Abstract
Using enrichment procedures, a lipolytic strain was isolated from a stinky tofu brine and was identified as Bacillus amyloliquefaciens (named B. amyloliquefaciens Nsic-8) by morphological, physiological, biochemical tests and 16S rDNA sequence analysis. Meanwhile, the key enzyme gene (named lip BA) involved in ester metabolism was obtained from Nsic-8 with the assistance of homology analysis. The novel gene has an open reading frame of 645 bp, and encodes a 214-amino-acid lipase (LipBA). The deduced amino acid sequence shows the highest identity with the lipase from B. amyloliquefaciens IT-45 (NCBI database) and belongs to the family of triacylglycerol lipase (EC 3.1.1.3). The lipase gene was expressed in Escherichia coli BL21(DE3) using plasmid pET-28a. The enzyme activity and specific activity were 250 ± 16 U/ml and 1750 ± 153 U/mg, respectively. The optimum pH and temperature of the recombinant enzyme were 9.0 and 40 °C respectively. LipBA showed much higher stability under alkaline conditions and was stable at pH 7.0–11.0. The Km and Vmax values of purified LipBA using 4-nitrophenyl palmitate as the substrate were 1.04 ± 0.06 mM and 119.05 ± 7.16 μmol/(ml min), respectively. After purification, recombinant lipase was immobilized with the optimal conditions (immobilization time 3 h at 30 °C, with 92 % enzyme recovery) and the immobilized enzyme was applied in biodiesel production. This is the first report of the lipase activity and lipase gene obtained from B. amyloliquefaciens (including wild strain and recombinant strain) and the recombinant LipBA with the detailed enzymatic properties. Also the preliminary study of the transesterification shows the potential value in biodiesel production applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipases (triacylglycerol acylhydrolase, EC 3.1.1.3) are a class of enzymes that can catalyze the hydrolysis of long-chain triacylglycerols into fatty acids and glycerol. They are widely distributed in bacteria, yeasts, fungi, plants and animals (Pahoja and Sethar 2002). Among these, various microbial lipases produced by Bacillus sp. showing conspicuous commercially significant due to its numerous unique characteristics: region specificity, chiral selectivity, and substrate specificity (Schmid and Verger 1998). The lipases are widely used in various industries like food, dairy, chemical, textile, pharmaceutical, cosmetic, detergent production, and especially in biodiesel production and synthesis of new polymeric materials. As containing so many applications, cloning the novel enzyme with distinct features such as thermostable, alkaline, high activities and stabilities in organic solvents is of interest for industrial applications (Niehaus et al. 1999; Pennisi 1997). Jaeger et al. (1999) classified the bacterial lipases into six families (families I–VI) and family I was further classified into six subfamilies (subfamilies I.1–I.6). Lipases from Bacillus sp. were considered to be the lidless and small (actually the smallest lipases known). These lipases seem to be well-suited for biotechnological applications, the synthesis of chiral drugs in particular.
Lipases from Bacillus species have attracted more attention since they have the potentials to be used in food and chemistry industries. Although many Bacillus lipases (Kim et al. 2002) genes from Bacillus subtilis, Bacillus pumilus (Cho et al. 2000), Bacillus stearothermophilus (Kim et al. 2000), Bacillus thermocatenulatus (Schmidt-Dannert et al. 1996) and Bacillus thermoleovorans (Cho et al. 2000) have been cloned and sequenced, there is no report of Bacillus amyloliquefaciens lipase including wild strain lipase activity, lipase gene cloning from B. amyloliquefaciens and recombinant strain. Recently, the increased global demand for biofuels has prompted the search for alternatives to edible oils for biodiesel production (Hama and Kondo 2013). Biodiesel is produced by transesterification of oils or fats with chemical catalysts or lipase (Tan et al. 2010). Enzyme immobilization provides an excellent base for increasing availability of enzyme to the substrate with greater turnover over a considerable period of time (Datta et al. 2013). Although many immobilized microbial lipases have been used in the production of biodiesel from vegetable oil (Hama and Kondo 2013). There is no report of transesterification catalyzed by Bacillus amyloliquefaciens lipase.
Previously, we have obtained a lipolytic strain which was identified as B. amyloliquefaciens (named B. amyloliquefaciens Nsic-8). In this study, a novel lipase gene (lip BA) was obtained from B. amyloliquefaciens and in vivo functional expression of the active lipase was successfully achieved. The enzyme characterizations including the enzyme activity/stability, optimum temperature, optimum pH and substrate specificity were also described through protein purification. Meanwhile, recombinant lipase LipBA was immobilized and the biodiesel production using immobilized LipBA lipase was studied with preliminary results in this research. Also the optimal conditions of immobilization and transesterification were analyzed in our research. To our best knowledge, this is the first report of lipase gene cloning from B. amyloliquefaciens and the recombinant enzyme with detailed enzymatic properties. This alkaline-adapted and thermal-adapted lipase showed potential value in industrial applications according to the enzyme characterizations especially in the detergent industry.
Materials and methods
Bacterial strain and plasmids
An alkaline thermostable lipase-producing bacterial strain used in this study was isolated from a stinky tofu brine by three steps (Kumar et al. 2011) and identified as B. amyloliquefaciens. Escherichia coli DH5α (Invitrogen) and plasmid pMD19-T (TaKaRa) were used for gene cloning and sequencing. Plasmid pET-28a (Novagen) was the vector used to construct the protein expression plasmid in E. coli BL21 (DE3).
Chemicals, strain screening and culture conditions
Methyl heptadecanoate and the substrate 4-nitrophenyl palmitate were purchased from Fluka and Sigma, respectively. Methanol (AR grade), n-hexane (AR grade) and olive oil (saponification value 175–195) used for transesterification and all other chemicals (analytical grade) were purchased at local market. Yeast extract and tryptone were obtained from Shanghai Sangon Biotechnology Co. Ltd (Shanghai, China).
In this work, enrichment culture technique was applied. LB medium added with 1 % olive oil emulsion was used to isolate potential bacterial strains. One ml sample (stinky tofu brine) was added to a flask with 100 ml sterile distilled water. With activating on a rotary for 40 min, 0.1 ml activation culture broth was inoculated into 100 ml enriched medium. Then the medium was shaken at 120 rpm, 30 °C for 48 h. After several rounds of enrichment, inocula was serially diluted and plated onto Rhodamine B agar plates. The microbes showing obviously hydrolysis circle were isolated, purified and transferred to maintenance slants. The strains showing high ratios of hydrolysis circles were selected and identified by 16S rDNA sequence analysis.
Gene cloning of lipase and DNA manipulation/propagation
Based on the information of Bacillus sp. lipase in GeneBank, degenerate primes (BA-L-U: ATGAAACAWATAAAAARCAAAATYC. BA-L-D: TTAATTYGTATTTTGTCCGCCGCCG) for the lipase gene sequence were designed. According to NCBI search, the following gene sequences of lipase were analyzed to design primers: Bacillus amyloliquefaciens strain E1PA (GI:511774067), Bacillus amyloliquefaciens SQR9 (GI:631799361), Bacillus amyloliquefaciens TA208 (GI:328551700) and Bacillus amyloliquefaciens XH7 (GI:341825903).
The DNA manipulations were carried out following the standard procedures. The Bacillus amyloliquefaciens Nsic-8 was harvested after overnight growth in LB medium and DNA extraction was performed using the DNA Mini kit (Axygen). The primes (forward primer P-NOSIG-F(5′-GCGGGATCCTCCTCAGGGCATAACCCT-3′, reverse primer P-NOSIG-R(5′-CTACTCGAGTTAATTTGTATTTTGTCC-3′, underlined nucleotides indicate restriction enzyme sites) were used for lipase gene (without signal peptide) amplification, and it was carried out by the following steps: initial denaturation at 94 °C for 5 min, 35 cycles of 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min, and a final extension of 8 min at 72 °C.The PCR products were cloned into pMD-l9T simple vector after recovering by DNA gel extraction kit (Axygen, China), and then transferred into E. coli DH5α. The nucleotide sequence and predicted amino acid sequence were analyzed by the programs of Blast (NCBI). The open reading frame (ORF) was predicted using the NCBI ORF Finder tool (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The signal peptide was predicted by the SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/). Enzyme Mw and pI were predicted using the ExPASy proteomic server program compute pI/Mw (http://web.expasy.org/compute_pi/). Multiple sequence alignments were performed using DNAMAN and CLUSTAL W. The unrooted phylogenetic tree was constructed using the MEGA program and the three-dimensional structure of this enzyme was predicted by the SWISS-MODEL server (http://swissmodel.expasy.org/SWISS-MODEL.html).
Gene expression of lipase in E. coli
Plasmid pET-28a was used for gene expression in E. coli. After digestion by BamHI/XhoI, the lip BA gene was reclaimed and connected with pET-28a vector, which were digested by the same restriction endonuclease. The positive clones were selected by restriction enzyme digestion and finally confirmed by sequencing (Fig. s-1 in supplementary materials). The recombinant plasmid pET-28-lip BA was transformed into E. coli BL21 (DE3). Recombinant cell (BL21-pET-28-lip BA) was grown to saturation in LB medium supplemented with appropriate antibiotic (Kanamycin 50 μg/ml).
The recombinant lipase strain was incubated in 5 ml Luria–Bertani (LB) medium containing kanamycin (50 μg/ml) at 37 °C for 12 h and then transferred into 50 ml LB medium for propagation. IPTG (0.1 mM) was added to the medium until the absorbance at 600 nm was 0.6. With the cultivation condition at 20 °C for 18 h, the crude enzyme was collected by ultrasonic broken after the strains were harvested by centrifugation (12,000×g, 10 min).
Purification of lipase and SDS–PAGE analysis
After induction by IPTG, cells were separated by centrifugation, dispersed in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and heated to 100 °C. Finally, proteins were separated by SDS–PAGE (12.5 % acrylamide/bis-acrylamide) and stained with coomassie blue.
The previous articles described the methods for heterologous protein expression and Ni–NTA purification procedures in our group (Tan et al. 2010). After lysed by sonication, the crude enzyme was passed through a 0.22 μm filter and then applied to a Ni–NTA sperflow column (1 ml, Qiagen). After equilibrated with the lysis buffer (NPI 10: 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0), the column was subsequently washed with 10 ml of wash buffer (NPI 20: 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0) to remove the impurity protein. The fusion protein (His-tagged LipBA) was eluted with a linear gradient of washing buffer (from NPI 50 to NPI 250). The eluted protein was desalted and concentrated by ultrafiltration using a 50 ml Amicon Ultra Centrifugal Filter Device with a molecular weight cut-off of 10 kDa (Millipore, USA). The purified enzyme was resuspended in sodium phosphate buffer (pH 7.0) containing 20 % glycerol and stored at −40 °C. The crude extract and the pure enzyme were analyzed by SDS–PAGE. All purification steps were carried out at 4 °C. Protein concentration was determined by the Bradford method with bovine serum albumin (BSA) as the standard.
Enzymatic properties analysis
Enzyme activities of purified LipBA solution were assayed by measuring the absorbance at 405 nm of liberated p-nitrophenol. 1 U is defined as the amount of enzyme releasing 1 μmol p-nitrophenol per min under the assay conditions. The reaction mixture (0.5 ml) contained 50 μl of 4-Nitrophenyl palmitate solution (final concentration of 25 mM in the solution of isopropanol and dimethyl sulfoxide with volume ratio 3:1) as the substrate, 440 μl of lipase assay buffer (50 mM Glycine-NaOH buffer, pH 9.0) and 10 μl of appropriately diluted enzyme sample. The reaction mixture was incubated at 40 °C for 5 min, cooled in ice-bath and the absorbance was measured at 405 nm.
The pH optimum for the enzyme activity was studied over a range from pH 2–10 for 5 min (40 °C). The pH stability of the enzyme was determined by incubating the enzyme in different buffers for 12 h and incubated at 40 °C. The following buffer systems were used: pH 2.0–3.0 with 50 mM glycine–HCl,pH 4.0–5.0 with 100 mM citric acid-sodium citrate, pH 6.0–7.0 with 200 mM sodium phosphate, pH 8.0 with 50 mM Tris–HCl, 9.0–10.0 with 50 mM glycine-NaOH and pH 11.0 with 50 mM sodium hydrogen phosphate-NaOH. The temperature optimum for the enzyme activity was assayed at 10–50 °C (pH 9.0). The thermal stability of LipBA was evaluated by assaying its residual activity after incubation of the enzyme at various temperatures for 12 h in sodium phosphate buffer (pH9.0). The effects of metal ions on the lipase activity were determined with various metal ions (1 and 8 mM) such as Fe2+, Mg2+, Ca2+, Cu2+, Zn2+, Co2+, Ni2+ and Mn2+ (pH 9.0). Meanwhile, effects of surfactants (Tween 20, Tween 80, TritonX-100, sodium dodecyl sulfate (SDS), EDTA and DTT at the concentration of 1.0 mM) and various organic solvents (Methanol, Ethanol, Acetonitrile, Benzene, n-hexane, Chloroform, DMSO, Acetone, Are propanol and Isopropanol) (15 % (v/v) and 25 % (v/v)) were measured using the spectrophotometric assay as above. The reaction mixtures containing the enzyme sample were incubated at 40 °C for 60 min in 50 mM glycine-NaOH buffer, pH 9.0. The enzyme sample without any additives was considered as control (100 %).
Immobilized lipase by embedded in sodium alginate
The conditions of the immobilized lipase using sodium alginate as carrier were studied. First, purified lipase solution (10, 20, 30, 40 and 50 ml) was mixed with 50 ml sodium alginate (0.5 g) solution (dissolved with hot distilled water). Then, the mixed solution was dropped into aseptic CaCl2 (1 %) solution using an injector. After immobilized for different time (0–6 h, every 30 min) with shaking (20–60 °C), filtration, washing and drying, the immobilized lipase was finally obtained (Datta et al. 2013). Then the residual lipase activity and thermal stability of the immobilized enzyme was measured.
Preliminary study of enzymatic transesterification reaction
The immobilized lipase was used for transesterification reaction. The reaction mixture containing 5.0 ml n-hexane, 0.5 mM olive oil, 1.5 mM methanol (divided into three times to add), 5 mg immobilized lipase (about 500 U) and 20 μl H2O. After shaking with 200 rpm at a certain temperature (20–60 °C) for 24 h, biodiesel products were detected in the samples. As control, the industrial enzyme Novozym 435 (lipase B from Candida Antarctica) was tested under the same conditions. To confirm a suitable organic solvent for the lipased-catalyzed transesterification, various organic solvents (Isooctane, n-heptane, n-hexane, Cyclohexane, Acetone and Benzene) were examined. A series of temperature (20, 25, 30, 35, 40, 45 and 50 °C) were tested simultaneously.
The products were analyzed by thin-layer chromatography (TLC) and gas chromatography (GC) respectively. Silica gel TLC plates were used for TLC analysis. After activated at 110 °C for 1 h, prepared samples were spotted onto the plate. The plate was placed in a thin-layer chromatography (TLC) chamber containing a solvent system of N-hexane and Diethyl (19:1). After the plate was dried, the products bands were dyed by solid iodine. For GC analysis, HP-5 capillary column (Agilent, 30.0 m × 320 um × 0.25 um) was used. The temperature of vaporizer and detection room was 260 and 280 °C, respectively. First, the column temperature kept 180 °C for 1 min, then heated up to 280 °C with a speed of 10 °C/min for detection.
Results
Strain identification and lipase gene cloning
Strain Nsic-8 was identified as Bacillus amyloliquefaciens by 16S rDNA phylogenetic analysis and the morphology (Table 1). The GeneBank accession numbers for the Bacillus amyloliquefaciens Nsic-8 lipase gene is KF040967. The lipase gene lip BA was amplified by primers BA-L-U/BA-L-D.
Sequence analysis revealed that the sequence of the lip BA ORF uses ATG as the start codon and the G+C content (%) of lip BA is 48.2 mol%. The lip BA gene consists of 645 nucleotides and encodes a deduced protein of 214 amino acids. Analysis of signal peptide showed that a possible signal peptide of 32 amino acids in the N-terminal region was found and the peptide bond between 32th and 33th amino acids (SKA-SS) would be cleaved by signal peptidase (SignalP Server). The molecular weight of LipBA was estimated to be 22.76 kDa (19.22 kDa without the signal peptide), and the pI value was calculated to be 9.74 (9.66 without the signal peptide) by the ExPASy compute pI/Mw program algorithm.
Using the neighbor-joining method (CLUSTAL W), the lip BA gene in Bacillus amyloliquefaciens Nsic-8 was aligned with the other confirmed lipase from other Bacillus sp.. An unrooted phylogenetic tree was constructed using the MEGA program and the phylogeny relationships of closely related microorganisms were shown in Fig. 1a. Homology analysis revealed that LipBA in Bacillus amyloliquefaciens shared the most identity with predicted triacylglycerol lipase in Bacillus amyloliquefaciens CAU B946 (GI: 375360932) and 78 % identical to the lipase in Bacillus sp. JS GI: 489334049 (74 % to Bacillus pumilus GI:169639781, GI:489310171, 73 % to B. licheniformis GI:51507670, 69 % to B. megaterium GI:515136309, 68 % to B. megaterium QM B1551 GI:294500508 and B. megaterium GI:18857878). Also, LipBA contains a single catalytic domain of the alpha/beta hydrolase family and belongs to the family of triacylglycerol lipase (EC 3.1.1.3) (Fig. 1b). The catalytic triad Ser110, Asp166, and His189 residues were seen in the regions. The conserved region, Ala-Xxx-Ser-Xxx-Gly (the feature of the lipase sequences from Bacillus sp.) is boxed. The sequence of signal peptide is underlined (Fig. 1c). Meanwhile, as no report of this lipase has been made, the secondary and three-dimensional structure of LipBA was predicted by the SWISS-MODEL server and the protein structure was viewed by PdbViewer (Fig. s-2 in supplementary materials). The catalytic triad Ser110, Asp166, and His189 residues and the distributions of α-helix/β-sheet were seen in the Fig. s-2.
The amino acid residues analysis. a Rooted phylogenetic tree of LipBA. The consensus amino acid residues of LipBA and some homologous lipase were aligned with FASTA. The sequences used in this alignment were obtained from GenBank as following. 1: Lipase from Bacillus amyloliquefaciens subsp. plantarum CAU B946 (Accession No: GI375360932), 2: Lipase from Bacillus amyloliquefaciens Nsic-8 (this study), 3: Lipase from Bacillus sp. JS (Accession No:GI386756854), 4: Lipase from Bacillus megaterium (Accession No: GI515136309), 5: Lipase from Bacillus megaterium QM B1551 (Accession No: GI294500508), 6: Lipase from Bacillus megaterium (Accession No: GI18857878), 7: Lipase from Bacillus pumilus (Accession No: GI169639781), 8: Lipase from Bacillus pumilus (Accession No: GI489310171), 9: Lipase from Bacillus licheniformis (Accession No: GI51507670). b Putative conserved domains in LipBA. c Conserved sequence alignment of LipBA. The structures are denoted as follows: filled triangle, the catalytic site (Ser110, Asp166 and His189). The reserved amino acid motif AHSXG is boxed. The consensus amino acid residues for the signal peptide are shown in underscore character
Expression and purification of the recombinant enzyme in E. coli
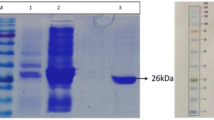
Recombinant strain (BL21-pET-28-lip BA) was grown to saturation in LB medium supplemented with appropriate antibiotic to express the recombinant protein. The SDS-PAGE results showed that the recombinant protein appeared mostly as inclusion bodies (induction temperature: 37 °C, induction time 18 h) in recombinant strains BL21-pET-28a-lip BA and no lipase activity was detected. When the induction temperature dropped to 30 °C, the protein was expressed both in precipitation and supernatant. Using low temperature induction (20 °C), the recombinant protein appeared mostly as the soluble protein (Fig. 2a). As shown in Fig. 2b, the expressed protein LipBA increased with the extension of the culture time within a certain range at 20 °C. However, when cells were fermented for more than 18 h, there was no more significant accumulating of the recombinant protein. So we chose 18 h as the culture time to collect recombinant strains (BL21-pET-28a-lip BA). The optimal expression conditions were found to be an IPTG concentration of 0.1 mM, an induction temperature of 20 °C and an induction time of 18 h. The optimal enzyme activity of LipBA was 250 ± 16 U/ml. Through Ni–NTA purification procedures, the specific activity of purified LipBA was 1750 ± 153 U/mg (about 7-purification fold). The purified LipBA migrate a single band on SDS–PAGE with an apparent molecular mass of about 20.0 kDa, which was identical to the calculated value.
SDS–PAGE analysis of the recombinant lipase and protein purification. a The SDS–PAGE analysis of the recombinant protein with different temperature (BL21-pET-28-lip BA, 20, 30 and 37 °C for 16 h culture). lane M standard marker proteins; lane 1 cell lysate (20 °C); lane 2 precipitation of cell lysate (20 °C); lane 3 supernatant of cell lysate (20 °C); lane 4 cell lysate (30 °C); lane 5 precipitation of cell lysate (30 °C); lane 6 supernatant of cell lysate (30 °C); lane 7 cell lysate (37 °C); lane 8 precipitation of cell lysate (37 °C); lane 9 supernatant of cell lysate (37 °C). b The SDS–PAGE analysis of the recombinant protein with different induction time (BL21-pET-28-lip BA, culture temperature 20 °C). lane M standard marker proteins; lanes 1–5 samples of the recombinant lipase (LipBA) cultured for 0, 6, 12, 18 and 24 h, respectively; lane 6 the purified LipBA protein
Biochemical characterization of the purified recombinant lipase
The optimum temperature of the purified LipBA was examined by assaying enzyme activity at different temperatures (20, 25, 30, 35, 40, 45, 50, 55 and 60 °C) in 50 mM Glycine-NaOH buffer (pH 9.0). The purified LipBA exhibited higher lipase activities over a temperature range of 30–45 °C (over 80 % of the highest activity), among which the highest specific enzyme activity was at 40 °C. Residual activities were determined with standard assay conditions. The LipBA was entirely stable at 20–60 °C and more than 50 % of its activity was retained after 12 h in 60 °C (Fig. 3a).
Characterization of recombinant LipBA. a The temperature optimum and thermal stability of the recombinant lipase activity. (filled triangle) Optimum temperature of the recombinant lipase. (filled circle) Thermostability of the recombinant lipase. b The pH optima and pH stability of the recombinant lipase activity. (filled square) Optimum pH of the recombinant lipase. (filled diamond) pH stability of the recombinat lipase
The effect of pH on lipase activity was determined using 4-nitrophenyl palmitate as substrate at various pH at 40 °C. The purified LipBA exhibited higher lipase activities over a pH range of 7.0–11.0, among which the highest specific enzyme activity was at pH 9.0. The activity of LipBA decreased significantly below pH 6.0 and only about 30 % of the maximal activity in this condition (pH 6.0). Stability of the purified enzyme was investigated in buffer solutions over the pH range of 2.0–11.0. The enzyme solution was incubated at 4 °C for 12 h and the residual activity was determined at pH 9.0 (Fig. 3b). Results showed that the lipase is an alkaline pH stability enzyme which was most stable at pH 7.0–11.0, among which the highest stability was at pH 9.0 (retaining 70 % activity).
The effects of different metal ions and chemical reagents on the lipase were examined (Table 2) . At low concentration (1 mM), Fe2+, Mg2+, Ca2+, Cu2+, Zn2+, Co2+, Ni2+ and Mn2+ partly inhibited or had no effects on the enzyme activity. However, at high concentration (8 mM), all of the metal ions showed significantly inhibited effects especially for Cu2+ (only 9 % of the highest activity). From the results of surfactants tests, the lipase activities had no significantly affected by EDTA and DTT, but significantly inhibited by Tween 20 and Tween 80 (Fig. 4). From the organic solvents test, the enzyme is fairly stable in alkanes, but is highly denatured in hydrophilic solvents such as acetone or short chain (C1–C3) alcohol (Methanol, Ethanol, Are propanol and Isopropanol). Also we found that the lipase was activated by 15 % (v/v) n-hexane (121 %), but inhibited by the concentration of 25 % (v/v) (73 %) (Fig. 4).
Immobilized lipase by embedded in sodium alginate
As shown in Fig. 5, the optimal temperature of immobilization was found to be 30 °C. Meanwhile, the optimal immobilization time of LipBA was 3 h at 30 °C, with 92 % enzyme recovery was detected. The high enzyme recovery indicted that LipBA immobilized by embedded in sodium alginate was practical. The immobilized ability of 50 ml sodium alginate (0.5 g) solution was 30 ml lipase solution (45 mg protein). The thermostability of the immobilized lipase was studied by incubating the enzyme at 60 °C for 12 h, the residue activities of the immobilized lipase and free lipase were 75 and 52 %, respectively. Moreover, the immobilized lipase had good operational stability, which could be repeatedly used for three times with the relative activity as high as 90 %. Furthermore, the immobilized lipase was used for enzymatic transesterification reaction.
Enzymatic transesterification reaction
In biodiesel production, we applied the immobilized LipBA as biocatalyst. The TLC analysis revealed that band of methl ester (biodiesel) was detected in silica gel (Fig. 6a), also the results were further verified by gas chromatography analysis (Fig. 6b). In biodiesel production, three influence factors of the immobilized recombinant LipBA on the transesterification was investigated including temperature, reaction time and organic solvents. The results indicated that the optimal temperature of LipBA transesterification reaction was 35 °C (Fig. 6c). The conversion rate reached maximum after 18 h with n-hexane as organic solvents reaction system. Transesterification activities results showed that LipBA exhibited about equal conversion in non-polar solvents with only minor differences between them (Fig. 6d). However, immobilized enzyme LipBA led to very low transesterification in polar organic solvents such as acetone. The conversion increased with the response time extended, but the transesterification reaction was no longer continued after 18 h (Fig. 6e).
Determination of the kinetic parameters
The kinetic parameters were determined from Lineweaver–Burk plots (Fig. s-3 in supplementary materials). The values of Km and Vmax for 4-nitrophenyl palmitate were calculated to be 1.04 ± 0.06 mM and 119.05 ± 7.16 μmol/(ml min) by measuring the absorption at 405 nm for several concentrations of the substrate over a range of 0.2–2.0 mM, respectively. The data shows that the recombinant enzyme has high catalytic capacity.
Discussion
Lipase is an important enzyme which is used especially in biodiesel production and synthesis of new polymeric materials. Cloning of novel lipase genes with distinct features, especially from easily grown bacteria, is of interest for industrial applications. Bacillus species are well known for their ability to produce and secrete a large number of useful extracellular enzymes. Stinky tofu is a well-known and popular traditional fermented Chinese snack. Previous studies indicated that stinky brine fermentation is a type of alkaline fermentation because of the production of NH3 and referred to stinky tofu as an alkaline-fermented food. The pH of the food rises to 8.0–9.0 due to ammonia production during the fermentation process and Bacillus sp. commonly participates in the fermentation process. Selecting new bacterial strains or improving bacterial strains is a prerequisite and effective solution in industrial applications and will be important for maximal lipase production. In this work, a strain with the grease degradation ability was isolated from stinky tofu brine and identified as Bacillus amyloliquefaciens. Although many lipases from Bacillus sp. have been intensively investigated (Kim et al. 2002; Kamijo et al. 2011; Gupta et al. 2004), there was no report of lipase gene expression from Bacillus amyloliquefaciens. We obtained the lipase gene from the strain Bacillus amyloliquefaciens isolated from a stinky tofu brine and the mature lipase gene without the N-terminal signal peptide was successfully expressed in E. coli BL21. The novel recombinant enzyme LipBA with detailed enzymatic properties has preferable research significance.
Protein sequence alignment showed that LipBA belongs to lipase family I.4. Although, as representative strains in this subfamily, lipases from B. subtilis and B. pumilus have been studied for a long time, the novel lipase from Bacillus amyloliquefaciens was reported for the first time in this paper. Many of the lipases act on their substrates at the lipid-water interface (called interfacial activation). This activation was enhanced by a lid-like polypeptide, which covers the active site of the lipase. Meanwhile, not all lipases show this interfacial activation. There are lipase family members that lack the lid and do not show activation at oil–water interfaces. From the three-dimensional structure of LipBA, there was no lid above the catalytic triad residues (Ser110, Asp166, and His189) (Fig. s-2 in supplementary materials). These small, lidless lipases seem to be well-suited for biotechnological applications (Jaeger et al. 1999). Homology analysis revealed that LipBA in Bacillus amyloliquefaciens shared the 60–80 % identity with predicted triacylglycerol lipase in Bacillus sp. The homology results reveal the remarkable research significance of the novel recombinant enzyme including enzyme characterizations and kinetic parameter. The LipBA was stable at pH 7.0–11.0 compared with that of B. alcalophilus (pH 10.0–10.5) (Gupta et al. 2004), B. licheniformis (pH 9–11) (Gupta et al. 2004), B. alcalophilus (pH 10.6) (Ghanem et al. 2000) and B. subtilis (pH 7.0–9.5) (Ma et al. 2006). The optimum temperature (40 °C) of the recombinant lipase is similar to that of lipase from B. pumilus B106 (50 °C) (Zhang et al. 2009) and B. subtilis (35 °C) (Lesuisse et al. 1993). As for the thermal stability, B. pumilus lipase B26 retained 100 % activity after 15 min at 70 °C (Kim et al. 2002), B. subtilis lipase 168 retained 100 % activity after 30 min at 40 °C (Lesuisse et al. 1993). In this study, lipase LipBA kept 53 % residue activity at 60 °C for 12 h. Even at 70 °C for 12 h, the enzyme attained 46 % residue activity. The results indicated that the recombinant LipBA is thermotolerant and was favorable for industrial or diagnostic use (Sugihara et al. 1991).
In this study, various metal ions (Fe2+, Mg2+, Ca2+, Cu2+, Zn2+, Co2+, Ni2+ and Mn2+) were used to test the influences on the enzyme activity. All the tested metal ions (1 mM) were found to inhibit the enzyme activity. Analysis of the effects of Ca2+ on the lipase activity indicated that LipBA is a Ca2+-independent lipase. The presence of Ca2+ (1 mM) strongly inhibited the enzyme activity (62 %). Meanwhile, many lipases were stimulated by Ca2+, such as Bacillus cereus C7 lipase (1 mM, 200 %) (Dutta and Ray 2009), B. subtilis lipase (10 mM, 116 %) (Ma et al. 2006) and Pseudomonas xuorescens lipase. Although most lipases have the phenomenon of interfacial activation, some lipases lack it, such as B. subtilis lipase and Candida antarctica lipase B, which do not have a lid domain. On the other hand, the activity of Bacillus pumilus lipase B26 (Ca2+-independent lipase) was almost constant at a wide range of Ca2+ concentration (Kim et al. 2002). The protein structure that LipBA do not contain calcium binding sites may provide the presence of this reason.
In this research, LipBA was inhibited by various surfactants especially for Tween 20 and Tween 80. However, among the reported lipases, the influences of the surfactants on enzyme activities are quite different. Neda Akbari found that only SDS and EDTA had significant effect on Pseudomonas sp. lipase activity. The CALB (lipase from Candida antarctica ZJB09193) activities were enhanced in the presence of Tween-20 and sorbitol, inhibited by SDS and Tween-80 (Liu et al. 2012). We chose many organic solvents with different log P to measure the value of the enzyme as a biocatalyst in organic solvents. For it is useful to compare activity in organic solvent to that in aqueous buffer (Snellman and Colwell 2008). Log P is a measure of the polarity of an organic solvent, which is defined as the logarithm of its partition coefficient in a standard n-octane/water two phase system. Laane et al. (1987) concluded that the solvent parameter that correlates best with enzyme activity was log P. In this study, various solvents with different log P were used to test the enzyme stability (Table 3). Methanol (log P: −0.69), ethanol (log P: 0.32), acetonitrile (log P: −0.34), chloroform (log P: 1.97) and acetone (log P: −0.24) inhibited the enzyme activity. The results can be explained that organic solvents with log P values <2.0 are considered to be unfavorable for biocatalysis. However, the lipase LipBA was activated by 15 % (v/v) n-hexane (log P 3.6), but inhibited by the concentration of 25 % (v/v). According to the reported enzymes, n-hexane slightly enhanced the Bacillus sphaericus 205y lipase activity (Rahman et al. 2003) and Bacillus megaterium lipase activity (Lima et al. 2004). On the other hand, the enzyme is fairly stable in alkanes and long chain alcohols but is highly denatured in hydrophilic solvents such as acetone or short chain (C1-C3) alcohol.
Immobilized lipase as the biocatalyst draws high attention because that process is “greener”. Cross-linking of alginate with divalent ions (like Ca2+) and glutaraldehyde improves the stability of enzymes. So the material sodium alginate was used for fabrication of immobilization in our experiments. In this research, the immobilized lipase LipBA with 92 % enzyme recovery showed better temperature tolerance (about 44 % improvement in 60 °C for 12 h) and operational stability (90 % residual enzyme activity through 3 batches used). Several methods are used for immobilization and various factors influence the recovery rate of immobilized enzymes activities (Datta et al. 2013). Candida rugosa lipase adsorbed on biodegradable poly (3-hydroxybutyrate-co-hydroxyvalerate) showed 94 % residual activity after 4 h at 50 °C and reusability till 12 cycles (Cabrera-Padilla et al. 2012). Rhizopus oryzae Lipase immobilized on the HPMCPVA (a blend of hydroxypropyl methyl cellulose (HPMC) and polyvinyl alcohol (PVA)) showed 86–94 % immobilized efficiency, which was very high without any washing procedure (Dhake et al. 2011). Increasing environmental concerns have led to the use of immobilized biocatalysts for biodiesel production (Datta et al. 2013). Enzyme immobilization provides an excellent base for increasing availability of enzyme to the substrate with greater turnover over a considerable period of time. The maximum biodiesel yield of lipase from Thermomyces lanuginosus was 97 % at 50 °C in 24 h reaction (Dizge et al. 2009). The immobilized lipase of P. cepacia can catalyze biodiesel production with the final conversion was around 67 % (Noureddini et al. 2005). The immobilized of Pseudomonas fluorescens can catalyze vegetable oil and cooking oil with yield higher than 87 % (Salis et al. 2008). Although many microbial lipases in biodiesel production had been studied, few lipases from Bacillus sp. were investigated. The biodiesel yield of lipase from Bacillus subtilis reached about 90 % after 72 h reaction in a solvent-free system (Ying and Chen 2007). The transesterification activity of the isolated Bacillus sp. lipase showed 76 % of fatty acid methyl esters yield in 40 h (Sivaramakrishnan and Muthukumar 2012). In our study, the transesterification yield of olive oil was much lower than industrial enzyme Novozym 435 (lipase B from Candida Antarctica) (Fig. 6a). In this research, we found that the enzyme activity was inhibited by n-hexane (25 % concentration). Meanwhile, the enzyme is highly denatured in short chain (C1–C3) alcohol such as methanol. In the reaction system of transesterification, n-hexane and methanol were used as solvent and reactant respectively. This may be the reasons of the low conversion in transesterification. Furthermore, in non-aqueous systems, there is an optimum water content required for providing conformational flexibility. The immobilized lipase used in our study may be contains too many water, and lead to the low conversion in transesterification. As no report of lipase cloning from Bacillus amyloliquefaciens, we just provide the useful exploration in LipBA transesterification.
In this study, we obtained the novel lipase gene (lip BA) from the strain Bacillus amyloliquefaciens Nsic-8 isolated from a stinky tofu brine and the gene was functional expressed in E. coli. The lipase gene (lip BA) is the first reported lipase gene cloned from Bacillus amyloliquefaciens. Meanwhile, the detailed report of LipBA enzymatic properties and the preliminary study of recombinant enzyme immobilization supplied the novel data for B. amyloliquefaciens lipase research. Also the alkaline thermostable lipase reveals the potential value in industrial applications. Future investigations will focus on the application of this enzyme in the enantioseparation and studies on structure–function relationships. In addition, enzyme directed evolution including DNA-shuffling and site directed mutagenesis will be studied.
References
Cabrera-Padilla RY, Lisboa MC, Fricks AT, Franceschi E, Lima AS, Silva DP, Soares CM (2012) Immobilization of Candida 419 rugosa lipase on poly(3-hydroxybutyrate-co-hydroxyvalerate): a new eco-friendly support. J Ind Microbiol Biotechnol 420(39):289–298
Cho AR, Yoo SK, Kim EJ (2000) Cloning, sequencing and expression in Escherichia coli of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiol Lett 186:235–238
Datta S, Christena LR, Rajaram YRS (2013) Enzyme immobilization: an overview on techniques and support materials. 424. Biotechnology 3:1–9
Dhake KP, Tambade PJ, Qureshi ZS, Singhal RS, Bhanage BM (2011) HPMC-PVA Film Immobilized Rhizopus oryzae Lipase as a Biocatalyst for Transesterification Reaction. Acs Catalysis 1:316–322
Dizge N, Keskinler B, Tanriseven A (2009) Biodiesel production from canola oil by using lipase immobilized onto hydrophobic microporous styrene–divinylbenzene copolymer. Biochem Eng J 44:220–225
Dutta S, Ray L (2009) Production and characterization of an alkaline thermostable crude lipase from an isolated strain of Bacillus cereus C7. Appl Biochem Biotech 159:142–154
Ghanem EH, Al-Sayed HA, Saleh KM (2000) An alkalophilic thermostable lipase produced by a new isolate of Bacillus alcalophilus. World J Microbiol Biotechnol 16:459–464
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hama S, Kondo A (2013) Enzymatic biodiesel production: an overview of potential feedstocks and process development. Bioresour Technol 135:386–395
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol 53:315–351
Kamijo T et al (2011) Molecular and enzymatic characterization of a subfamily I.4 lipase from an edible oil-degrader Bacillus sp. HH-01. Antonie Van Leeuwenhoek 99:179–187
Kim MH, Kim HK, Lee JK, Park SY, Oh TK (2000) Thermostable lipase of Bacillus Stearothermophilus: high-level production, purification, and calcium-dependent thermostability. Biosci Biotech Biochem 64:280–286
Kim HK, Choi HJ, Kim MH, Sohn CB, Oh TK (2002) Expression and characterization of Ca2+-independent lipase from Bacillus pumilus B26. Biochim Biophys Acta 1583:205–212
Kumar R, Mahajan S, Kumar A, Singh D (2011) Identification of variables and value optimization for optimum lipase production by Bacillus pumilus RK31 using statistical methodology. New Biotechnol 28:65–71
Laane C, Boeren S, Vos K, Veeger C (1987) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 30:81–87
Lesuisse E, Schanck K, Colson C (1993) Purification and preliminary characterization of the extracellular lipase of Bacillus subtilis 168, an extremely basic pH-tolerant enzyme. Eur J Biochem 216:155–160
Lima VM, Krieger N, Mitchell DA, Baratti JC, Filippis ID, Fontana JD (2004) Evaluation of the potential for use in biocatalysis of a lipase from a wild strain of Bacillus megaterium. J Mol Catal B Enzym 31:53–61
Liu Z-Q, Zheng X-B, Zhang S-P, Zheng Y-G (2012) Cloning, expression and characterization of a lipase gene from the Candida antarctica ZJB09193 and its application in biosynthesis of vitamin A esters. Microbiol Res 453(167):452–460
Ma J, Zhang Z, Wang B, Kong X, Wang Y, Cao S, Feng Y (2006) Overexpression and characterization of a lipase from Bacillus subtilis. Protein Expres Purif 45:22–29
Niehaus F, Bertoldo C, Kahler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Noureddini H, Gao X, Philkana R (2005) Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour Technol 96(769–777):14
Pahoja VM, Sethar MA (2002) A review of enzymatic properties of lipase in plants, animals and microorganisms. Pak J Appl Sci 2:474–484
Pennisi E (1997) Biotechnology: in industry, extremophiles begin to make their mark. Science 276:705–706
Rahman RN, Chin JH, Salleh AB, Basri M (2003) Cloning and expression of a novel lipase gene from Bacillus sphaericus 205y. Mol Genet Genomics 269:252–260
Salis A, Pinna M, Monduzzi M, Solinas V (2008) Comparison among immobilised lipases on macroporous polypropylene toward biodiesel synthesis. J Mol Catal B Enzym 54:19–26
Schmid RD, Verger R (1998) Lipases: interfacial enzymes with attractive applications. Angew Chem Int Edit 37:1608–1633
Schmidt-Dannert C, Rúa ML, Atomi H, Schmid RD (1996) Thermoalkalophilic lipase of Bacillus thermocatenulatus. I. 470 Molecular cloning, nucleotide sequence, purification and some properties. Biochim Biophys Acta 1301:105–114
Sivaramakrishnan R, Muthukumar K (2012) Isolation of thermo-stable and solvent-tolerant Bacillus sp. lipase for the production of biodiesel. Appl Biochem Biotech 166:1095–1111
Snellman EA, Colwell RR (2008) Transesterification activity of a novel lipase from Acinetobacter venetianus RAG-1. Antonie Van Leeuwenhoek 94:621–625
Sugihara A, Tani T, Tominaga Y (1991) Purification and characterization of a novel thermostable lipase from Bacillus sp. J Biochem 109:211–216
Tan T, Lu J, Nie K, Deng L, Wang F (2010) Biodiesel production with immobilized lipase: a review. Biotechnol Adv 28:628–634
Ying M, Chen G (2007) Study on the production of biodiesel by magnetic cell biocatalyst based on lipase-producing Bacillus subtilis. Appl Biochem Biotech 137:793–803
Zhang HZ, Zhang FL, Li ZY (2009) Gene analysis, optimized production and property of marine lipase from Bacillus pumilus B106 associated with South China Sea sponge Halichondria rugosa. World J Microb Biot 25:1267–1274
Acknowledgments
This research was financially supported by the Fundamental Research Funds for the Central Universities of China (No. WF1214047), the National Natural Science Foundation of China (No. C050203-31200596), the National High Technology Research and Development Program of China (No. 2013AA102109) and the National major science and technology projects of China (No. 2012ZX09304009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, X., Ma, J., Wei, Dz. et al. Functional expression of a novel alkaline-adapted lipase of Bacillus amyloliquefaciens from stinky tofu brine and development of immobilized enzyme for biodiesel production. Antonie van Leeuwenhoek 106, 1049–1060 (2014). https://doi.org/10.1007/s10482-014-0274-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-014-0274-5