Abstract
This work evaluated the effect of the cowpea (Vigna unguiculata L. Walp) extract concentration on mead production. Fermentations were carried out in 500-mL Erlenmeyer flasks containing 250 mL of honey wort (30 °Brix), supplemented with 1 g L−1 of ammonium sulfate and 0.1 g L−1 of magnesium chloride and the cowpea extract (5 and 30 g L−1), inoculated with 106 cells mL−1, and incubated at 30 °C for 240 h. Higher cell growth ((cells mL−1): 11.1 × 107, 11.3 × 107, and 19.6 × 107; substrate consumption (%): 86.0, 90.0, and 85.0) and ethanol production ((v v−1 %): 15.0, 15.5, and 14.1) for yeasts Safbrew T-58, Premier Blanc, and Premier Cuvée, respectively, were obtained with 30 g L−1 of bean extract. S. bayanus Premier Blanc had the best metabolic activity with lowest glycerol production (8.5 g L−1) and highest ethanol volumetric yields (0.51–1.52 h−1) after 48 h of fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey is a very complex matrix, because, during production, variables not controlled by man can interfere, such as climate, flowering, presence of sucking insects, and other factors. It is made from the flower’s nectar (floral honey) or the excretions of sucking insects from the live parts of the plants (honeydew honey) [1]. Honey is composed mostly of carbohydrates, and among them, fructose and glucose represent about 85–95%. Proteins, enzymes, amino acids, vitamins, minerals, aromatic components, and polyphenols constitute a small fraction of honey [2, 3].
Mead is a beverage with an alcoholic strength between 8 and 18% v v−1, produced from the fermentation of a diluted honey solution [4]. Due to the high concentration of sugars, low pH and low concentration of minerals present in the honey, mead production takes a long time, which causes problems with the quality of the drink, such as the production of undesirable flavors and aroma [5]. To solve this problem, the fermentation conditions must be optimized [4]. Thus, studies have been developed about selecting strains of Saccharomyces cerevisiae [6], using immobilized yeasts [7], assessing the effect of temperature [4, 8], and verifying the nutrient supplementation effect from pollen addition [9, 10], nitrogen and organic acids [4, 8, 11], salts or vitamins [12], and fruit pulp [13,14,15].
The cowpea (Vigna unguiculata L. Walp) is part of the amylaceous legume group and a very common crop in the semi-arid areas of the Brazilian Northeast [16]. In general, beans contain about 18 and 35% protein [17], which is a complex mixture of globulins, albumins, prolamins, and glutelins in proportions differing between species, varieties, and cultivars. Cowpea is a valuable source of dietary fiber, vitamins (thiamine and pyridoxine) and minerals such as magnesium, phosphorus, calcium, iron, potassium, copper, manganese, and zinc [18, 19]. This type of bean is used to produce flour for preparing bakery products [20] and for cotyledons and cotyledon flour to prepare acarajé and abará, as well as cooked to consume the cowpea beans [21].
Considering the context, the main objective of this work is to evaluate the effect of the concentration of cowpea (Vigna unguiculata L. Walp) extract as a nutritional supplement for yeast in the production of mead.
Materials and Methods
Raw Materials
Floral honey was obtained from the Cooperativa de Apicultores do Tucano (COOAPIT), Bahia, Brazil, while cowpea beans were purchased from the local trade in Feira de Santana, Bahia, Brazil.
Preparation of Cowpea Extract
The cowpea beans were ground and mixed in distilled water. The mixture was autoclaved at 121 °C for 30 min. The product was centrifuged at 3500 rpm for 10 min using the Excelsa Baby I® benchtop centrifuge (model 206 BL, brand FANEM) and stored in sterile plastic bottles. The cowpea bean extract (CBE) had pH 6.10 ± 0.01, 0.24 ± 0.12% w w−1 reducing sugars, 0.53 ± 0.02% w w−1 proteins, 45.73 ± 1.62 mg L−1 assimilable nitrogen, 124.0 ± 1.4 mg kg−1 calcium, 54.0 ± 1.5 mg kg−1 magnesium, 34.0 ± 0.1 mg kg−1 iron, 40.0 ± 0.0 mg kg−1sodium, 1351.0 ± 21.4 mg kg−1 potassium, and 3.2 ± 0.7 mg kg−1 phosphorus.
Yeast Strains
Three freeze-dried commercial yeast strains kept at refrigeration temperature (10 °C) were used: Safbrew T-58 (ScST58) of Saccharomyces cerevisiae, Fermentis brand, and Premier Blanc (SbPB) and Premier Cuvée (SbPC) of Saccharomyces bayanus, Red Star brand.
Inoculum Preparation
The yeasts were weighed according to manufacturer’s instructions, propagated in previously sterilized Erlenmeyer flasks containing honey diluted at 30 °Brix, and shaken (Tecnal TE-420) at 150 rpm under the conditions of 30 °C for 24 h, to provide a cell concentration of 107 cells mL−1.
Fermentation Tests
The fermentation tests were carried out in 500-mL Erlenmeyer flasks, containing 225 mL of the wort (30 °Brix) compound of honey (pH 3.34 ± 0.05; 80.0 ± 0.1 °Brix; 0.264 ± 0.026% m m−1 ash; 0.7 ± 0.1% proteins; total phenolic compounds of 276.4 ± 10.0 mg kg−1 gallic acid, 80.3 ± 1.8% m m−1 total sugars, and 71.7 ± 1.4% w w−1 reducing sugars), diluted in previously sterilized water (1 atm, 121 °C), supplemented with ammonium sulfate (1 g L−1), magnesium (0.1 g L−1), and cowpea extract (0, 5, and 30 gL−1). The pH was adjusted to 5.0 using calcium carbonate.
Inoculation with the yeast strains was carried out by transferring 25 mL of the inoculum, corresponding to 10% of the total volume of the fermentation medium (250 mL) to reach an initial concentration of 106 cells mL−1 [15, 22]. The airlock valve filled with 70% ethyl alcohol was coupled to each Erlenmeyer flask. Soon after, the bottles were placed in a BOD (biochemical oxygen demand) greenhouse at 30 °C for 240 h.
The experiments were carried out in triplicate, and for each yeast strain, the three CBE (0, 5, and 30 g L−1) were evaluated.
Analytical Monitoring of the Fermentative Process
The fermentation monitoring was performed every 24 h, from the collection of samples, determining the following parameters: cell concentration (cells mL−1) from counting performed in the Neubauer chamber (1/400 mm2 × 1/10 mm). For determining viable and nonviable cells, the International Coloring Method was implemented using methylene blue, according to the Amorim et al. [15]. Concentrations of soluble solids (°Brix) and ethanol (% v v−1) were determined using a portable digital refractometer (Reichert Tecnal AR-200), according to Amorim et al. [15].
Determination of Sugars, Organic Acids, and Glycerol Concentrations
The glucose, fructose, sucrose, ethanol, and glycerol concentrations during fermentation were determined through high-performance liquid chromatography (HPLC) (Waters 2414, USA) employing a BIORAD AMINEX HPX-87H (300 mm × 7.8 mm) column and a RID 6A refractive index detector, using 0.005 M H2SO4 as an eluent, at an 0.6 mL min−1 flow rate and 45 °C column temperature.
Fermentation Kinetics
The volumetric productivity in ethanol (Qp, g L−1 h−1) was calculated using Eq. (1).
where P0 and P correspond to the initial and final concentrations of ethanol, and t0 and t correspond to the initial and final fermentation times.
The specific rate of cell growth (μX, h−1) was calculated according to Eq. (2).
where X corresponds to cell concentration and dX/dt corresponds to the derivative of cell concentration with respect to time.
The specific rate of substrate consumption (μSi, gsubstrate gcell−1 h−1) was calculated using Eq. (3).
where X corresponds to cell concentration, Si represents the determined carbohydrates (glucose, fructose, and sucrose) and dS/dt corresponds to the derivative of the substrate consumption, in relation to time.
The specific rate of product formation (μP, gethanol gcell−1 h−1) was calculated according to Eq. (4).
where X corresponds to cell concentration, P represents the formed product (ethanol), and dP/dt corresponds to the derivative of ethanol production with respect to time.
The derivatives dx/dt, dS/dt, and dP/dt were calculated according to the method proposed by Le Duy and Zajic [23].
Results and Discussion
Influence of Cowpea Extract on Cell Growth of S. cerevisiae Safbrew T-58, S. bayanus Premier Blanc and Premier Cuvée during Mead Production
Figure 1 a–c shows the cell growth profile of S. cerevisiae Safbrew T-58 (ScST58) and S. bayanus Premier Blanc (SbPB) and Premier Cuvée (SbPC) during the fermentation of honey wort, supplemented with different CBE (0 g L−1, 5 g L−1, 30 g L−1). In general, the cellular growth of yeasts was favored by increased supplementation with cowpea extract. The increase in the cell growth of SbPC (7 times), ScST58 (7 times), and SbPB (5 times) was verified, utilizing a higher CBE (30 g L−1) for 24 h (Fig. 1a–c). The inappropriate amount of assimilable nitrogen in the fermentative process can led to poor growth of the yeast [11].
Following this duration (24 h), a gradual increase in cell growth of yeasts ScST58 and SbPB (Fig. 1a–c) was clearly observed in the control trials (absence of cowpea supplementation), in relation to the trials with an increased supplementation with CBE, from 5 to 30 g L−1. This fact evidences the positive influence of the added cowpea extract as a source of proteins and essential amino acids [24] for the metabolic activities of these yeasts. Since honey lacks the essential components for the development of yeast, it compromises the fermentation process [5].
However, at the maximum concentration (30 g L−1) of cowpea extract, the SbPC yeast presented higher cell growth (19.6 × 107 cells mL−1, 168 h) than the ScST58 (11.1 × 107 cells mL−1, 96 h) and the SbPB (11.3 × 107 cells mL−1, 120 h) (Fig. 1a–c).
In fermentation control trials, without supplementation with cowpea extract in the wort, the SbPC (15.4 × 107 cells mL−1 at 72 h) obtained the highest growth, followed by the SbPB (9.4 × 107 cells mL−1 at 216 h) and the ScST58 (7.25 × 107 cells mL−1 at 96 h). Similar cell growth (16 × 107 cells mL−1 at 60 h) to that observed in the SbPC was reported by Balogu and Towobola [25], with a S. cerevisiae strain in the wort composed of a mixture of honey and coconut water (pH 3.5, 25 °C, and inoculum of 1.0 × 106 cells mL−1).
The SbPC presented growth (15.0 × 107 cells mL−1 at 162 h), similar to the control test (absence of cowpea extract), with the use of 5 g L−1 of CBE (Fig. 1c). Thus, independent of the supplementation with CBE, the SbPC, commercially destined for mead production, exhibited the highest cell growth in the fermented wort. The SbPB, used to produce mead, independent of the supplementation with CBE (0, 5, and 30 g L−1), showed its maximum growth potential in the wort, with higher cultivation times (216 h, 192 h, and 216 h, respectively) (Fig. 1b).
Supplementation with higher concentrations of nitrogen source (e.g., 1.015 g L−1 diammonium phosphate) favored the cell growth of the S. cerevisiae strain, with a reduction in culture time from 600 to 264 h [11]. These assays were conducted in the wort with approximately 22.25 °Brix, pH from 3.50 to 4.72 at 22 °C. The authors also reported that these effects, in relation to favoring cell growth and reducing cultivation time, were not observed when the wort was supplemented with organic acids. Pereira et al. [12] also reported that the honey wort (23.2–23.4 °Brix) supplementation with salts and/or vitamins showed no alteration in the cell growth profile of the S. cerevisiae strains (Lalvin QA23 and Lalvin IVC D47) in mead production using pH, varying between 3.67 and 3.78 and inoculum of 105 cells mL−1.
Lower values of cell growth (3.2 × 107 cells mL−1) after 288 h of fermentation, in wort supplemented with 10, 20, and 30% pineapple pulp were reported in the mead production, under the conditions of 30 °Brix, pH 4.5, 30 °C, and inoculum of 3 × 106 cells mL−1, by the S. cerevisiae Montrachet [14]. On the other hand, under the same conditions used by Mascarenhas et al. [14], higher values of cell growth (7.6 × 109 cells mL−1) after 288 h of fermentation in the mead production, using tamarind pulp concentrations of 10, 20, and 30%, were verified by Anunciação et al. [13]. Four regions of higher cell growth (~ 2 × 108 cells mL−1) after 48, 168, 216, and 288 h of fermentation using 17%, 24%, 10%, and 18% acerola pulp concentration, with respect to the S. cerevisiae AWRI 796 was reported by Amorim et al. [15].
Despite the observation of higher cell growth in the initial 24 h of culture, the ScST58, commercially destined for brewing, presented lower cell growth at the end in the fermented wort (Fig. 1a). Although ScST58 is a brewer’s yeast, it was tested in mead production because it had excellent performance in beer production with high alcohol content (8.5 to 11.5% v v−1). According to Róldan et al. [10] and Pereira et al. [6], different strains of S. cerevisiae used in the production of wine, champagne, and beer were also successfully used in the production of mead.
Kinetic Study About the Supplementation of Cowpea Extract in Mead Production Using S. cerevisiae Safbrew T-58, S. bayanus Premier Blanc and Premier Cuvée
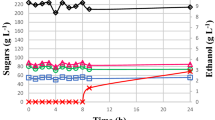
Figure 2 a–c illustrates the glucose, fructose, sucrose, and ethanol concentration (g L−1) profiles during the fermentation of honey wort using ScST58, SbPB, and SbPC without, or supplemented with 5 g L−1 and 30 g L−1 of CBE.
The sugars D-glucose (~ 84.9 g L−1) and D-fructose (~ 143.83 g L−1) initially presented in honey wort were consumed simultaneously by all yeasts regardless of supplementation with cowpea (Fig. 2a–c). Higher intakes of glucose (97.4%) and fructose (71.4%) were verified in the fermentation by SbPB in the medium, without the addition of extracts (Table 1). The glucose consumption (96 to 99%) by this yeast was independent of the extract concentration in the medium, but an increase of fructose consumption by up to 95.5% occurred with the highest CBE (30 g L−1), producing a mead containing 0.9 g L−1 glucose and 5.9 g L−1 fructose. On the other hand, lower intakes of glucose (89.7%) and fructose (57.8%) were obtained in the medium without the addition of extract, fermented by ScST58 brewer’s yeast. The addition of a higher CBE intensified the metabolism of sugars glucose (99.8%) and fructose (85.6%) by this brewer’s yeast, obtaining a mead containing 0.2 g L−1 glucose and 35.3 g L−1 fructose. A 10.9% and 30.5% increase in the metabolism of glucose and fructose sugars, respectively, was observed in the SbPC fermentation, resulting in a mead containing 0.1 g L−1 glucose and 17.8 g L−1 fructose (Fig. 2c). Gomes et al. [4] suggest that mead containing 27.6 g L−1 fructose is not suitable, since a high concentration of sugars at the end of fermentation may increase the chances of product refermentation. The availability of a high concentration of fermentable sugars in the mead could favor contamination by undesirable microorganisms as well as the appearance of non-characteristic odors of the product due to the formation of lactic and acetic acids (vinegar off-character).
In relation to sucrose, regardless of the supplementation of honey wort with CBE, sucrose consumption was low (between 9.5 and 27.7%) in all the experimental conditions. Sucrose could be converted into its monomers (glucose and fructose) by means of a hydrolytic process, which may be chemical by the use of acids or enzymatic by the action of the invertase enzyme being consumed by yeast [26, 27].
The SbPB showed higher total sugar consumption (90%), followed by the ScST58 (86%) and the SbPC (85%). Similar values of substrate consumption (88.5%) during the fermentation of honey wort (21 °Brix) using S. cerevisiae at pH 4.5 and 25 °C were reported by Ilha et al. [28]. Gomes et al. [29] also found high substrate consumption (80%) in the fermentation of honey wort in sweet mead production. These authors also obtained a simultaneous consumption of glucose and fructose, as observed by Pereira et al. [6]. Similar substrate consumption (82%) during the fermentation of honey wort using two commercial strains (ICV D47 and QA23) of S. cerevisiae was verified by Pereira et al. [5].
Higher ethanol concentrations of 118.4 g L−1 (15.0% v v−1), 122.3 g L−1 (15.5% v v−1), and 111.2 g L−1 (14.1% v v−1) in the fermentation by ScST58, SbPB, and SbPC, respectively, were obtained using higher extract concentration (Fig. 2c). In the trials without supplementation with cowpea, lower concentrations of ethanol (89.2 g L−1 (11.3% v v−1), 94.7 g L−1 (12.0% v v−1), and 101.8 g L−1 (12.9% v v−1)) were obtained. Similar values of alcoholic contents in S. bayanus (94.5 g L−1) and S. cerevisiae (130.4 g L−1) cultures, in multiflorous honey and honeydew honey at 36 °Brix, temperature of 18 to 20 °C after 720 h, were reported by Czabaj et al. [30]. According to Kempka and Mantovani [9], the mead production using wort (16 °Brix) with the honey from the wild type, angico, and honeydew cultivated by S. cerevisiae at pH 5.45 and 36 °C for 168 h, showed a decrease in the values of total soluble solids by 58.5, 54.5, and 42.9%, respectively. In these cases, ethanol concentrations of 71.0 g L−1, 63.1 g L−1, and 51.3 g L−1 were obtained, respectively. Amorim et al. [15] reported that the addition of 10–30% acerola pulp enhanced substrate consumption (55–58%) and ethanol production (15.2–16.6% v v−1 or 120–127 g L−1) after 288 h of fermentation.
Varela et al. [31] reported that worts with higher concentrations of assimilable nitrogen source (300 mg L−1) resulted in wine production with higher alcohol content (12.7% v v−1) after 700 h of culture at pH 3.5 and 28 °C. Ukpabi [32] also found similar ethanol concentration (12.7–15.0%) in mead produced with floral honey, from the nectar of several manioc species (Manihot esculenta).
Lower glycerol concentrations (4.5 g L−1) were verified in the medium fermented by SbPB, without addition of the extract. However, increasing the extract concentration in the fermentation medium produced the greatest increase (almost 50%) in the concentration of glycerol by this yeast. Glycerol is the largest by-product formed during the fermentation process for the production of ethanol. This alcohol is produced by the yeast to maintain the oxidation balance-reduction and to counteract osmotic stress [33].
The highest concentrations of glycerol by the ScST58 (8.49 g L−1), SbPB (10.08 g L−1), and SbPC (10.97 g L−1) were produced with the maximum supplementation of cowpea extract (30 g L−1). Similar concentrations of glycerol (7.41–9.45 g L−1) in mead from honey wort have been verified by Czabaj et al. [30]. Hernandez et al. [34] showed that the nitrogen source had an effect on the sugar consumption as well as the rate of production, for both glycerol (8.5–9.5 g L−1 and 7–10 g L−1) and ethanol (approx. 200 g L−1 and 180–240 g L−1), by the yeasts Lalvin QA23 and Fermiblanc Arom, respectively, at 16 days. Lower concentrations of glycerol in sweet (5.1 g L−1) and dry (5.96 g L−1) mead after 79 h and 198 h of cultivation, respectively, were reported by Gomes et al. [29]. Mead containing between 4.2 and 9.3 g L−1 glycerol after 200 h of culture in clear and dark honey wort containing two types of nutritional supplements at 27 °C for three strains of S. cerevisiae, two isolates of honey, and one commercial wine producer, were reported by Pereira et al. [6].
The concentration of glycerol in relation to the ethanol obtained ranged from 5 to 10% at the end of the fermentation (240 h), regardless of the yeast used in this work. The glycerol levels are generally founded in the range of 7 to 10% of the produced ethanol [33]. Glycerol is a non-volatile compound and does not contribute to the aromatic characteristics of the beverages, but contributes to its softness and viscosity, observed at concentrations greater than 25 g L−1 [33].
Higher values of volumetric productivity in ethanol (2.25, 1.80, and 1.30 g L−1 h−1) in the honey worts fermented by ScST58, SbPB, and SbPC, respectively, were obtained, with the maximum concentration of CBE (30 g L−1).
Lower values of Qp (0.19 and 0.27 g L−1 h−1) were reported in fermentations with immobilized and free S. cerevisiae cells after 480 and 288 h of culture, respectively, in wort containing 24 °Brix and supplemented with pollen at 30 °C [22]. Higher volumetric productivity in ethanol (23.1 g L−1 h−1) in the mead production, through immobilized yeasts of Hansenula anomala in honey wort at pH 4.5 and temperature between 27 and 35 °C, after 110 days of cultivation in a packed bed reactor, was reported by Qureshi and Tamhane [35].
Figure 3a–c shows the profile of the specific rates of cell growth, substrate consumption, and ethanol production during the fermentation process of honey must using the commercial yeasts Safbrew T-58, Premier Blanc, and Premier Cuvée for the concentrations of cowpea extract, at 0 g L−1 (Fig. 3a), 5 g L−1 (Fig. 3b), and 30 g L−1 (Fig. 3c).
Profile of the specific rates of cell growth, substrate consumption; and ethanol production during the fermentation process of honey must using the commercial yeasts Safbrew T-58, Premier Blanc, and Premier Cuvée for the concentrations of cowpea extract, at 0 g L−1 (a), 5 g L−1 (b), and 30 g L−1 (c)
SbPC achieved the maximum specific cell growth rate (μxmax, 2.14 ± 0.002 h−1) with 5 g L−1 of CBE supplementation in 96 h of culture (Table 2, Fig. 3b). However, it was the yeast with the lowest specific rates of substrate consumption and ethanol production (Table 2), independent of the supplementation with the cowpea extract (Fig. 3a–c). Thus, SbPC presented the best cell growth (19.6 × 107 cells mL−1) and lowest concentration of ethanol (109.7 g L−1, 14.1% v v−1), with the supplementation of 30 g L−1 cowpea extract at 240 h.
The highest μxmax (0.63 ± 0.001 h−1) of SbPB was obtained at 216 h of culture (Fig. 3c), using the highest supplementation of cowpea extract (30 g L−1). Higher specific rates of substrate consumption (μsmax, 1.97 ± 0.005 gsubstrate gcell−1 h−1) and ethanol production (μpmax, 0.33 ± 0.001 gethanol gcell−1 h−1) were obtained by the SbPB in 24 h and 48 h, respectively. Consequently, the highest ethanol concentration (121.3 g L−1, 15.5% v v−1) was obtained with the highest supplementation of CBE (30 g L−1) at 240 h.
ScST58 exhibited the lowest μxmax values independent of supplementation. However, this strain presented high values of μsmax and μpmax, even without the supplementation of cowpea extract (Table 2). Furthermore, this strain had the highest μpmax (0.50 ± 0.001 gethanol gcell−1 h−1) in 24 h with a higher supplementation of cowpea (30 g L−1) (Fig. 3c). In general, an increase in the μsmax and μpmax was verified with the supplementation using cowpea extract, except the fermentations carried out by strain Premier Cuvée.
Specific growth rates between 0.18 and 0.20 h−1, for seven S. cerevisiae strains submitted or not to osmotic stress (20% v v−1 glucose plus 20% v v−1 fructose add to YPD liquid medium), were reported by Pereira et al. [6]. Lower specific growth rates (0.15 and 0.16 h−1) of S. cerevisiae Lalvin IVC D47 and Lalvin QA23 strains, respectively, after 96 h in honey wort using initial conditions of pH 3.7, 267 mg L−1 of ammonium phosphate at 22 °C, were reported by Pereira et al. [5]. Similar specific growth rates (0.14 and 0.22 h−1) for eight wine-producing S. cerevisiae strains in wort, supplemented with concentrations between 67 and 670 mg L−1 of nitrogen, were verified by Barbosa et al. [36]. According to these authors, the maximum values of the specific rate of cell growth for each strain were not altered with the use of the evaluated nitrogen concentrations.
Conclusion
The higher concentration of cowpea extract had a stimulatory effect on the metabolic activities of all yeasts, primarily the Saccharomyces bayanus Premier Blanc, resulting in the better utilization of substrate for the production of ethanol, cell growth, glycerol, and higher volumetric productivity. Thus, cowpea can be used as an alternative raw material in the production of mead.
References
Campos, G., Della-Modesta, R. C., Silva, T. J. P., Baptista, K. E., Gomides, M. F., & Godoy, R. L. (2003). Classificação do mel em floral ou mel de melato. Ciências e Tecnologia de Alimentos, 23, 1–5.
Finola, M. S., Lasagno, M. C., & Marioli, J. M. (2007). Microbiological and chemical characterization of honeys from central Argentina. Food Chemistry, 100, 1649–1653.
Won, S. R., Lee, D. C., Ko, S. H., Kim, J. W., & Rhee, H. I. (2008). Honey major protein characterization and its application to adulteration detection. Food Research International, 41, 952–956.
Gomes, T., Barradas, C., Dias, T., Verdial, J., Morais, J. S., Ramalhosa, E., & Estevinho, L. M. (2013). Optimization of mead production using response surface methodology. Food and Chemical Toxicology, 59, 680–686.
Pereira, A. P., Mendes-Ferreira, A., Oliveira, J. M., Estevinho, L. M., & Mendes-Faia, A. (2013). A high-cell-density fermentation of Saccharomyces cerevisiae for the optimization of mead production. Food Microbiology, 33(1), 114–123.
Pereira, A. P., Dias, T., Andrade, J., Ramalhosa, E., & Estevinho, L. M. (2009). Mead production: Selection and characterization assays of Saccharomyces cerevisiae strains. Food and Chemical Toxicology, 47(8), 2057–2063.
Navrátil, M., Sturdik, E., & Gemeiner, P. (2001). Batch and continuous mead production with pectate immobilized, ethanol-tolerant yeast. Biotechnology Letters, 23, 977–982.
Iglesias, A., Pascoal, A., Choupina, A. B., Carvalho, C. A., Féas, X., & Estevinho, L. M. (2014). Developments in the fermentation process and quality improvement strategies for mead production. Molecules, 19(8), 12577–12590.
Kempka, A. P., & Mantovani, G. Z. (2013). Produção de hidromel utilizando méis de diferentes qualidades. Revista Brasileira de Produtos Agroindustriais, 15, 273–281.
Roldán, A., Van Muiswinkel, G. C. J., Lasanta, C., Palacios, V., & Caro, I. (2011). Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chemistry, 126, 574–582.
Mendes-Ferreira, A., Cosme, F., Barbosa, C., Falco, V., Inês, A., & Mendes-Faia, A. (2010). Optimization of honey must preparation and alcoholic fermentation by Saccharomyces cerevisiae for mead production. International Journal of Food Microbiology, 144(1), 193–198.
Pereira, A. P., Mendes-Ferreira, A., Estevinho, L. M., & Mendes-Faia, A. (2015). Improvement of mead fermentation by honey-must supplementation. Journal of the Institute of Brewing, 121, 405.
Anunciação, A. S., Martins, B. J. A., Amorim, T. S., Carvalho, G. B. M., & Martinez, E. A. (2017). Polpa de tamarindo (Tamarindus indica L.) na produção de hidromel. Revista Brasileira de Agrotecnologia, 7, 441–445.
Mascarenhas, A. M. O., Amorim, T. S., Anunciação, A. S., Albinati, F. L., & Martinez, E. A. (2017). Produção de hidromel: efeito da concentração da polpa de abacaxi (Ananas mill). Revista Brasileira de Agrotecnologia, 7, 436–440.
Amorim, T. S., Lopes, S. B., Bispo, J. A. C., Bonafe, C. F. S., Carvalho, G. B. M., & Martínez, E. A. (2018). Influence of acerola pulp concentration on mead production by Saccharomyces cerevisiae AWRI 796. LWT- Food Science and Technology, 97, 561–569.
Oliveira, O. M. S., Silva, J. F., Gonçalves, J. R. P., & Klehm, C. S. (2010). Weed coexistence with cowpea cultivars in the Amazonas floodplain. Planta Daninha, 28, 523–530.
Neves, V. A., Pereira, D. D., Shoshima, A. H. R., & Tavano, O. L. (2003). Características da solubilidade proteica e isolamento da globulina principal de caupí (Vigna unguiculata (L.) Walp.) cultivar BR 14-mulato. Alimentos e Nutrição, 14, 47–55.
Frota, K. M. G., Soares, R. A. M., & Arêas, J. A. G. (2008). Composição química do feijão caupi (Vigna unguiculata L. Walp), cultivar BRS-Milênio. Ciência e Tecnologia de Alimentos, 28, 470–476.
Iqbal, A., Khalil, I. A., Ateeq, N., & Khan, M. S. (2006). Nutritional quality of important food legumes. Food Chemistry, 97, 331–335.
Frota, K. M. G., Morgano, M. A., Silva, M. G., Araújo, M. A. M., & Moreira-Araújo, R. S. R. (2010). Utilização da farinha de feijão-caupi (Vigna unguiculata L. Walp) na elaboração de produtos de panificação. Ciência e Tecnologia de Alimentos, 30, 44–50.
Brito, E. S. (2008). Feijão Caupi. Fortaleza: Embrapa Agroindústria Tropical.
Martinez, A. M., Vivas, G. J., & Quicazan, M. C. (2016). Evaluation of alcoholic fermentation during the production of mead using immobilized cells in kappa-carrageenan. Chemical Engineering Transactions, 49, 19–24.
Le Duy, A., & Zajic, J. E. A. (1973). Geometrical approach for differentiation of an experimental function at a point: Applied to growth and product formation. Biotechnology and Bioengineering, 15, 805–815.
Gupta, P., Singh, R., Malhotra, S., Boora, K. S., & Singal, H. R. (2014). Cowpea [Vigna unguiculata (L.) Walp.] seed proteins: Heterogeneity in total proteins and protein fractions. Legume Research, 37, 62–67.
Balogu, T. V., & Towobola, O. (2017). Production and quality analysis of wine from honey and coconut milk blend using Saccharomyces cerevisiae. Fermentation, 3, 1–9.
Lima, U. A., Basso, L. C., & Amorim, H. V. (2001). Biotecnologia Industrial: Processos Fermentativos e Enzimáticos. São Paulo: Blücher.
Schmidell, W., Lima, A. U., Aquarone, E., & Borzani, W. (2001). Biotecnologia Industrial. São Paulo: Blücher.
Ilha, E. C., Bertoldi, F. C., Reis, V. D. A., & Sant’anna, E. (2008). Rendimento e Eficiência da Fermentação Alcoólica na Produção de Hidromel. Corumbá: Embrapa Pantanal.
Gomes, T., Dias, T., Cadavez, V., Verdial, J., Morais, J. S., Ramalhosa, E., & Estevinho, L. M. (2015). Influence of sweetness and ethanol content on mead acceptability. Polish Journal of Food and Nutrition Sciences, 65, 137–142.
Czabaj, S., Kawa-Rygielska, J., Kucharska, A. Z., & Kliks, J. (2017). Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules, 22, 803.
Varela, C., Pizarro, F., & Agosin, E. (2004). Biomass content governs fermentation rate in nitrogen-deficient wine musts. Applied and Environmental Microbiology, 70(6), 3392–3400.
Ukpabi, U. J. (2006). Quality evaluation of meads produced with cassava (Manihot esculenta) floral honey under farm conditions in Nigeria. Tropical and Subtropical Agroecosystems, 6, 37–41.
Scanes, K. T., Hohmann, S., & Prior, B. A. (1998). Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. South African Journal for Enology Viticulture, 19, 17–23.
Hernandez, C. Y., Serrato, J. C., & Quicazan, M. C. (2015). Evaluation of physicochemical and sensory aspects of mead, produced by different nitrogen sources and commercial yeast. Chemical Engineering Transactions, 43, 1–6.
Qureshi, N., & Tamhane, D. V. (1987). Production of mead by immobilized cells of Hansenula anomala. Applied Microbiology and Biotechnology, 27, 27–30.
Barbosa, C., Lage, P., Vilela, A., Mendes-Faia, A., & Mendes-Ferreira, A. (2014). Phenotypic and metabolic traits of commercial Saccharomyces cerevisiae yeasts. AMB Express, 4, 39.
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), Financial Code – 001, and Fundação de Amparo à Pesquisa do Estado de Bahia (FAPESB), Brazil. We thank the Graduate Program in Biotechnology (PPGBiotec) of the State University of Feira de Santana (UEFS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The presenting author of this manuscript in journal Applied Biochemistry and Biotechnology is Geiza Suzart Araújo.
Rights and permissions
About this article
Cite this article
Araújo, G.S., Gutiérrez, M.P., Sampaio, K.F. et al. Mead Production by Saccharomyces cerevisiae Safbrew T-58 and Saccharomyces bayanus (Premier Blanc and Premier Cuvée): Effect of Cowpea (Vigna unguiculata L. Walp) Extract Concentration. Appl Biochem Biotechnol 191, 212–225 (2020). https://doi.org/10.1007/s12010-020-03267-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03267-0