Abstract
Amongst HIV+ individuals, sleep complaints have been recognized as common and debilitating; but have rarely been formally assessed or compared to controls using validated sleep tools. In this study we conducted structured interview for sleep disorders, polysomnography, 2-week home (ambulatory) monitoring and validated sleep/functional questionnaires. 56 % (14/25) of HIV+ participants and 0 % (0/19) of controls fulfilled the diagnostic criteria for insomnia. Insomnia severity scores were correlated with fatigue and anxiety symptoms. Sleep latency on 2-week actigraphy was significantly longer (P = 0.027) for HIV+ participants and associated with lower MOS-HIV scores. Sleep quality was significantly reduced in HIV+ participants based on validated questionnaires of overall sleep quality (P = 0.0017) and insomnia related symptoms (P < 0.001) even after adjusting for education and affective symptoms. HIV+ individuals are suffering with under-diagnosed sleep disorders that are negatively impacting quality of life and functional capabilities. Further studies aimed at improving recognition of sleep disorders and implementation of efficacious medical and behavioral treatment could improve functioning and disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in the medical care and available therapeutics (e.g., combination antiretroviral therapy [cART]) for HIV+ individuals have significantly reduced morbidity and mortality [1, 2]. However, HIV+ individuals now face new challenges of living decades with chronic and indolent HIV-related conditions [3]. Existing studies suggest that between 30 and 73 % of the seropositive HIV population complain of poor sleep quality with associated fatigue [1]. The large range in estimated prevalence (mainly self-reported) is likely due to the variety of tools utilized to assess sleep quality, including both validated (e.g., Pittsburgh sleep quality index) [4] and unvalidated sleep questionnaires, or “sleep-related” subitems from a variety of mood questionnaires [5, 6]. Furthermore, because these sleep quality measures do not take into account common nosologic criteria, such as the International Classification of Sleep Disorders-2 [7] or the current DSM-IV [8], the true rates of sleep disorders in HIV remain largely unknown.
Very few studies evaluating sleep in HIV+ participants have utilized polysomnography (PSG), which is essential to properly diagnose the full range of sleep disorders. The few that have conducted PSG in HIV+ individuals have found anomalies in both sleep continuity and architecture [e.g., reduced sleep efficiency and rapid eye movement (REM) sleep percentage, delayed sleep onset, and increased slow wave sleep], which corroborate the self-report studies [6, 9]. Two more recent investigations, however, have failed to replicate these findings [9, 10]. Additional studies have reported a strong relationship between sleep disordered breathing, sleep fragmentation and subjective complaints of poor sleep quality and daytime sleepiness in HIV. However, one of these studies was retrospective, reporting the symptoms of HIV+ patients who were referred for a polysomnogram (PSG) while another was prospective that evaluated daytime symptoms based solely on the Epworth Sleepiness Scale [5, 11].
Thus far, investigations of sleep disorders in HIV+ individuals have focused primarily on sleep apnea or insomnia. To our knowledge, the other common sleep disorders [e.g., restless legs syndrome (RLS), circadian rhythm sleep disturbance (CRSD), poor sleep hygiene] have never been evaluated in an HIV+ cohort utilizing validated and multi-method assessment tools. A systematic evaluation of all sleep conditions in those with HIV is particularly important considering the mounting evidence linking sleep disorders to conditions associated with increased morbidity (e.g., depression, anxiety, psychomotor slowing, metabolic syndrome) and mortality (e.g., malignancy, heart failure, stroke) in other clinical populations [12–14].
Formal evaluation of the sleep characteristics and patterns is the first step towards evaluating the impact that disturbed sleep may have on daytime functioning, disease progression, and overall clinical management for seropositive HIV individuals. Therefore, we set out to characterize the sleep continuity and architecture, and the rate of sleep disorders in HIV+ individuals stable on cART, as compared with seronegative controls, using a multi-method assessment including: PSG, a structured clinical sleep interview, and actigraphy. As a secondary exploratory analysis, we sought to determine possible associations between sleep and daytime function. We focused this initial study on HIV+ Black males, comparing them to Black male seronegative controls that were matched on age and body mass index. We wanted to investigate this relationship in HIV+ male subjects compared to controls since Black males continue to be disproportionately affected by HIV. Moreover sleep patterns, behaviors and even attitudes regarding sleep can be influenced by a number of sociocultural and demographic variables (e.g., age, gender, ethnicity, education) which further underscores the importance of evaluating this relationship in comparison to a control group [15].
Methods
This was a prospective study specifically designed to study the associations between HIV status, sleep disorders and day-time functions. Study data were collected between August 2008 and April 2011 for the HIV+ group and between May 2010 and April 2011 for the control group. All of the seropositive HIV participants were recruited at Johns Hopkins Medical Institutions (JHMI) from an established HIV-research cohort at JHU [the Northeastern AIDS Dementia (NEAD)], Central Nervous System HIV Antiretroviral Therapy Effects Research (CHARTER) and other available HIV+ research cohorts. Control participants were recruited from other JHMI research cohorts, advertisements, and from personal referral of established participants. This study was approved by the JHMI IRB and all participants provided informed consent prior to enrollment. All control participants underwent an HIV test to confirm seronegative status. HIV+ participants were required to have a relatively low HIV viral load (<3,000 copies/ml). All HIV participants were on various cART regimens (Table 1). However, participants whose cART regimen included efavirenz were excluded from the study due to its potential sleep-altering effects [16]. Participants were also excluded if they had recent changes in any of their medications, or had an unstable affective disorder as determined by the study team. Because of ethical concerns, we did not withdraw participants from other potential medications that may affect sleep or pain management, but instead only included those who were stable on these medications for at least 60 days. To remain in the study, all participants had to refrain from using recreational drugs for the duration of the 2-week protocol. Urine toxicology screens were conducted at the initial screening, prior to admission for the PSG, at the midpoint visit for the two-week ambulatory monitoring, and again at the exit interview. Participants who tested positive for recreational substances were withdrawn from the study. All of the participants engaged in the same study procedures with the exception of the MOS-HIV, which was completed only by the HIV participants (Table 2).
Clinical Sleep Disorder Diagnoses
Structured Interview for Sleep Disorders (SIS-D)
To diagnose sleep disorders, we administered the Structured Interview for Sleep Disorders (SIS-D). This interview generates diagnoses for the full range of sleep disorders. The SIS-D is the only published interview that demonstrates sound reliability and validity based on PSG and expert interviews [17]. Kappa statistics assessing inter-rater reliability for the sub-classifications of insomnia and hypersomnia for example, range between 0.84 and 0.86. A modified version of the SIS-D was used to reflect current DSM-IV Disorders such as sleep apnea and periodic limb movement disorders, that also require polysomnographic data for definitive diagnosis, were determined by integrating the PSG data with the clinical interview from the SIS-D data.
Polysomnogram (PSG)
The PSG is a standard measure of sleep quality and duration and is the gold standard diagnostic tool necessary to definitively diagnose the majority of intrinsic primary sleep disorders including: sleep apnea, periodic limb movement disorder, and REM sleep behavioral disorder [18]. Sleep on a single-night PSG was recorded using standardized procedures and was visually scored according to the 2007 American Academy of Sleep Medicine (AASM) Manual for Scoring Sleep [19]. A certified sleep specialist reviewed and finalized all of the studies, which were conducted and scored by a registered technician. Sleep disordered breathing events were identified as either apneas (as defined by cessation of air flow for >10 s evident on both nasal cannula and thermistor) or hypopneas (as defined by reduction of air flow by at least 30 % of the baseline on either the nasal cannula or thermistor for >10 s associated with >4 % desaturation from the baseline) based on Centers for Medicare and Medicaid Services (CMS) guidelines [20] and the recommended criteria of the AASM [19].
Actigraphy
To objectively assess sleep continuity in the home environment, all participants wore a MiniMitter Actiwatch 2 (Respironics, Bend, Oregon) device continuously on the non-dominant wrist for two consecutive weeks. The device is a lightweight wristwatch, containing an omni-directional accelerometer which integrates and records the occurrence (frequency) and degree of motion. Data are stored as activity counts within a series of 1-min epochs. The algorithm utilized by the software to calculate standard sleep continuity parameters has demonstrated reliability and validity and has been found to discriminate sleep from wake states in healthy participants and participants with insomnia [21].
Participants were asked to push an event marker on the device to indicate lights out and times out-of-bed during their major sleep period. These event markers, along with movement data derived from actigraphy were utilized to establish the major rest interval during which the scoring algorithm was applied to score sleep onset latency (SL), wake after sleep onset time (WASO), sleep efficiency (SE), and total sleep time (TST).
Procedures to Establish Clinical Diagnoses
All study participants completed detailed medical and sleep assessments [22] along with validated questionnaires and tasks related to measures of daytime functioning (See Tables 2, 3). Inquiries regarding current and past history of any potential sleep-related complaints and any co-morbid medical and psychiatric conditions that could affect sleep quality, including clinical symptoms of sensory neuropathy (due to high prevalence amongst individuals with HIV) were made in the first visit based on the medical and sleep history interview along with the physical examination.
Statistical Approach
We first compared participants with and without HIV with respect to demographic and clinical characteristics using t tests or Wilcoxon rank-sum tests for continuous variables and Fisher’s exact test for categorical variables. Next, we performed analyses of covariance (ANCOVAs) for continuous variables and logistic regressions for categorical variables to compare the two groups’ sleep-related characteristics while controlling for education and trait anxiety. In multivariable-adjusted analyses, we controlled for education and trait anxiety based on our knowledge of the literature and on the fact that both of these were associated with HIV status and at least one outcome at the P < 0.05 level. We chose not to adjust for state anxiety due to its high degree of correlation with trait anxiety.
Although several analyses were conducted, the analyses did not control for multiple comparisons because such an approach increases Type II error (i.e., decreases the likelihood of observing a significant relationship when one actually exists). The relationship between sleep and overall function in HIV+ patients represents a relatively unexplored area of research. Our objective was to take an exploratory analytical approach to investigating this potential relationship in the hopes of paving the way for future studies.
Results
Participant Characteristics
Participants were all Black men; 25 were HIV+ and 19 were seronegative controls (Table 3). Within the HIV+ group, 2 participants tested positive for recreational drugs during the second week study visit, and were subsequently dropped from the study. Participants in the control group were more likely to have obtained a degree beyond high school (P = 0.035). The two groups did not differ significantly in terms of age, body mass index or Beck Depression Inventory (BDI) [23] scores. The HIV+ participants scored significantly higher on both the state (P = 0.007) and trait (P < 0.001) portions of the State Trait Anxiety Inventory (STAI) [24]. The groups also differed significantly on signs and symptoms of neuropathy with the HIV+ group reporting more neuropathy (P = 0.017). All of the individuals in the control group with neuropathy denied associated pain; however, 5 (31.3 %) of the 16 individuals with neuropathy in the HIV+ group reported significant associated neuropathic pain. The groups did not differ significantly in terms of medication use (FDA-approved and non-approved sleep aids, sedating prescription medication, alerting prescription medication, and medications with non-specific effects on sleep). The central nervous system (CNS) penetration quotients of the cART medications used by the HIV participants [25] were also calculated (see Table 1) with a mean penetrance of 7.8 ± 2.2.
HIV Status and Self-Reported Sleep and Daytime Functioning
In unadjusted analyses, the HIV+ group demonstrated significantly higher mean Pittsburgh Sleep Quality Index (PSQI) scores compared to controls (P = 0.001) (Table 4). This difference remained significant after adjusting for education and STAI trait scores (P = 0.017). HIV+ participants also reported significantly higher scores on the insomnia severity index (ISI) compared to controls (P < 0.001). This difference also remained significant after adjusting for trait scores (P < 0.001). On the fatigue severity scale (FSS), the groups differed in unadjusted (P = 0.007) but not education- and anxiety-adjusted analyses.
HIV Status and Actigraphic Variables
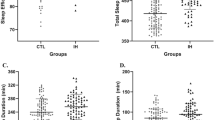
The HIV+ group demonstrated significantly longer mean sleep latency times over the 2 week monitoring period, compared to controls (P = 0.027), but this difference became non-significant after adjusting for education and trait anxiety (P = 0.312) (Table 5).
HIV Status and Polysomnographic Variables
Few significant differences were found between the HIV+ and control groups on sleep variables recorded by PSG (Table 6). Unadjusted analysis revealed significantly higher apnea rates in the HIV+ group compared to controls (P = 0.029) but was not significant after adjusted comparisons. The two groups did not differ significantly on PSG scores pertaining to Total Sleep Time (TST), Sleep latency (SL), Sleep Efficiency (SE), time awake after initial sleep onset (WASO), percent time spent in N2, N3, or REM sleep. There was a trend for the HIV+ group to experience shorter REM latency than the control group (P = 0.074). This difference became significant after adjustment for education and trait anxiety (P < 0.001).
HIV Status and Clinical Sleep Disorders
Fifty-six percent of the HIV+ participants (14/25) were diagnosed with clinical insomnia while none of the controls demonstrated symptoms consistent with this diagnosis (P < 0.001) (Table 7). Out of 25 HIV+ participants, 1 was found to have a parasomnia (i.e., night terrors), 2 were found to have circadian rhythm sleep disorder (CRSD), 2 were found to have Restless Legs Syndrome (RLS), and 4 were found to have poor sleep hygiene. In the control group, 1 participant was found to have a parasomnia.
Sleep Variables and Functional/Psychiatric Variables in Participants with HIV
Significant exploratory bivariate correlations (all P < 0.05) were found between ISI scores and scores on the FSS (Spearman’s rho = 0.51), STAI State (Spearman’s rho = 0.50) and STAI Trait (Spearman’s rho = 0.56) examinations. Unadjusted analysis of mean actigraphic sleep latency (SL) was negatively correlated (P < 0.05) with the MOS-HIV (Spearman’s rho = −0.51). None of the sleep variables measured by PSG or any of the other variables measured using self-report or actigraphy were significantly correlated with any of the other functional/psychiatric variables.
Discussion
Sleep plays a pivotal role in several neurobiological processes, including immune system modulation, cognitive function, pain sensitivity, and mood stability [26–28]. Sleep disruption might therefore exacerbate common HIV-related complications such as cognitive impairment, painful peripheral neuropathy and chronic fatigue. To our knowledge, this is the first study to comprehensively describe sleep quality, sleep architecture and the presence of the full range of clinical sleep disorders in Black HIV+ men compared to age-, gender-, and ethnic matched seronegative controls. Most of the previous investigations in the PRE and POST-cART era have objectively evaluated sleep disturbance in HIV+ individuals strictly by PSG assessments in order to identify the presence of sleep-disordered breathing. Previous reports have suggested a link between “cART associated lipodystrophy” and the increased prevalence of sleep apnea amongst HIV+ individuals treated with cART [29]. Although the HIV+ participants demonstrated a significantly higher sleep apnea index compared to controls, the HIV+ subjects stable on cART in this study had an overall mean sleep disordered breathing index (apnea-hypopnea index) in the “mild range” which arguably is well within the range one would find in the otherwise “healthy” general population [30, 31].
Limitations to our study included a small sample size which limited our power. Conducting this multi-method design requires extraordinary financial and administrative resources; this may partially explain why similar studies have yet to be conducted on a larger scale. Nonetheless, this study with a relatively small sample size did demonstrate significant differences in sleep patterns and presence of clinical sleep disorders that warrant further investigation in a larger sample. In addition, our sample included only seropositive HIV Black males and seronegative Black male controls, thus generalizing from our results should be done with caution. However, socio-demographic variables including ethnicity, culture and gender, have been shown to strongly impact indices of sleep-quality such as sleep duration, sleep onset, sleep continuity, and even sleep disorder prevalence. For instance, several population-based studies have found higher rates of sleep-disordered breathing and sleep architecture differences in Black and minority participants compared to their white counterparts [15]. Moreover, ethnicity may even factor into one’s neurophysiologic response to the research setting and methods. For instance, one study reported a “location by ethnicity” interaction whereby Blacks had significantly more slow-wave sleep at home compared to the hospital setting while for white participants the reverse response was observed [32]. Even though Blacks disproportionately suffer with HIV (U. S. African Americans comprise 14 % of the population yet account for 44 % of all new HIV infections) [33, 34], very few studies have taken into account the potential influence of socio-cultural factors in the manifestation of sleep disorders, much less their impact on disease management and progression. This point is further underscored by the fact that both HIV+ participants and controls in our study demonstrated sleep durations and efficiencies far below recommended guidelines (based primarily on normative studies utilizing white cohorts). Thus, behavioral, environmental and socio-cultural variables may certainly influence the expression of these sleep complaints [7, 35].
Sleep disturbances and disorders may be among the most prominent, yet unrecognized challenges facing HIV+ individuals and their providers. Despite the presence of insomnia in 56 % of our HIV participants, none of them reported being on an FDA-approved sleep aid or having undergone behavioral treatment (the most accepted therapy) [36, 37] for insomnia (Table 3). This suggests that HIV health care providers may not be fully cognizant of the magnitude of this issue. Thus, investigation and recognition of the impact of sleep disturbance on HIV+ individuals could potentially have substantial functional consequences therefore setting the stage for new practice guidelines and treatment algorithms in the post-cART era.
References
Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69(3):376–80.
McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4(9):543–55.
Low Y, Preud’homme X, Goforth HW, Omonuwa T, Krystal AD. The association of fatigue with depression and insomnia in HIV-seropositive patients: a pilot study. Sleep. 2011;34(12):1723–6.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Lo Re V 3rd, Schutte-Rodin S, Kostman JR. Obstructive sleep apnoea among HIV patients. Int J STD AIDS. 2006;17(9):614–20.
Wiegand M, Moller AA, Schreiber W, Krieg JC, Holsboer F. Alterations of nocturnal sleep in patients with HIV infection. Acta Neurol Scand. 1991;83(2):141–2.
American Academy of Sleep Medicine. The international classification of sleep disorder. Diagnostic and coding manual. Westchester: American Academy of Sleep Medicine; 2005.
American PA. Diagnostic and statistical manual of mental disorders. Washington, DC: American PA; 1994.
Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosom Med. 2005;67(2):260–9.
Omonuwa TS, Goforth HW, Preud’homme X, Krystal AD. The pharmacologic management of insomnia in patients with HIV. J Clin Sleep Med. 2009;5(3):251–62.
Epstein LJ, Strollo PJ Jr, Donegan RB, Delmar J, Hendrix C, Westbrook PR. Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep. 1995;18(5):368–76.
Norra C, Kummer J, Boecker M, Skobel E, Schauerte P, Wirtz M, et al. Poor sleep quality is associated with depressive symptoms in patients with heart disease. Int J Behav Med. 2011;19(4):526–34.
Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, et al. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2011;153(1):107–12.
Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135(2):563–72.
Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–18.
Moyle G, Fletcher C, Brown H, Mandalia S, Gazzard B. Changes in sleep quality and brain wave patterns following initiation of an efavirenz-containing triple antiretroviral regimen. HIV Med. 2006;7(4):243–7.
Schramm E, Hohagen F, Grasshoff U, Riemann D, Hajak G, Weess HG, et al. Test-retest reliability and validity of the structured interview for sleep disorders according to DSM-III-R. Am J Psychiatry. 1993;150(6):867–72.
Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521.
Iber C, Ancoli-Israel S, Cheeson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007.
CMS manual system: sleep testing for obstructive sleep apnea (OSA) (Internet). Available from http://www.cms.gov/transmittals/downloads/R103NCD.pdf.
Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–9.
Gamaldo CE, McArthur JC. The evaluation and diagnosis of “insomnia” in relation to sleep disturbance prevalence and impact in early-treated HIV-infected persons. Clin Infect Dis. 2012;55(10):1429–30.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Spielberger CD, Gorsuch RL, Lushene RE. Manual for state-trait anxiety inventory. Palo Alto: Consulting Psychologist Press; 1970.
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70.
Salas RE, Gamaldo CE. Adverse effects of sleep deprivation in the ICU. Crit Care Clin. 2008;24(3):461–76, v–vi.
Wright KP Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18(4):508–21.
Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28(8):981–9.
Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27(3):251–9.
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(1073-449; 1073-449; 9):1217–39.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43.
Stepnowsky CJ Jr, Moore PJ, Dimsdale JE. Effect of ethnicity on sleep: complexities for epidemiologic research. Sleep. 2003;26(3):329–32.
HIV among African Americans (Internet). Available from http://www.cdc.gov/hiv/topics/aa/.
Enhanced comprehensive HIV prevention planning for the Baltimore Towson metropolitan statistical area workbook #1 (Internet). Available from http://www.cdc.gov/hiv/strategy/echpp/pdf/baltimoreEchpp_plan_1.pdf.
The 2010 sleep in America poll (internet) (2010). Updated 03/08.
Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–64.
Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(0002-953; 0002-953; 1):5–11.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5.
Smith MT, Wegener ST. Measures of sleep: the insomnia severity index, medical outcomes study (MOS) sleep scale, Pittsburgh sleep diary (PSD), and Pittsburgh sleep quality index (PSQI). Arthritis Rheum. 2003;49(S5):S184.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–3.
Grossman HA, Sullivan PS, Wu AW. Quality of life and HIV: current assessment tools and future directions for clinical practice. AIDS Read. 2003;13(12):583–90, 595–7.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110.
Acknowledgments
This publication was made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. This study was also supported by award 5P30MH075673-S02 from the National Institute of Mental Health (NIMH) (PI JCM), a Developmental Grant from JHU NIMH Center for Novel Therapeutics of HIV-associated Cognitive Disorders (to author CG), and a Developmental Grant from JHU Center for Mind–Body Research (CMBR) (PI Jennifer Haythornthwaite PI to author CG). The recruitment of participants was assisted by an existing cohort, funded by NIMH, the Central Nervous System HIV Antiretroviral Therapy Effects Research (CHARTER)]. The authors thank Ms. Bernadette Clark and Ms. Rebecca Clark for their editing assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gamaldo, C.E., Spira, A.P., Hock, R.S. et al. Sleep, Function and HIV: A Multi-Method Assessment. AIDS Behav 17, 2808–2815 (2013). https://doi.org/10.1007/s10461-012-0401-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-012-0401-0