Abstract
The Eastern Mediterranean deep sea is one of the most oligotrophic regions in the world’s ocean. With the aim to classify bacteria from this special environment we isolated 107 strains affiliating to the Gammaproteobacteria, Alphaproteobacteria, Firmicutes, Actinobacteria and Bacteroidetes from sediments of the Eastern Mediterranean Sea. As determined by 16S rRNA gene sequence analysis, Actinobacteria and Firmicutes, in particular members of the genus Bacillus, were dominant and represented a remarkable diversity with 27 out of a total of 33 operational taxonomic units obtained from the untreated sediment. The considerable percentage of operational taxonomic units (42%) which may be considered to be new species underlines the uniqueness of the studied environment. In order to selectively enrich bacteria which are adapted to the deep-sea conditions and tolerate broad pressure ranges, enrichments were set up with a sediment sample under in situ pressure and temperature (28 MPa, 13.5°C) using N-acetyl-d-glucosamine as substrate. Interestingly Gammaproteobacteria were significantly enriched and dominant among the strains isolated after pressure pre-incubation. Obviously, Gammaproteobacteria have a selective advantage under the enrichment conditions applied mimicking nutrient supply under pressure conditions and cope well with sudden changes of hydrostatic pressure. However, under the continued low nutrient situation in the Eastern Mediterranean deep-sea sediments apparently Firmicutes and Actinobacteria have a clear adaptative advantage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies on microbial communities in marine deep-sea sediments frequently focus on highly productive spots, like hydrothermally active regions. However, the major fraction of deep-sea sediments represents extremely oligotrophic environments subjected to high hydrostatic pressures. This holds especially true for the Eastern Mediterranean sea (Lampadariou et al. 2009). The extreme depletion of nutrients in surface waters results in low primary production. Only a minor fraction of the produced organic matter (1%) is reaching the deep-sea sediments (Lampitt and Antia 1997). Organic carbon values of the Southern Cretan Margin sediments were found to be in the range of 0.07–1.55% of sediment dry weight (Polymenakou et al. 2008). The Eastern Mediterranean deep sea is furthermore characterised by comparably high temperatures of approximately 14°C opposite to most other deep-sea environments with temperatures of 2–4°C (Emig and Geistdoerfer 2004). The sediments of the Eastern Mediterranean deep sea thus combine different environmental features (extreme oligotrophy, high hydrostatic pressure of up to 52 MPa at the deepest point and comparably high deep-water temperature) which may be in favour of the development of unique types of bacteria. T-RFLP analysis revealed high values of bacterial diversity within Eastern Mediterranean sediments of the deep Ionian sea (Luna et al. 2004). Also environmental 16S rRNA gene libraries of Western Mediterranean deep-sea sediments revealed high bacterial diversity, while Archaea appear to be of minor abundance and diversity (Danovaro et al. 2009). Dominating bacterial sequences from deep-sea sediments of the Southern Cretan Margin were assigned to the Acidobacteria (18%), Gammaproteobacteria (13%), Planctomycetes (11%), Actinobacteria (11%), Alphaproteobacteria (10%) and Deltaproteobacteria (9%) (Polymenakou et al. 2009).
There is however, a considerable lack of cultivation-based studies of bacteria that is essential to enlighten their ecological role in natural environments (Das et al. 2006). Studies of cultured bacterial diversity from deep-sea environments are generally performed at atmospheric pressure and there are only few studies using in situ hydrostatic pressure incubation (e.g. Yayanos et al. 1979; Kato et al. 1995, 1996, 1998, 1999; Yanagibayashi et al. 1999). In contrast, low nutrient concentrations prevailing in marine deep-sea habitats have been taken into account in cultivation-based studies since the 1990s (Rueger and Tan 1992; Rappé et al. 1999). The use of low nutrient media turned out to significantly improve the cultivation efficiency (Carlucci et al. 1986; Rueger and Tan 1992; Koch 2001), for the growth of copiotrophic and fast growing bacteria is retarded in low-nutrient media giving a chance for growth of oligotrophic bacteria.
In the present study bacteria from Mediterranean deep sea sediments were isolated and characterised by culture-dependent studies using different media with low nutrient content and as well enrichments under in situ hydrostatic pressure with the chitin monomer N-acetyl-d-glucosamine (NAG) as supplement.
Materials and methods
Sample collection
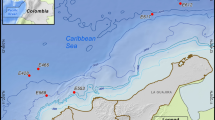
All samples were obtained southwards of Crete during Meteor cruise 71 leg 2 in December 2006–January 2007. Sediment was collected from the Ierapetra basin (IB) at 4400 m (34°30.296N, 26°11.507E) and the Herodotos plain (HP) at 2800 m (33°42.989N, 26°20.329E) using a multiple corer from the Senckenberg Research Institute in Wilhelmshaven, Germany (DZMB, German Center for Marine Biodiversity Research) which has the possibility to hold 12 cores of 9.5 cm inner diameter. Of each sediment core the uppermost 5 cm were aseptically sub sampled. The deep-sea water column [>500 m referring to Kontoyiannis et al. (1999)] was sampled at the same locations by a rosette sampler equipped with 24 Niskin Bottles connected to a CTD probe. Sampling depth were 500, 1200, 2500 and 4000 m.
Isolation and cultivation
For standard isolation of aerobic bacteria from the sediment, dilutions of 10−1–10−4 in sterile Mediterranean seawater were prepared and plated on five different agar media. MW-medium: 1.5% agar added to Mediterranean seawater; MWY-medium: 1.5% BactoTM agar (BD, Germany), 0.01% BactoTM yeast extract (BD, Germany) in Mediterranean seawater; TSB0.1-medium: 1.5% BactoTM agar (BD, Germany), 0.01% BBLTM tryptic soy broth (BD, Germany), 3% NaCl in Aquademin; CFB-medium: 1.5% BactoTM agar (BD, Germany), 0.1% BBLTM tryptone (BD, Germany), 0.05% BactoTM yeast extract (BD, Germany), 0.05% CaCl2 × 2H2O (Merck, Germany), 0.05% MgCl2 × 7H2O (Merck, Germany) in Mediterranean seawater; Chitin-medium: 1.5% BactoTM agar (BD, Germany), 0.2% chitin (Fluka Biochemica, Switzerland) in Mediterranean seawater. Water samples of different depths were treated equally using dilutions of 100 and 10−1. Agar plates (two parallels each) were incubated at onboard room temperature for up to three months and checked frequently for growth.
Pre-incubation of sediment at in situ hydrostatic pressure
In order to selectively enrich deep-sea bacteria that are adapted to broad ranges of hydrostatic pressure and spontaneous nutrient input, a sediment sample from 2800 m depth (Herodotos Plain) was pre-incubated at 28 MPa (13.5°C) in supplemented sea water and N-acetyl-d-glucosamine prior to plating on identical media and incubation at identical conditions as described above. For this purpose 10 ml of sediment were transferred into a plastic bag with 9 ml of sterile-filtered seawater (taken from the multicorer) and 1 ml solution of N-acetyl-d-glucosamine (100 μM). Using compression-proof steel tubes connected to a pumping system, which generates hydrostatic pressure by pumping water into the tubes in situ hydrostatic pressure (28 MPa) was impressed. A manometer attached to the pumping system allowed the adjustment of the respective hydrostatic pressure within the tubes. After reaching the desired pressure, the pump was disconnected and the tubes were incubated for 6 days at in situ temperature (13.5°C). During the incubation, maintenance of the hydrostatic pressure within the tubes was checked frequently by attachment of the manometer. After this incubation, 10−1 and 10−2 dilutions were plated directly on the above-mentioned agar media and incubated under the standard conditions used throughout these experiments.
Using a binocular microscope, all colonies appearing morphologically different were transferred to fresh agar medium until pure cultures were obtained. Strains were checked for purity by microscopy and identified by 16S rRNA gene sequencing. For long term storage, all strains were kept at −80°C using the Cryobank system (Mast Diagnostica GmbH, Germany).
Testing the growth properties of selected strains
Selected strains of all operational taxonomic units (strains are marked by an asterisk in Table 1) were grown (i) in nutrient-rich Marine Broth medium at in situ temperature (ii) in oligotrophic MW-medium (containing solely autoclaved oligotrophic Mediterranean seawater) using room temperature and (iii) in low-nutrient medium MWY (containing autoclaved oligotrophic Mediterranean Sea water with 0.01% yeast extract) at in situ temperature of 13.5°C.
DNA extraction, PCR and sequencing
DNA extraction, PCR and 16S rRNA gene sequencing was performed according to Gärtner et al. (2008). 16S rRNA gene sequences determined during this study were deposited in the EMBL Nucleotide Sequence Database and were assigned accession no. FM992709–FM992846 and FN179280.
Sequence analysis
Sequences were edited by ChromasPro v.1.33 and were compared to the NCBI database using BLAST [see http://blast.ncbi.nlm.nih.gov/Blast.cgi (Altschul et al. 1990)]. Subsequently, all sequences were aligned to the ARB database (see http://www.arb-home.de, (Ludwig 2004)) using the integrated aligner function. Type strains most closely related to the isolates according to BLAST were added to the ARB database when not already present. Alignments were refined manually and aligned sequences were added to the ARB tree using the quick-add-marked function (Parsimony). According to the results from Stackebrandt and Ebers 2006strains showing sequence similarities <98.7% were assigned to different operational taxonomic units (OTUs) using MOTHUR software (http://www.mothur.org/).
Phylogenetic trees
Phylogenetic trees were calculated applying maximum likelihood analysis using PHYML (Guindon and Gascuel 2003). Maximum Likelihood analysis was performed assuming the GTR model (Keane et al. 2006) with an optimised gamma distribution parameter alpha and 100 bootstrap replicates.
Results
The majority of the strains isolated during this study were affiliated to bacterial genera commonly found in the ocean. The 107 strains were grouped into 49 operational taxonomic units (OTUs showing ≥98.7% sequence similarity). A considerable percentage of 42% of the OTUs can be considered to represent new species (Table 1). The high proportion of presumably new bacteria is considered to underline the special features of the Mediterranean deep sea.
Phylogenetic affiliation of the strains
Strains isolated from the sediment
Using low-nutrient media, colony numbers of up to 2.1 × 102 CFU/cm3 sediment were determined. Just slightly higher cell numbers (2.4 × 102 CFU/cm3) sediment were obtained with sediment samples using the CFB medium which had the highest nutrient content. Altogether 65 strains were obtained from the Eastern Mediterranean sediment (37 strains from sediment of the Ierapetra basin and 28 strains from the Herodotos plain). The strains isolated from the IB and HP sediments revealed a similar distribution among phylogenetic groups (Figure S1). The majority of strains affiliated to the Firmicutes (34 of 65 strains, 52%) and Actinobacteria (21 of 65 strains, 32%) with only few representatives of the Alpha- and Gammaproteobacteria (Figs. 1, 2). Firmicutes and Actinobacteria were dominant among the isolated strains of the untreated sediment and represented highly diverse groups with 17 OTUs of Firmicutes and 10 OTUs of Actinobacteria (Table 1). Especially the genus Bacillus was isolated with a remarkable high number of OTUs (16 OTUs). Some of the Firmicutes and Actinobacteria were exclusively isolated using Mediterranean Sea Water medium (MW-medium) without additional nutrients (OTUs 22, 23, 24 and 30). Strains with close relationship to known species (≥98.7%) are summarised in Table 1. Fifteen strains represented thirteen putative new species. These strains affiliated to the genera Micromonospora (strains S32a and S29), Pseudonocardia (strains S82 and S78), Streptomyces (strains S06 and D92), Halobacillus (strain S40) and Bacillus (strains S54, D89, S33, D94, S10, S11, D87 and S44x).
a Maximum likelihood tree of the 16Sr RNA gene sequences of all Gram-negative strains obtained from sediment and water samples. Numbers on nodes represent bootstrap percentages > 50, calculated from 100 replicates. S Isolate from sediment’ PE isolate from pressure experiment, W isolate from water column. b Maximum likelihood tree of the 16Sr RNA gene sequences of Actinobacteria strains obtained from sediment and water samples. Numbers on nodes represent bootstrap percentages > 50, calculated from 100 replicates. S Isolate from sediment, PE isolate from pressure experiment, W isolate from the water column. c Maximum likelihood tree of the 16Sr RNA gene sequences of Firmicutes strains obtained from sediment and water samples. Numbers on nodes represent bootstrap percentages > 50, calculated from 100 replicates. S Isolate from sediment, PE isolate from pressure experiment, W isolate from the water column
Strains isolated from the deep-sea water
To demonstrate differences between strains inhabiting the sediment and the deep water, isolates from deep water samples of both stations (cell counts were <1.7 × 101 CFU/ml deep-sea water, 19 strains obtained in total) were obtained and compared to those isolated from the sediment. According to their phylogenetic position, strains isolated from the deep water differed substantially from those isolated from the sediments. The 19 isolates grouped in 12 different OTUs: 3 (5 strains) Gammaproteobacteria, 2 (3 strains) Bacteroidetes, 4 (8 strains) Alphaproteobacteria, 3 (3 strains) Firmicutes (Fig. 1, 2; Table 1). Members of the Bacteroidetes were exclusively obtained from the deep water and all of them showed sequence similarities below 98.7% to known species. Other isolates from the deep sea water possibly can be assigned to new species assigned to the genera of Pseudomonas (strains W01 and W29), Citreicella (strain N108), Paracoccus (strain W14), Leeuwenhoekiella (strain W32) and Pontibacter (strain W27).
Strains isolated after hydrostatic pressure incubation
Pre-incubation was performed to selectively enrich bacteria supposed to be well adapted to the extreme conditions of the deep Mediterranean (high deep-sea temperatures, elevated hydrostatic pressure, oligotrophy). Noteworthy, CFU were increased at least tenfold compared to plating of sediment samples without pre-incubation. The 23 strains obtained after hydrostatic pressure incubation grouped into 15 different OTUs (10 Gammaproteobacteria, 3 Firmicutes, 1 Alphaproteobacteria and 1 Actinobacteria). Interestingly, the hydrostatic pressure pre-incubation yielded a considerable increase in the number of Gammaproteobacteria (78% of all isolates after hydrostatic pressure incubation of the sediment from Herodotos plain compared to 14% from this untreated sediment (Figs. 2, S1; Table 1). In contrast, the proportion of Firmicutes strains was drastically reduced (13% after hydrostatic pressure incubation compared to 50% from untreated sediment, Table 1, Figs. 2, S1). Noteworthy, 8 OTUs were exclusively obtained after this treatment and could not be isolated from the untreated sediment (Fig. S2). Most of these (7) are likely to represent new species and were assigned to the genera Alteromonas (strains D42, D39, D33, D45, D47 and D56) and Bacillus (strain D04).
Growth under selected in situ conditions
The strains isolated during this study originate from media with different nutrient concentrations. All tested strains were able to grow in oligotrophic Mediterranean Sea water medium and also on Marine Broth medium with elevated nutrient concentrations. All strains could grow at room temperature as well as at the lower in situ temperature of 13.5°C. Apparently growth at the lower temperature was dependent on the medium and nutrient concentration. Cultivation experiments using MWY, which contained 0.01% yeast extract, revealed that the great majority of the selected strains (93%) grew well under experimental low-nutrient conditions and in situ temperature of 13.5°C. Strains that did not grow under these conditions were Bacillus sp. D29a, Corynebacterium sp. S35 and Staphylococcus sp. W15. These strains did grow, however, at 13.5°C when the nutrient concentration was increased. Thus, most of the isolates were able to grow under in situ conditions (temperature and nutrient conditions) prevailing in the Eastern Mediterranean deep sea. This includes strains of Alteromonas spp. (D33, D42, D47 and D56), Pseudomonas sp. D43 and Sphingobium sp. D53, which were enriched and isolated exclusively after incubation at in situ hydrostatic pressure and in situ temperature with N-acetyl-d-glucosamine as energy and nutrient source. Most of the strains (14/23) obtained from the in situ hydrostatic pressure enrichment were isolated from low-nutrient seawater media (MW, MWY or Chitin-medium).
Discussion
Isolation from sediment samples
Gram-positive bacteria were clearly dominant among the strains isolated from the Eastern Mediterranean deep-sea sediment. This is in accordance to the frequent isolation of mainly Firmicutes and Actinobacteria from diverse marine habitats (Marteinsson et al. 1996; Colquhoun et al. 1998; Yanagibayashi et al. 1999; Siefert et al. 2000; Süß et al. 2004; Jensen et al. 2005; Koepke et al. 2005; Pathom-aree et al. 2006; Gontang et al. 2007; Stevens et al. 2007; Prieto-Davó et al. 2008). Members of the Firmicutes are moreover considered to be the most frequently isolated strains from subsurface sediments (D’ Hondt et al. 2004).
In contrast to the high isolation frequency of Gram-positive bacteria, they were of minor relevance in 16S rRNA gene libraries of Mediterranean deep-sea sediments (Heijs et al. 2008). Actinobacteria accounted for 4–28% of total sequences in clone libraries of Cretan margin sediments, while Firmicutes accounted for a maximum of 3% (Polymenakou et al. 2009). Both taxonomic groups might well be under-represented in 16S rRNA gene based molecular approaches, most likely because they often resist commonly applied DNA extraction techniques or may be missed due to primer biases (McVeigh et al. 1996; Mincer et al. 2005; Carrigg et al. 2007). Nevertheless, it appears that Actinobacteria and Firmicutes represent only a small fraction of the microbial community. On the other hand, quantitative occurrence does not give any information about the ecological relevance of these groups. It was shown e.g. that spores of Bacillus strains are capable for manganese oxidation in hydrothermal sediments of Guaymas basin (Rosson and Nealson 1982; Dick et al. 2006). The high abundance of Firmicutes isolated during this study and in particular the remarkable high diversity of different operational taxonomic units of the genus Bacillus demonstrates the importance of culture-dependent techniques as an additional tool to assess the microbial diversity.
Members of the genus Bacillus are ubiquitary, but very little is known about the metabolic activity of Bacillus sp. in marine sediments. Bacillus species frequently recovered from different marine environments are B. marinus, B. badius, B. subtilis, B. cereus, B. licheniformis, B. firmus, B. lentus and B. pumilis (Ivanova et al. 1999). B. marinus e.g. was isolated from tropical Atlantic, Antarctic and Arctic deep-sea sediments and turned out to be psychrophilic or psychrotolerant (Rueger et al. 2000). Closest phylogenetic relatives to isolates assigned to Bacillus obtained during this study originated from various environments and varied in their physiological properties. Though inference of physiological properties from those of closest phylogenetic relatives is possible to a limited extent, with some probability it may be used to delineate key properties of genetic relatives. With consideration of these limitations, it is interesting to see that Bacillus licheniformis as closest relative to isolate D94 is known for its specific strategy to cope with nutrient limitation (Hoi et al. 2006), which is important for adaptation to the extreme oligotrophy prevailing in the deep Eastern Mediterranean.
Interestingly, the Mariana Trench sediment was also dominated by Gram-positive bacteria and Bacillus sp. accounted for 25% thereof (Takami et al. 1997) (Figs. 1c,b). In addition, isolates from the present study assigned to Micromonospora and Streptomyces have close relatives to those recovered from the Mariana Trench (Pathom-aree et al. 2006) (Fig. 1b). This close affiliation of strains from different deep-sea sediments indicates their possibly wide distribution in deep sea sediments. More distantly related OTUs of our isolates might represent bacteria specifically adapted to the conditions of the Mediterranean Sea.
Isolation after enrichment under in situ hydrostatic pressure
Hydrostatic pressure incubation of the sediments clearly shifted the composition towards Gammaproteobacteria which is considered to reflect their ecological importance in the nutrient degradation of the deep sea. Apparently, they cope very well with changes of hydrostatic pressure as they have been decompressed during sampling, recompressed to in situ hydrostatic pressure and again decompressed for cultivation. It has already been demonstrated that decompression during retrieval of deep-sea samples affects microbial activity and microbial production rates (Seki and Robinson 1969; Jannasch et al. 1976; Bianchi et al. 1999). Especially cell membranes were shown to be damaged by changing hydrostatic pressure (Chastain and Yayanos 1991; Pagan and Mackey 2000; Park and Clark 2002). The gammaproteobacterial strains recovered by this approach might be assumed to be barotolerant. The majority of gammaproteobacterial OTUs (six out of ten) were exclusively isolated after this enrichment and are regarded as specifically selected by this treatment. This refers in particular to strains affiliating to the genus Alteromonas, which is commonly found in marine habitats including the deep sea (Yanagibayashi et al. 1999; Li et al. 1999a, b; Lopez-Lopez et al. 2005). Most of the barophilic bacterial strains identified to date (bacteria with optimal growth at hydrostatic pressure >0.1 MPa) affiliate to the Gammaproteobacteria (Yayanos et al. 1979; Kato et al. 1995; Delong et al. 1997; Kato et al. 1999; Lauro and Bartlett 2008). Lopez-Lopez et al. 2005 compared shallow water strains of Alteromonas macleodii to those obtained from the deep water by detailed sequence analyses of the 16S rRNA gene, the internal transcribed spacer and of house-keeping genes such as gyrB and rpoB. They demonstrated characteristic differences between deep-sea and shallow-water strains and ascribed them to different ecotypes (Lopez-Lopez et al. 2005; Lauro et al. 2007; Lauro and Bartlett 2008). This hints towards the existence of specifically adapted bacterial ecotypes in the deep sea (Lauro and Bartlett 2008).
In our experiments, the pressurised sediment was supplemented with N-acetyl-d-glucosamine (NAG), which is known to induce bacterial degradation of chitin, the (1-4)-linked β-homopolymer of NAG and a structural component of many organisms including fungi, protists, animals and plants. Chitin, as a valuable carbon and nitrogen source, is reported to be mainly degraded by chitinolytic bacteria, e.g. members of the genera Pseudomonas, Aeromonas, Xanthomonas, Serratia, Cytophaga, Arthrobacter as well as Bacillus and is supposed to be the most abundant biopolymer in the marine environment (Gooday 1990). It can be assumed that many bacteria adapted to the oligotrophic conditions of the Eastern Mediterranean deep sea are able to use a broad spectrum of available carbon sources, including chitin and its degradation products such as NAG. All strains from the pressurised enrichment were grown with NAG as sole carbon source and 11 additional strains of the untreated sediment were isolated from solid chitin media. These strains belong to the Gammaproteobacteria, the Firmicutes and the Actinobacteria. Apparently, the ability to degrade chitin and derivatives thereof is a beneficial and a widely distributed property of bacteria inhabiting the deep sea.
Growth at low nutrient concentrations
All strains tested during this study grew easily well with minor nutrient concentrations and as well at in situ temperature of 13.5°C, conditions prevailing in the deep Mediterranean Sea. The bacterial isolates apparently are well adapted to low nutrient concentrations of oligotrophic deep-sea sediments as well as to spontaneous and occasional nutrient supply due to seasonal changes or sinking carcasses. An example is represented by isolates assigned to Alteromonas (D33, D42, D47 and D56). As demonstrated by their growth on low nutrient media, they coped well with minute nutrient concentrations but also successfully competed with others at in situ hydrostatic pressure in the presence of N-acetyl-d-glucosamine. Members of the Gammaproteobacteria are frequently appointed to be r-strategists that can stand long periods of starvation but they outcompete others when nutrients become available (Fuchs et al. 2000; Pinhassi and Berman 2003). This is in accordance with D’ Hondt et al. (2004) who showed that Gammaproteobacteria are common in sediments of the ocean margin where concentrations of organic matter and net metabolic rates are high. As it was already shown for the Cretan Sea, bacterial abundance and biomass are sensitive to sudden changes in nutrient availability (Danovaro et al. 2000). Our data indicate that Gammaproteobacteria might considerably contribute to detectable changes in the composition of the bacterial communities in the Eastern Mediterranean deep-sea sediments.
In contrast Actinobacteria and Firmicutes dominated the strains obtained from the untreated sediment and some of these were exclusively isolated using low nutrient media. The suitability of low-nutrient media for the isolation of Gram-positive bacteria was also approved by other culture-dependent studies (D’ Hondt et al. 2004; Jensen et al. 2005; Gontang et al. 2007). The lifestyle of these bacteria apparently is adapted to minute nutrient concentrations (k-strategists) and under such conditions gives them advantage over the fast growing Gammaproteobacteria. This is supported by a study of D’ Hondt et al. (2004) who consistently isolated Actinobacteria and Firmicutes from sediments of ocean margins and open ocean sites exhibiting low concentrations of organic matter and net metabolic rates. As biopolymer degradation was shown to be of special importance in the Mediterranean deep Sea (Martin-Cuadrado et al. 2009), Actinobacteria might be considered as important players in the degradation of complex biopolymers of the studied environment.
References
Altschul S, Gish W, Miller W, Myers E, Lipman D (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bianchi A, Garcin J, Tholosan O (1999) A high-pressure serial sampler to measure microbial activity in the deep sea. Deep-Sea Res Part I 46:2129–2142
Carlucci AF, Shimp SL, Craven DB (1986) Growth characteristics of low-nutrient bacteria from the north-east and central Pacific Ocean. FEMS Microbiol Lett 38:1–10
Carrigg C, Rice O, Kavanagh S, Collins G, O′Flaherty V (2007) DNA extraction method affects microbial community profiles from soils and sediment. Appl Microbiol Biotechnol 77:955–964
Chastain RA, Yayanos AA (1991) Ultrastructural changes in an obligately barophilic marine bacterium after decompression. Appl Environ Microbiol 57:1489–1497
Colquhoun JA, Heald SC, Li L, Tamaoka J, Kato C, Horikoshi K, Bull AT (1998) Taxonomy and biotransformation activities of some deep-sea actinomycetes. Extremophiles 2:269–277
D’ Hondt S, Jorgensen BB, Miller DJ, Batzke A, Blake R, Cragg BA, Cypionka H, Dickens GR, Ferdelman T, Hinrichs KU (2004) Distributions of microbial activities in deep subseafloor sediments. Science 306:2216–2221
Danovaro R, Marrale D, Dell’ Anno A (2000) Bacterial response to seasonal changes in labile organic matter composition on the continental shelf and bathyal sediments of the Cretan Sea. Prog Oceanogr 46:345–366
Danovaro R, Corinaldesi C, Luna GM, Magagnini M, Manini E, Pusceddu A (2009) Prokaryote diversity and viral production in deep-sea sediments and seamounts. Deep Sea Res Part II 56:738–747
Das S, Lyla PS, Khan SA (2006) Marine microbial diversity and ecology: importance and future perspectives. Cur Sci 90:1325–1335
Delong EF, Franks DG, Yayanos AA (1997) Evolutionary relationships of cultivated psychrophilic and barophilic deep-sea bacteria. Appl Environ Microbiol 63:2105–2108
Dick GJ, Lee YE, Tebo BM (2006) Manganese (II)-oxidizing Bacillus spores in Guaymas basin hydrothermal sediments and plumes. Appl Environ Microbiol 72:3184
Emig C, Geistdoerfer P (2004) The Mediterranean deep-sea fauna: historical evolution, bathymetric variations and geographical changes. Carnets de Geologie/Netbooks on Geology, Article 2004/01 (CG2004_A01_CCE-PG)
Fuchs BM, Zubkov MV, Sahm K, Burkill PH, Amann R (2000) Changes in community composition during dilution cultures of marine bacterioplankton as assessed by flow cytometric and molecular biological techniques. Environ Microbiol 2:191–201
Gärtner A, Wiese J, Imhoff JF (2008) Amphritea atlantica gen. nov., sp. nov., a gammaproteobacterium from the Logatchev hydrothermal vent field. Int J Syst Evol Microbiol 58:34–39
Gontang EA, Fenical W, Jensen PR (2007) Phylogenetic diversity of Gram-positive bacteria cultured from marine sediments. Appl Environ Microbiol 73:3272–3282
Gooday GW (1990) Physiology of microbial degradation of chitin and chitosan. Biodegradation 1:177–190
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Heijs SK, Laverman AM, Forney LJ, Hardoim PR, van Elsas JD (2008) Comparison of deep-sea sediment microbial communities in the Eastern Mediterranean. FEMS Microbiol Ecol 64:362–377
Hoi LT, Voigt B, Jürgen B, Ehrenreich A, Gottschalk G, Evers S, Feesche J, Maurer KH, Hecker M, Schweder T (2006) The phosphate starvation response of Bacillus licheniformis. Proteomics 6:3582–3601
Ivanova EP, Vysotskii MV, Svetashev VI, Nedashkovskaya OI, Gorshkova NM, Mikhailov VV, Yumoto N, Shigeri Y, Taguchi T, Yoshikawa S (1999) Characterization of Bacillus strains of marine origin. Int Microbiol 2:267–271
Jannasch HJ, Wirsen CO, Taylor CD (1976) Undecompressed microbial populations from the deep sea. Appl Environ Microbiol 32:360–367
Jensen PR, Gontang E, Mafnas C, Mincer TJ, Fenical W (2005) Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ Microbiol 7:1039–1048
Kato C, Sato T, Horikoshi K (1995) Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers Conserv 4:1–9
Kato C, Inoue A, Horikoshi K (1996) Isolating and characterizing deep-sea marine microorganisms. Trends Biotechnol 14:6–12
Kato C, Li L, Nogi Y, Nakamura Y, Tamaoka J, Horikoshi K (1998) Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl Environ Microbiol 64:1510–1513
Kato C, Yanagibayashi M, Nogi Y, Horikoshi K (1999) Analyses of microbial diversity in the sediment obtained from Japan Trench at a depth of 7326 m and high pressure cultivation. JAMAREC J Deep Sea Res Biol 15:47–52
Keane TM, Creevey CJ, Pentony MM, Naughton TJ, Mclnerney JO (2006) Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6:29. doi:10.1186/1471-2148-6-29
Koch AL (2001) Oligotrophs versus copiotrophs. Bioassays 23:657–661
Koepke B, Wilms R, Engelen B, Cypionka H, Sass H (2005) Microbial diversity in coastal subsurface sediments: a cultivation approach using various electron acceptors and substrate gradients. Appl Environ Microbiol 71:7819–7830
Kontoyiannis H, Theocharis A, Balopoulos E, Kioroglou S, Papadopoulos V, Collins M, Velegrakis AF, Iona A (1999) Water fluxes through the cretan arc straits, Eastern Mediterranean Sea: March 1994 to June 1995. Prog Oceanogr 44:511–529
Lampadariou N, Tselepides A, Hatziyanni E (2009) Deep-sea meiofaunal and foraminiferal communities along a gradient of primary productivity in the eastern Mediterranean Sea. Scientia Marina 73:337–345
Lampitt RS, Antia AN (1997) Particle flux in deep seas: regional characteristics and temporal variability. Deep Sea Res Part I 44:1377–1403
Lauro FM, Bartlett DH (2008) Prokaryotic lifestyles in deep sea habitats. Extremophiles 12:15–25
Lauro FM, Chastain RA, Blankenship LE, Yayanos AA, Bartlett DH (2007) The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl Environ Microbiol 73:838–845
Li L, Guenzennec J, Nichols P, Henry P, Yanagibayashi M, Kato C (1999a) Microbial diversity in Nankai trough sediments at a depth of 3843 m. J Oceanogr 55:635–642
Li L, Kato C, Horikoshi K (1999b) Bacterial diversity in deep-sea sediments from different depths. Biodivers Conserv 8:659–677
Lopez-Lopez A, Bartual SG, Stal L, Onyshchenko O, Rodriguez-Valera F (2005) Genetic analysis of housekeeping genes reveals a deep-sea ecotype of Alteromonas macleodii in the Mediterranean Sea. Environ Microbiol 7:649–659
Ludwig W (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Luna GM, Dell’ Anno A, Giuliano L, Danovaro R (2004) Bacterial diversity in deep Mediterranean sediments: relationship with the active bacterial fraction and substrate availability. Environ Microbiol 6:745–753
Marteinsson VT, Birrien JL, Jeanthon C, Prieur D (1996) Numerical taxonomic study of thermophilic Bacillus isolated from three geographically separated deep-sea hydrothermal vents. FEMS Microbiol Ecol 21:255–266
Martin-Cuadrado AB, Ghai R, Gonzaga A, Rodriguez-Valera F (2009) CO Dehydrogenase genes found in metagenomic fosmid clones from the Deep Mediterranean Sea. Appl Environ Microbiol 75:7436–7444
McVeigh HP, Munro J, Embley TM (1996) Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J Ind Microb Biotech 17:197–204
Mincer TJ, Fenical W, Jensen PR (2005) Culture-dependent and culture-independent diversity within the obligate marine actinomycete genus Salinispora. Appl Environ Microbiol 71:7019–7028
Pagan R, Mackey B (2000) Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential-and stationary-phase cells and variation among strains. Appl Environ Microbiol 66:2829–2834
Park CB, Clark DS (2002) Rupture of the cell envelope by decompression of the deep-sea methanogen Methanococcus jannaschii. Appl Environ Microbiol 68:1458–1463
Pathom-aree W, Stach J, Ward A, Horikoshi K, Bull A, Goodfellow M (2006) Diversity of actinomycetes isolated from Challenger deep sediment (10.898 m) from the Mariana trench. Extremophiles 10:181–189
Pinhassi J, Berman T (2003) Differential growth response of colony-forming alpha- and gamma-proteobacteria in dilution culture and nutrient addition experiments from Lake Kinneret (Israel), the Eastern Mediterranean Sea, and the Gulf of Eilat. Appl Environ Microbiol 69:199–211
Polymenakou PN, Lampadariou N, Tselepides A (2008) Exo-enzymatic activities and organic matter properties in deep-sea canyon and slope systems off the southern Cretan margin. Deep-Sea Res Part 1 55:1318–1329
Polymenakou PN, Lampadariou N, Mandalakis M, Tselepides A (2009) Phylogenetic diversity of sediment bacteria from the southern Cretan margin, Eastern Mediterranean Sea. Syst Appl Microbiol 32:17–26
Prieto-Davó A, Fenical W, Jensen PR (2008) Comparative actinomycete diversity in marine sediments. Aquat Microb Ecol 52:1–11
Rappé MS, Connon SA, Vergin KL, Giovannoni SJ (1999) Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418:630–633
Rosson RA, Nealson KH (1982) Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol 151:1027–1034
Rueger HJ, Tan TL (1992) Community structures of cold and low-nutrient adapted heterotrophic sediment bacteria from the deep eastern tropical Atlantic. Mar Ecol Prog Ser 84:83–93
Rueger HJ, Fritze D, Sproer C (2000) New psychrophilic and psychrotolerant Bacillus marinus strains from tropical and polar deep-sea sediments and emended description of the species. Int J Syst Evol Microbiol 50:1305–1313
Seki H, Robinson DG (1969) Effect of decompression on activity of microorganisms in seawater. Internationale Revue der gesamten Hydrobiologie und Hydrographie 54:201–205
Siefert JL, Larios-Sanz M, Nakamura LK, Slepecky RA, Paul JH, Moore ERB, Fox GE, Jurtshuk J (2000) Phylogeny of marine Bacillus isolates from the Gulf of Mexico. Curr Microbiol 41:84–88
Stackebrandt E, Ebers J (2006) Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 33:152–155
Stevens H, Brinkhoff T, Rink B, Vollmers J, Simon M (2007) Diversity and abundance of Gram-positive bacteria in a tidal flat ecosystem. Environ Microbiol 9:1810–1822
Süß J, Engelen B, Cypionka H, Sass H (2004) Quantitative analysis of bacterial communities from Mediterranean sapropels based on cultivation-dependent methods. FEMS Microbiol Ecol 51:109–121
Takami H, Inoue A, Fuji F, Horikoshi K (1997) Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett 152:279–285
Yanagibayashi M, Nogi Y, Li L, Kato C (1999) Changes in the microbial community in Japan Trench sediment from a depth of 6292 m during cultivation without decompression. FEMS Microbiol Lett 170:271–279
Yayanos AA, Dietz AS, Van Boxtel R (1979) Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science 205:808–810
Acknowledgments
We thank the captain and crew of RV Meteor as well as the scientific party of M71/2 for any support during the cruise. We are grateful to Dr. Jörg Süling for onboard support and fruitful discussion. We thank the colleagues from the IKMB (Institute of Clinical Molecular Biology) in Kiel (Germany) for sequencing. We are also thankful for the valuable suggestions of the unknown reviewers. This work was supported by a grant of the Deutsche Forschungsgemeinschaft and the Kieler Wirkstoff-Zentrum am IFM-GEOMAR granted by the Ministry of Science, Economic Affaires and Transport of the State of Schleswig–Holstein (Germany) in the frame of “Future Program for Economy” which is co-financed by EFRE.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10482_2011_9599_MOESM1_ESM.png
Supplementary material 1 Total number of strains obtained from the two sampling sites (Ierapetra Basin (IB), 4400m and Herodotos Plain (HP), 2800 m) and their phylogenetic affiliation compared to the pressurised sediment enrichment (PNG 6 kb)
10482_2011_9599_MOESM2_ESM.png
Supplementary material 2 Number of unique and shared OTUs from the water (n = 12), the sediment (n = 33) and the pressurised sediment enrichment (n = 15) (PNG 26 kb)
Rights and permissions
About this article
Cite this article
Gärtner, A., Blümel, M., Wiese, J. et al. Isolation and characterisation of bacteria from the Eastern Mediterranean deep sea. Antonie van Leeuwenhoek 100, 421–435 (2011). https://doi.org/10.1007/s10482-011-9599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9599-5