. The inoculated fruit (treated and control) were placed in covered plastic trays to maintain a high relative humidity and kept at either 20 or 4 °C in the dark for either 7 or 30 days. Five pear fruit were used as a single replicate and each treatment had three replicates. The test was repeated twice.
Based on the results of the preliminary screening, yeast isolates with biocontrol activity were selected for further evaluation. The biocontrol activity of the selected yeast antagonists was compared with a yeast antagonist previously isolated from pear fruits, identified as Cryptococcus laurentii and demonstrated to have a good biocontrol activity against blue mold of pear fruit (Yu et al. 2008; Zheng et al. 2007). This yeast was used as a positive control in further experiments to evaluate the biocontrol efficacy of the newly isolated yeasts. In these subsequent tests, 12 fruits were used as a single replicate and each treatment had three replicates. The experiment was performed twice.
Identification and phenotypic characterization of the selected yeast strains
The potential antagonistic yeast isolate, YC1, was identified by analyzing the sequence of the D1/D2 domain of the large-subunit (LSU) of ribosomal DNA (rRNA gene). DNA was extracted from the yeast using an Ezup column yeast genomic DNA extraction kit (Sangon Biotech, Shanghai) following the provided protocol. Polymerase chain reaction (PCR) amplification was done with external primers NL1 5′-GCATATCAATAAGCGGAGGAAAAG-3′ and NL4 5′-GGTCCGTGTTTCAAGACGG-3′ as described by Kurtzman and Robnett (1998). The extracted PCR products were 585 bp, and were sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). Sequence similarity was conducted querying the obtained sequences against the NCBI database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Biocontrol activity of YC1 at low temperature
The biocontrol activity of the selected Tibetan yeast, YC1, was evaluated at 4 °C. Fruit samples were prepared as described in “Evaluation of biocontrol activity on pear fruit” section. The concentration of the washed, cell suspensions of YC1 were adjusted to 1 × 106, 1 × 107, 1 × 108, and 1 × 109 cells/ml with sterile distilled water. Fifty microliter of the different concentrations of the yeast suspensions were pipetted into different wounds. The same volume of sterile distilled water was used as a control. After the wounds had dried, 30 µl of a spore suspension of P. expansum at a concentration of 1 × 104 spores/ml was pipetted into each wound. The inoculated fruit were kept for 30 days in the same conditions described in “Evaluation of biocontrol activity on pear fruit” section. Twelve fruits were used as a bioreplicate in each treatment and there were three replicates. The overall test was conducted twice.
Evaluation of different preparations of YC1 against P. expansum in pear fruits stored at low temperature
In order to explore the mechanism by which YC1 inhibits P. expansum infection of pear fruits stored at low temperature, the biocontrol activity of different preparations of YC1 were evaluated. Fruit samples were prepared as described in “Evaluation of biocontrol activity on pear fruit” section. The different preparations were: (1) sterile distilled water which served as a control; (2) 1 × 108 unwashed cells/ml in culture medium (NYDB); (3) 1 × 108 washed cells/ml (as described in “Evaluation of biocontrol activity on pear fruit” section); (3) cell-free culture filtrate (supernatant from yeast grown in NYDB, centrifuged, and then filtered through a 0.45 μm sterile, Millipore filter); and, (4) autoclaved yeast cells, sterilized at 121 °C by moist heat sterilization for 20 min. Thirty microliter of a water suspension of pathogen spores (1 × 104 spores/ml) were pipetted into each wound after first administering one of the above preparations into the wound and allowing them to air dry. The fruit were handled and stored as described in “Evaluation of biocontrol activity on pear fruit” section (4 °C for 30 days). There were three replicates (fruit) per treatment, and the experiments were conducted twice.
Inhibition of spore germination
The inhibitory effect of YC1 on spore germination of P. expansum was assessed in potato dextrose broth (PDB) using the method described by Wang et al. (2012). Aliquots (100 µl) of a condial suspension of P. expansum (1 × 107 spores/ml) were transferred to 15 ml glass tubes containing 5.0 ml PDB. Preparations of YC1 cells were prepared as described in “Biocontrol activity of YC1 at low temperature” section. Aliquots (100 µl) of the various preparations were then added to the tubes containing the pathogen stores. The preparations that contained yeast cells were adjusted 1 × 108 cells/ml prior to adding the aliquots to the tubes. The tubes were then cultured for 72 h at 4 °C on a rotary shaker (QYC 2102, Shanghai FUMA) at 150 rpm. Subsequently, percent germination of 200 spores from each sample was assessed using a light microscope. Each treatment consisted of three replicates and the experiment was conducted twice.
Nutrient competition
Competition for nutrients between YC1 and P. expansum was assessed using the method described by Vero et al. (2002) and modified by Bautista-Rosales et al. (2013). Fruit samples were prepared as described in “Evaluation of biocontrol activity on pear fruit” section. Competition for carbon and nitrogen sources was evaluated separately. Carbon sources included 2 % glucose, fructose, sucrose, maltose, or galactose. Nitrogen sources include 0.3 % of NaNO3, KNO3, NH4NO3, or NH4SO4. Additionally, MgSO4 and FeCl3 were also used to evaluate competition for magnesium and iron. Fifty microliter of a yeast suspension (108 cells/ml), 30 µl of a pathogen (104 spores/ml), and 50 µl of one of the nutrient solutions were pipette into each wound. Sterile distilled water was used instead of the nutrients as a control. Competition for nutrients was assessed by comparing lesion diameter between the various treatments. Each treatment was replicated three times and the experiment was performed twice.
Population dynamics of YC1 in pear wounds at low temperature
Fruit samples were wounded as described in “Evaluation of biocontrol activity on pear fruit” section. Two uniform wounds were made on the equator of pear fruits. One received 50 µl of C. laurentii (1 × 108 cells/ml) as a control, while the other received the same volume and concentration of YC1 cells. After the wounds had dried, the fruit was handled and stored at 4 °C as described in “Evaluation of biocontrol activity on pear fruit” section. A portion of wounded tissue (10 mm diameter × 5 mm deep) was removed at different time points (0, 3, 6, 9, 12, 15 and 18 days) with a sterile cork-borer and ground in 10 ml of sterile water using a sterile mortar and pestle. Cell counts were obtained by placing each sample in a hemocytometer and viewing through a light microscope (Yu and Zheng 2006). Each treatment consisted of three replicates and the experiment was conducted twice.
Enzyme activity
The activity of enzymes associated with biocontrol activity, chitinase and β-1,3-glucanase, were assayed in cultures of YC1 grown in NYDB for 10 days at 4 °C, by which time the yeast was in stationary phase. Enzyme activity was compared to the level of activity of C. laurentii grown under the same conditions. Ten milliliters of yeast culture medium was centrifuged at 5000g for 10 min and the supernatant was filtered through a 0.45 μm Millipore membrane. The resulting filtrate was then used to assay enzyme activity. Chitinase activity was assayed by measuring the reduction of N-acetyl glucosamine at 530 nm as described by Abeles et al. (1971) and modified by Zhang et al. (2013). One unit of chitinase activity was defined as 100 µg of N-acetyl glucosamine equivalents formulation per hour and described as U/ml. Each culture was replicated three times and the experiment was conducted twice.
β-1,-3-Glucanase activity was determined by measuring the amount of reducing sugars released from 0.2 % laminarin (w/v) in 50 mM, pH 5.0, potassium acetate buffer.as described by Ippolito et al. (2000) and El Ghaouth et al. (2003). One unit of β-1,3-glucanase activity was defined as the formation of 1 mg glucose equivalents per hour and described as U/ml. Each culture was replicated three times and the experiment was performed twice.
Cryoprotectants
Trehalose content
YC1 was cultured at 4 °C as described in “Evaluation of biocontrol activity on pear fruit” section. The intracellular content of trehalose was determined using the anthrone method described by Brin (1966) and modified by Wang et al. (2010a, b). The trehalose content in C. laurentii was also assessed. A weighed culture of YC1 yeast cells was centrifuged at 5000g for 10 min and the cells were then washed twice with sterile distilled water. The cells were then immersed in 1 ml of 0.5 M trichloroacetic acid (TCA) for 60 min on ice and shaken every 15 min. The samples were again centrifuged and then the supernatant was diluted twice with sterile distilled water. One milliliter aliquot was then mixed with 0.2 % anthrone solution prepared with analytical-grade, concentrated sulfuric acid. The samples were heated at 100 °C for 5 min and allowed to cool. Trehalose content was determined by measuring absorbance at 590 nm. Each treatment was replicated three times and the experiment was conducted twice.
Proline content
YC1 and C. laurentii were cultured at 4 °C as described in “Evaluation of biocontrol activity on pear fruit” section. After culturing for 10 days at 4 °C, the yeast cells were centrifuged at 5000g for 10 min and washed twice with sterile distilled water. Intracellular amino acids were extracted by boiling the centrifuged cells for 10 min as described by Momose et al. (2008). The samples were centrifuged again at 12,000g for 10 min and the amino acids in the supernatants were quantified using an amino acid analyzer (L8900, Hitachi, Japan). Proline content was expressed as a percentage of dry weight. Each culture had three replicates and the analysis was conducted twice.
Lipid composition
Total lipids were extracted from YC1 and C. laurentii cells using a chloroform–methanol (2:1, v/v) solvent system as described by Folch et al. (1957). Fatty acid methyl ester (FAME) extracts in the total lipid extract were prepared and separated by gas chromatography (GC, Shimadzu, Kyoto, Japan) as described by Yang et al. (2006).
Statistical analyses
Treatment effects were analyzed by one-way analysis of variance (ANOVA) using SPSS 20.0 (SPSS Inc. Chicago, Illinois, USA). Duncan’s multiple range tests was used to determine mean separation. Percentage data concerning decay incidence were previously transformed using the arcsine transformation (sin−1 \( \sqrt x \)) prior to analysis. However, for the ease of interpretation and comparisons with other published reports, the data is presented as untransformed percent data. A student’s t test was used to determine significant differences in the fatty acid data. Differences at the level p < 0.05 were considered statistically significant. Presented data represent the means from one individual experiment that was representative of two independent experiments.
Results
Selection of antagonistic yeasts against blue mold in pear fruit
All 46 yeast isolates obtained from soil samples taken in Tibet were screened in a preliminary test against blue mold in pear fruit at both 20 and 4 °C. Four of them exhibited better biocontrol activity than the others and were selected as candidates for further study and comparison with a strain of C. laurentii, obtained from Hangzhou (a coastal city at sea level), which had previously been selected as good yeast antagonist. All four isolates significantly inhibited decay incidence and reduced lesion diameter of blue mould infections of pear fruit at both 20 and 4 °C (Table 1). The biocontrol activity of yeast isolates HY1, LB2 and YC1 was similar to C. laurentii at 20 °C. YC1, however, exhibited the highest level of protection, among all isolates, against P. expansum at 4 °C. As a result, YC1 was selected for identification and further study.
Identification of the YC1 yeast isolate
YC1 was identified by analyzing the sequence of the large subunit D1/D2 domain of rRNA gene. A BLAST query of the obtained sequence for YC1 provided 100 % nucleotide identity with the large subunit rRNA gene sequence of R. mucilaginosa. The YC1 isolate was deposited in the China General Microbiological Culture Collection (http://www.cgmcc.net/) under the following accession number CGMCC10223.
Biocontrol efficacy of R. mucilaginosa (YC1) at low temperature
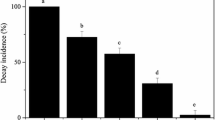
The biocontrol activity of YC1 was evaluated in pear fruit after 30 days storage at low temperature (4 °C). YC1 administered to the wounds significantly reduced decay incidence and lesion diameter compared to water-treated control fruit. The level of biocontrol activity was concentration dependent. Biocontrol efficacy increased as the yeast concentration increased. The highest level of inhibition was exhibited at 108 and 109 cells/ml (Fig. 1). At these higher concentrations decay incidence was reduced to 28.5 and 13.9 %, respectively.
Biocontrol activity of YC1 against blue mold of pear fruit stored at low temperature. Decay incidence (a) and lesion diameter (b) were evaluated after 30 days of storage at 4 °C. Data represent the mean ± SD (n = 3). Different letters represent significantly different values based on a Duncan’s multiple range test at P = 0.05
Biocontrol activity of different preparations
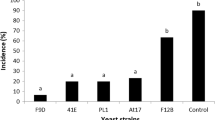
The biocontrol activity of different preparations of YC1, relative to a water control, was estimated in vivo. The biocontrol efficacy, as measured by both incidence and lesion diameter, of a washed cell suspension was significantly better than any of the other three different preparations at 4 °C (Fig. 2). Decay incidence using the washed cell suspension was reduced to 34.6 % while the other three preparations had nearly no impact on decay incidence. They were able, however, to reduce lesion diameter to varying degrees, relative to the water control (Fig. 2b).
Biocontrol activity of different preparations of YC1 against blue mold on pear fruit, relative to a untreated, water control. Decay incidence (a) and lesion diameter (b) were measured after 30 days of storage at 4 °C. Data represent the mean ± SD (n = 3). Different letters represent significantly different values based on a Duncan’s multiple range test at P = 0.05
Inhibitory effect of different preparations of YC1 on spore germination of P. expansum
The impact of co-incubation of different preparations of YC1 with conidia on spore germination of P. expansum is shown in Fig. 3. After 3 days incubation at 4 °C, Three of the preparations of YC1 significantly inhibited spore germination. Both the washed and unwashed yeast cell suspension preparations had the greatest impact on the germination of P. expansum at low temperature in vitro. The level of germination using these preparations was 15.7 and 30 %, respectively.
Inhibitory effect of different preparations of YC1 on pathogen spore germination. Spore germination was determined after 3 days of incubation and expressed as the percentage of germinated conidia where n ≥ 200 spores. Data represent the mean ± SD (n = 3). Different letters represent significantly different values based on a Duncan’s multiple range test at P = 0.05
Nutrient competition
An evaluation of the impact of the addition of different nutrients to fruit wounds that had been inoculated with both YC1 yeast cells and pathogen spores was assessed in order to evaluate the ability of the yeast and pathogen to compete for different nutrient sources. The lesion diameter of inoculated wounds was measured after 30 days of storage at 4 °C. Results indicated that YC1 could effectively compete against the pathogen for the various substrates (Tables 2, 3). The addition of different carbon sources had a variable effect on lesion size but none of the observed differences were statistically significant (Table 2). Supplemental iron improved the biocontrol activity. In contrast, nitrogen source had a significant impact on lesion size (Table 3). All of the different exogenous nitrogen sources and the magnesium sulfate, except NH4CO3, resulted in a significant increase in lesion diameter, relative to wounds treated with both the yeast and the pathogen without additional nutrients. KNO3 increased lesion diameter the most, a 139 % increase relative to the control. In contrast, NH4CO3 reduced it slightly, though the decrease was not statistically significant.
Population dynamics of YC1 in wounds of pear fruit
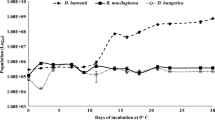
The population dynamics of YC1 and C. laurentii are shown in Fig. 4. Both grew rapidly at 4 °C during the first 3 days. The population continued to increase over the next 12 days reaching a maximum on day 15. The population of the two yeasts at that time was of 1.68 × 109 and 2.34 × 109 cells/ml, for YC1 and C. laurentii, respectively. After this phase, the population of both yeast species began to decrease. There were no significant differences between the populations of these two yeast strains during any of the measured time period.
Enzyme activity
Chitinase and β-1,3-glucanase activity in cell-free culture filtrates obtained from both YC1 and C. laurentii were assayed after 10 days of incubation in NYDB at 4 °C. Results indicated that both chitinase and β-1,3-glucanase activity was significantly higher in the culture filtrate obtained from R. mucilaginosa (YC1) cultures than from cultures of C. laurentii (Fig. 5). Chitinase activity and β-1,3-glucanase activity in YC1 culture filtrates were 37.88 and 1.22 times greater, respectively, than the activity observed in culture filtrates of C. laurentii.
Chitinase activity (a) and β-1,3-glucanase activity (b) in culture filtrates of YC1 and C. laurentii cultures after 10 days incubation in NYDB at 4 °C. Data represent the mean ± SD (n = 3). Different letters represent significantly different values based on a Duncan’s multiple range test at P = 0.05
Cryoprotectants (trehalose and proline)
Two intracellular, compatible solutes, trehalose and proline, were measured after 10 days of incubation at 4 °C in R. mucilaginosa (YC1) and C. laurentii. The trehalose content in YC1 was much higher than in C. laurentii (Fig. 6a). A very minor, but statistically significant, difference in proline content was also observed (Fig. 6b). Therefore, any biological significance in the differences in proline content is dubious.
Lipid composition in R. mucilaginosa (YC1) and C. laurentii
The main fatty acids present in the total lipids of R. mucilaginosa (YC1) and C. laurentii at low temperature (4 °C) are presented in Table 4. Most fatty acids in both yeast species were unsaturated fatty acids. No significant differences were observed in the total percentage of saturated and unsaturated fatty acids present in the two biocontrol yeast species. The oleic acid (C18:1) content in C. laurentii was higher than in YC1, while YC1 had two more types of polyunsaturated fatty acids than C. laurentii. Although the total percentage of unsaturated fatty acids was similar in the two yeast species, YC1 had more polyunsaturated fatty acids. The fatty acids present in C. laurentii consisted of mainly monounsaturated fatty acids.
Discussion
Biocontrol agents are exposed to many different kinds of environmental conditions when they are applied in the field or packing house (Lahlali and Jijakli 2009; Sui et al. 2015). Since most fruits are stored and shipped at low temperatures (0–5 °C) to extend their shelf life, cold stress is a common adversity to which yeast antagonists used to manage postharvest diseases would be exposed. Therefore, it is logical to try and identify biocontrol agents that are adapted to grow at low temperatures in order to maintain biocontrol activity, in cold storage (Spadaro et al. 2013; Spotts et al. 2002). Several reports have described attempts to obtain cold-adapted yeasts from the Antarctic, polar seas, and other cold places in order to screen them for biocontrol activity (Dunlap et al. 2007; Hernández-Montiel et al. 2010; Vero et al. 2013). The present study utilized a similar approach by obtaining yeast isolates collected from cold soils in the region of Tibet, and demonstrated that this approach could be used to identify effective, potential biocontrol agents. The use of wounded/inoculated pear fruit is an efficient method for identifying yeasts that could be potentially used to manage postharvest rots (Kaoud 2014). After a preliminary screening test, four yeast isolates with biocontrol activity were selected for further study. Their biocontrol activity was compared to a previously selected antagonistic yeast strain of C. laurentii which has been demonstrated to be an effective postharvest biocontrol agent (Yu et al. 2012; Zhang et al. 2007). The YC1 isolate was found to be as effective as C. laurentii at 20 °C, and exhibit better biocontrol activity than C. laurentii at 4 °C (Table 1). Subsequent large-subunit (LSU) ribosomal DNA (rRNA gene) D1/D2 domain sequence analysis identified YC1 as a strain of R. mucilaginosa. Although R. mucilaginosa has been previously demonstrated to be an effective postharvest biocontrol agent (Robiglio et al. 2011; Zhang et al. 2014, 2013), in-depth analysis of the characteristics of R. mucilaginosa that adapt it to low temperature and/or make it an effective biocontrol agent have been conducted.
The results presented in Fig. 1 indicate that the biocontrol activity of YC1 at low temperature gradually increases at higher concentrations. The concentration of 1 × 108 cells/ml was selected as optimum since biocontrol activity did not increase at 1 × 109 cells/ml, and administering such a high concentration of yeast would be problematic. Similar results have been obtained in studies of Rhodotorula yeasts (Dal Bello et al. 2008; Li et al. 2011).
The evaluation of the effect of different preparations of YC1 on biocontrol activity at low temperature, both in vitro and in vivo (Figs. 2, 3), demonstrated that a preparation consisting of a washed yeast cell suspension had the best biocontrol activity. This finding suggests that competition for space and nutrients represents a major biocontrol mechanism for YC1 at low temperature. This premise is consistent with the theory reported by Sharma et al. (2009). The ability cell-free culture filtrate obtained from YC1 cultures to reduce lesion diameter indicates that YC1 may also secrete some inhibitory or toxic metabolites. An array of different carbon and nitrogen sources, as well iron and magnesium, were added to the wound site in order to better understand the role of nutrient competition in the biocontrol activity of YC1. The results in Tables 2 and 3 indicate that YC1 appears to compete with P. expansum at low temperature, mainly for a source of nitrogen. This in agreement with a similar study conducted by Bautista-Rosales et al. (2013). This premise is based on the observations that the biocontrol efficacy of YC1 was reduced when wounds were supplemented with a variety of nitrogen sources.
A variety of analyses comparing R. mucilaginosa (YC1) and C. laurentii provided evidence for why YC1 exhibits better biocontrol activity than C. laurentii at low temperature. The results in Fig. 4 demonstrate that there was no significant difference in the population dynamics of these two yeast strains in wounds of pear fruit stored at 4 °C for a period of 18 days. This indicates that the growth potential of these two species, in vivo, at low temperature, is similar. The different biocontrol efficacy of these two species, however, was disparate, indicating something other than just competition for nutrients and the ability to grow at low temperature plays a role in their biocontrol activity. Differences between the two species in the level of activity of two enzymes (chitinase and β-1,3-glucanase) that have been reported to play an important role in biocontrol activity (Bautista-rosales et al. 2014; Choudhary et al. 2014; Lima et al. 2013; Lutz et al. 2013; Wang et al. 2015), suggest that these enzymes may play a significant role in the differences observed in biocontrol activity at 4 °C.
Trehalose and proline play an important role in enhancing cryo—and dehydrative—stress tolerance in both microorganisms and higher plants (Li and Tian 2007; Sasano et al. 2012; Takagi 2008; Trischuk et al. 2006). The results in Fig. 6 demonstrate that trehalose content in YC1 was much higher than in C. laurentii. In contrast, differences in proline content were minor at best, though statistically significant, suggesting that its role in defining any differences in stress tolerance between the two yeast species is dubious. In addition to the presence of protective, compatible solutes, the lipid fluidity of cell membranes also plays an important role in making yeast cells, and cells in general, more resistant to low temperature and freezing stress (Rossi et al. 2009; Steels et al. 1994). The composition of fatty acids was examined in both YC1 and C. laurentii. Results (Table 4) indicated that there is no obvious difference between the two species in their fatty acid composition, although YC1 does contain more polyunsaturated fatty acids, which may contribute to better fluidity of the cell membrane.
In conclusion, the present study indicates that the yeast, R. mucilaginosa (YC1), isolated from soil samples taken in Tibet, is cold-adapted and exhibits good biocontrol activity against blue mold of pear in cold storage. The better biocontrol activity exhibited by YC1, compared to another biocontrol yeast species, C. laurentii, may be attributed to the high level of chitinase and β-1,3-glucanase activity exhibited by YC1 relative to C. laurentii. Further studies will be required to better understand the commercial potential of YC1 as a yeast biocontrol agent used to manage postharvest diseases of harvested fruits stored at low temperature.
References
Abeles FB, Bosshart RP, Forrence LE, Habig WH (1971) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47:129–134. doi:10.1104/pp.47.1.129
Bautista-Rosales PU, Calderon-Santoyo M, Servín-Villegas R, Ochoa-Álvarez NA, Ragazzo-Sánchez JA (2013) Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloeosporioides in mangoes. Biol Control 65:293–301. doi:10.1016/j.biocontrol.2013.03.010
Bautista-rosales PU, Ricardo V, Servín-villegas R, Ang N, Ragazzo-s JA (2014) Biocontrol action mechanisms of Cryptococcus laurentii on Colletotrichum gloeosporioides of mango. Crop Prot 65:194–201. doi:10.1016/j.cropro.2014.07.019
Brin M (1966) Transketolase: clinical aspects. In: Wood WA (ed) Methods in enzymology. Academic Press, New York, pp 506–514. doi:10.1016/0076-6879(66)09101-8
Buzzini P, Margesin R (2014) Cold-adapted yeasts. Springer, Berlin. doi:10.1007/978-3-642-39681-6
Cao J, Zhang H, Yang Q, Ren R (2013) Efficacy of Pichia caribbica in controlling blue mold rot and patulin degradation in apples. Int J Food Microbiol 162:167–173. doi:10.1016/j.ijfoodmicro.2013.01.007
Castoria R, Morena V, Caputo L, Panfili G, De Curtis F, De Cicco V (2005) Effect of the biocontrol yeast Rhodotorula glutinis Strain LS11 on patulin accumulation in stored apples. Phytopathology 95:1271–1278. doi:10.1094/PHYTO-95-1271
Choudhary B, Nagpure A, Gupta RK (2014) Fungal cell-wall lytic enzymes, antifungal metabolite(s) production, and characterization from Streptomyces exfoliatus MT9 for controlling fruit-rotting fungi. J Basic Microbiol 54:1295–1309. doi:10.1002/jobm.201400380
Dal Bello G, Mónaco C, Rollan MC, Lampugnani G, Arteta N, Abramoff C, Ronco L, Stocco M (2008) Biocontrol of postharvest grey mould on tomato by yeasts. J Phytopathol 156:257–263. doi:10.1111/j.1439-0434.2007.01351.x
Droby S, Wisniewski M, Macarisin D, Wilson C (2009) Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol Technol 52:137–145. doi:10.1016/j.postharvbio.2008.11.009
Dunlap CA, Evans KO, Theelen B, Boekhout T, Schisler DA (2007) Osmotic shock tolerance and membrane fluidity of cold-adapted Cryptococcus flavescens OH 182.9, previously reported as C. nodaensis, a biocontrol agent of Fusarium head blight. FEMS Yeast Res 7:449–458. doi:10.1111/j.1567-1364.2006.00193.x
El Ghaouth A, Wilson CL, Wisniewski M (2003) Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology 93:344–348. doi:10.1094/PHYTO.2003.93.3.344
Folch J, Lees M, Slone Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509. doi:10.1371/journal.pone.0020510
Fravel DR (2005) Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337–359. doi:10.1146/annurev.phyto.43.032904.092924
Haïssam JM (2011) Pichia anomala in biocontrol for apples: 20 years of fundamental research and practical applications. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 99:93–105. doi:10.1007/s10482-010-9541-2
Hernández-Montiel LG, Ochoa JL, Troyo-Diéguez E, Larralde-Corona CP (2010) Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol Technol 56:181–187
Ippolito A, El Ghaouth A, Wilson CL, Wisniewski M (2000) Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol Technol 19:265–272. doi:10.1016/S0925-5214(00)00104-6
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441. doi:10.1146/annurev.phyto.40.120401.130158
Janisiewicz WJ, Jurick WM, Peter KA, Kurtzman CP, Buyer JS (2014) Yeasts associated with plums and their potential for controlling brown rot after harvest. Yeast 31:207–218. doi:10.1002/yea.3009
Kaoud HA (2014) Alternative methods for the control of Tuta absoluta. GJMAS J 2:41–46. doi:10.1111/jam.12495
Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 73:331–371. doi:10.1023/A:1001761008817
Lahlali R, Jijakli MH (2009) Enhancement of the biocontrol agent Candida oleophila (strain O) survival and control efficiency under extreme conditions of water activity and relative humidity. Biol Control 51:403–408
Lennox CL, Spotts RA, Booyse M (2004) Incidence of postharvest decay of “d”Anjou’ pear and control with a thiabendazole drench. Plant Dis 88:474–478. doi:10.1094/Pdis.2004.88.5.474
Li BQ, Tian SP (2007) Effect of intracellular trehalose in Cryptococcus laurentii and exogenous lyoprotectants on its viability and biocontrol efficacy on Penicillium expansum in apple fruit. Lett Appl Microbiol 44:437–442. doi:10.1111/j.1472-765X.2006.02080.x
Li RP, Zhang HY, Liu WM, Zheng XD (2011) Biocontrol of postharvest gray and blue mold decay of apples with Rhodotorula mucilaginosa and possible mechanisms of action. Int J Food Microbiol 146:151–156. doi:10.1016/j.ijfoodmicro.2011.02.015
Lima G, Castoria R, De Curtis F, Raiola A, Ritieni A, De Cicco V (2011) Integrated control of blue mould using new fungicides and biocontrol yeasts lowers levels of fungicide residues and patulin contamination in apples. Postharvest Biol Technol 60:164–172. doi:10.1016/j.postharvbio.2010.12.010
Lima JR, Gondim DMF, Oliveira JTA, Oliveira FSA, Gonçalves LRB, Viana FMP (2013) Use of killer yeast in the management of postharvest papaya anthracnose. Postharvest Biol Technol 83:58–64. doi:10.1016/j.postharvbio.2013.03.014
Liu J, Wisniewski M, Droby S, Tian S, Hershkovitz V, Tworkoski T (2011) Effect of heat shock treatment on stress tolerance and biocontrol efficacy of Metschnikowia fructicola. FEMS Microbiol Ecol 76:145–155. doi:10.1111/j.1574-6941.2010.01037.x
Liu J, Sui Y, Wisniewski M, Droby S, Liu Y (2013) Review: utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int J Food Microbiol 167:153–160. doi:10.1016/j.ijfoodmicro.2013.09.004
Lu H, Lu L, Zeng L, Fu D, Xiang H, Yu T, Zheng X (2014) Effect of chitin on the antagonistic activity of Rhodosporidium paludigenum against Penicillium expansum in apple fruit. Postharvest Biol Technol 92:9–15. doi:10.1016/j.postharvbio.2014.01.009
Lutz MC, Lopes CA, Sosa MC, Sangorrín MP (2012) A new improved strategy for the selection of cold-adapted antagonist yeasts to control postharvest pear diseases. Biocontrol Sci Technol 22:1465–1483. doi:10.1080/09583157.2012.735223
Lutz MC, Lopes CA, Rodriguez ME, Sosa MC, Sangorrín MP (2013) Efficacy and putative mode of action of native and commercial antagonistic yeasts against postharvest pathogens of pear. Int J Food Microbiol 164:166–172. doi:10.1016/j.ijfoodmicro.2013.04.005
Manso T, Nunes C (2011) Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol Technol 61:64–71. doi:10.1016/j.postharvbio.2011.02.004
Mari M, Bertolini P, Pratella GC (2003) Non-conventional methods for the control of post-harvest pear diseases. J Appl Microbiol 94:761–766. doi:10.1046/j.1365-2672.2003.01920.x
Momose Y, Hirayama K, Itoh K (2008) Competition for proline between indigenous Escherichia coli and E. coli O157:H7 in gnotobiotic mice associated with infant intestinal microbiota and its contribution to the colonization resistance against E. coli O157:H7. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 94:165–171. doi:10.1007/s10482-008-9222-6
Nally MC, Pesce VM, Maturano YP, Rodriguez Assaf LA, Toro ME, Castellanos de Figueroa LI, Vazquez F (2015) Antifungal modes of action of Saccharomyces and other biocontrol yeasts against fungi isolated from sour and grey rots. Int J Food Microbiol 204:91–100. doi:10.1016/j.ijfoodmicro.2015.03.024
Nunes CA (2011) Biological control of postharvest diseases of fruit. Eur J Plant Pathol 133:181–196. doi:10.1007/s10658-011-9919-7
Pantelides IS, Christou O, Tsolakidou M-D, Tsaltas D, Ioannou N (2015) Isolation, identification and in vitro screening of grapevine yeasts for the control of black aspergilli on grapes. Biol Control 88:46–53. doi:10.1016/j.biocontrol.2015.04.021
Parafati L, Vitale A, Restuccia C, Cirvilleri G (2015) Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol 47:85–92. doi:10.1016/j.fm.2014.11.013
Robiglio A, Sosa MC, Lutz MC, Lopes CA, Sangorrín MP (2011) Yeast biocontrol of fungal spoilage of pears stored at low temperature. Int J Food Microbiol 147:211–216
Romanazzi G, Lichter A, Gabler FM, Smilanick JL (2012) Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol 63:141–147. doi:10.1016/j.postharvbio.2011.06.013
Rossi M, Buzzini P, Cordisco L, Amaretti A, Sala M, Raimondi S, Ponzoni C, Pagnoni UM, Matteuzzi D (2009) Growth, lipid accumulation, and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS Microbiol Ecol 69:363–372. doi:10.1111/j.1574-6941.2009.00727.x
Sasano Y, Haitani Y, Ohtsu I, Shima J, Takagi H (2012) Proline accumulation in baker’s yeast enhances high-sucrose stress tolerance and fermentation ability in sweet dough. Int J Food Microbiol 152:40–43. doi:10.1016/j.ijfoodmicro.2011.10.004
Satyanarayana T, Kunze G (2009) Yeast biotechnology: Diversity and applications. Springer, Dordrecht. doi:10.1007/978-1-4020-8292-4
Sharma RR, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221. doi:10.1016/j.biocontrol.2009.05.001
Spadaro D, Gullino ML (2004) State of the art and future prospects of the biological control of postharvest fruit diseases. Int J Food Microbiol 91:185–194. doi:10.1016/s0168-1605(03)00380-5
Spadaro D, Lorè A, Garibaldi A, Gullino ML (2013) A new strain of Metschnikowia fructicola for postharvest control of Penicillium expansum and patulin accumulation on four cultivars of apple. Postharvest Biol Technol 75:1–8
Spotts RA, Cervantes LA, Facteau TJ (2002) Integrated control of brown rot of sweet cherry fruit with a preharvest fungicide, a postharvest yeast, modified atmosphere packaging, and cold storage temperature. Postharvest Biol Technol 24:251–257. doi:10.1016/S0925-5214(01)00155-7
Steels EL, Learmonth RP, Watson K (1994) Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 140:569–576. doi:10.1099/00221287-140-3-569
Sugar D, Basile SR (2011) Orchard calcium and fungicide treatments mitigate effects of delayed postharvest fungicide applications for control of postharvest decay of pear fruit. Postharvest Biol Technol 60:52–56. doi:10.1016/j.postharvbio.2010.11.007
Sui Y, Wisniewski M, Droby S, Liu J (2015) Responses of yeast biocontrol agents to environmental stress. Appl Environ Microbiol 81:2968–2975. doi:10.1128/AEM.04203-14
Takagi H (2008) Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl Microbiol Biotechnol 81:211–223. doi:10.1007/s00253-008-1698-5
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol 32:235–245. doi:10.1016/j.postharvbio.2003.11.005
Trischuk RG, Schilling BS, Wisniewski M, Gusta LV (2006) Freezing stress: systems biology to study cold tolerance. In: Physiology and molecular biology of stress tolerance in plants. Springer, Netherlands, pp 131–155. doi:10.1007/1-4020-4225-6_5
Vero S, Mondino P, Burgueño J, Soubes M, Wisniewski M (2002) Characterization of biocontrol activity of two yeast strains from Uruguay against blue mold of apple. Postharvest Biol Technol 26:91–98. doi:10.1016/S0925-5214(01)00199-5
Vero S, Garmendia G, Gonzalez MB, Bentancur O, Wisniewski M (2013) Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res 13:189–199. doi:10.1111/1567-1364.12021
Wang YF, Wang P, Xia JD, Yu T, Lou BG, Wang J, Zheng XD (2010a) Effect of water activity on stress tolerance and biocontrol activity in antagonistic yeast Rhodosporidium paludigenum. Int J Food Microbiol 143:103–108. doi:10.1016/j.ijfoodmicro.2010.07.035
Wang YF, Yu T, Xia JD, Yu DS, Wang J, Zheng XD (2010b) Biocontrol of postharvest gray mold of cherry tomatoes with the marine yeast Rhodosporidium paludigenum. Biol Control 53:178–182. doi:10.1016/j.biocontrol.2010.01.002
Wang J, Shi XG, Wang HY, Xia XM, Wang KY (2012) Effects of esterified lactoferrin and lactoferrin on control of postharvest blue mold of apple fruit and their possible mechanisms of action. J Agric Food Chem 60:6432–6438. doi:10.1021/jf300483v
Wang L, Jin P, Wang J, Jiang L, Zhang S, Gong H, Liu H, Zheng Y (2015) In vitro inhibition and in vivo induction of defense response against Penicillium expansum in sweet cherry fruit by postharvest applications of Bacillus cereus AR156. Postharvest Biol Technol 101:15–17. doi:10.1016/j.postharvbio.2014.11.007
Xu X-M, Jeffries P, Pautasso M, Jeger MJ (2011) Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 101:1024–1031. doi:10.1094/PHYTO-08-10-0216
Yang LF, Siriamornpun S, Li D (2006) Polyunsaturated fatty acid content of edible insects in Thailand. J Food Lipids 13:277–285. doi:10.1111/j.1745-4522.2006.00051.x
Yu T, Zheng XD (2006) Salicylic acid enhances biocontrol efficacy of the antagonist Cryptococcus laurentii in apple fruit. J Plant Growth Regul 25:166–174. doi:10.1007/s00344-005-0077-z
Yu T, Wang LP, Yin Y, Wang Y, Zheng XD (2008) Effect of chitin on the antagonistic activity of Cryptococcus laurentii against Penicillium expansum in pear fruit. Int J Food Microbiol 122:44–48. doi:10.1016/j.ijfoodmicro.2007.11.059
Yu T, Yu C, Lu HP, Zunun M, Chen FX, Zhou T, Sheng K, Zheng XD (2012) Effect of Cryptococcus laurentii and calcium chloride on control of Penicillium expansum and Botrytis cinerea infections in pear fruit. Biol Control 61:169–175. doi:10.1016/j.biocontrol.2012.01.012
Yu C, Zhou T, Sheng K, Zeng LZ, Ye CZ, Yu T, Zheng XD (2013) Effect of pyrimethanil on Cryptococcus laurentii, Rhodosporidium paludigenum, and Rhodotorula glutinis biocontrol of Penicillium expansum infection in pear fruit. Int J Food Microbiol 164:155–160. doi:10.1016/j.ijfoodmicro.2013.04.012
Zhang HY, Zheng XD, Yu T (2007) Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control 18:287–291. doi:10.1016/j.foodcont.2005.10.007
Zhang HY, Wang L, Dong Y, Jiang S, Zhang HH, Zheng XD (2008) Control of postharvest pear diseases using Rhodotorula glutinis and its effects on postharvest quality parameters. Int J Food Microbiol 126:167–171. doi:10.1016/j.ijfoodmicro.2008.05.018
Zhang D, Spadaro D, Garibaldi A, Gullino ML (2010) Selection and evaluation of new antagonists for their efficacy against postharvest brown rot of peaches. Postharvest Biol Technol 55:174–181. doi:10.1016/j.postharvbio.2009.09.007
Zhang H, Liu Z, Xu B, Chen K, Yang Q, Zhang Q (2013) Burdock fructooligosaccharide enhances biocontrol of Rhodotorula mucilaginosa to postharvest decay of peaches. Carbohydr Polym 98:366–371. doi:10.1016/j.carbpol.2013.06.008
Zhang H, Ge L, Chen K, Zhao L, Zhang X (2014) Enhanced biocontrol activity of Rhodotorula mucilaginosa cultured in media containing chitosan against postharvest diseases in strawberries: possible mechanisms underlying the effect. J Agric Food Chem 62:4214–4224
Zhao J, Xue Q-H, Shen G-H, Xue L, Duan J-L, Wang D-S (2012) Evaluation of Streptomyces spp. for biocontrol of gummy stem blight (Didymella bryoniae) and growth promotion of Cucumis melo L. Biocontrol Sci Tech 22:23–37. doi:10.1080/09583157.2011.636481
Zheng XD, Yu T, Chen RL, Huang B, Wu VCH (2007) Inhibiting Penicillium expansum infection on pear fruit by Cryptococcus laurentii and cytokinin. Postharvest Biol Technol 45:221–227. doi:10.1016/j.postharvbio.2007.03.001
Zhu R, Yu T, Guo S, Hu HAO, Zheng X (2015) Effect of the yeast Rhodosporidium paludigenum on postharvest decay and patulin accumulation in apples and pears. J Food Prot 78:157–163. doi:10.4315/0362-028X.JFP-14-218
Acknowledgments
Support for this project was provided by the Doctoral Program Foundation of the Ministry of Education of China (20100101110087) and the National Natural Science Foundation of China (30972051). Thank you to Dr. Michael Edward Wisniewski, USDA-ARS Appalachian Fruit Research Station (West Virginia, USA) for his advice and critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, H., Yan, F., Wilson, C. et al. The ability of a cold-adapted Rhodotorula mucilaginosa strain from Tibet to control blue mold in pear fruit. Antonie van Leeuwenhoek 108, 1391–1404 (2015). https://doi.org/10.1007/s10482-015-0593-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0593-1