Abstract
The conversion of secondary forests to larch plantations has resulted in soil degradation in Northeast China. Previous studies have proven that introducing native broadleaved tree species into larch plantations could improve soil quality, but it could not provide economic benefits in the short term. To gain short-term economic benefits, Aralia elata (Miq.) Seem., a native broadleaved shrub or small tree with high economic value, has been introduced into larch plantations and thus formed a larch-A. elata agroforestry system. However, the effect of this practice on degraded soil of larch plantations remains unclear. Here, we compared the soil chemical and microbial properties at four soil depths (humus, 0–10 cm, 10–20 cm, 20–30 cm) in paired stands of larch plantations and adjacent larch-A. elata agroforestry systems with different years since inter-planting (1, 3, 5, and > 10 years). The results showed that compared with larch plantations, most chemical and microbial properties significantly changed with inter-planting years in larch-A. elata agroforestry systems, especially at the humus layer and 0–10 cm soil layer. Particularly in the larch-A. elata agroforestry system with inter-planting for over 5 years, the soil chemical (mineral nitrogen, available phosphorus, and pH) and microbial (microbial biomass of C, N, and P, β-glucosidase, β-cellobiohydrolase, N-acetyl-β-glucosaminidase, acid phosphatase, phenol oxidase, and peroxidase) properties significantly increased by 5–97% in the humus layer and by 3–110% in 0–10 cm soil layer. Most of the chemical and microbial properties were mainly affected by the number of years since inter-planting, basal area, litterfall, and C/N ratio of the forest floor. Conclusively, inter-planting with A. elata could improve the soil chemical and microbial properties of larch plantations, especially after > 5 years since inter-planting, while providing economic benefits simultaneously in the short term.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Northeast China, extensive areas of secondary forests have been replaced by larch (Larix olgensis) plantations (LP) because of the growing demand for timber and other forest products since the 1950s (Mason and Zhu 2014). The conversion of secondary forests to LP monoculture has resulted in some adverse problems, including soil acidification and decline of soil nutrients such as soil carbon (C) and nitrogen (N), microbial biomass and enzyme activity (Yang et al. 2012, 2013). A feasible method to solve these problems is to convert LP monoculture plantations into mixed forests by introducing local broadleaved tree species (Zhu et al. 2010) Diao et al. (2020) have demonstrated that larch-broadleaved mixed forests, formed by introducing native broadleaved tree species (i.e., Fraxinus mandschurica Rupr.) to larch plantations, could improve the soil quality of LP. However, these practices cannot provide economic benefits in the short term due to the shift of forest management strategies, i.e., from pursuing timber production to providing ecological services (Li and Zhou 2000), which led to income reduction of local foresters.
Tree-based agroforestry, formed by inter-planting shrub or tree species of high and short-term economic value in existent monoculture plantations, can improve not only the local foresters’ income but also the soil quality of monoculture plantation, due to the changes in litter input and root activities (Chen et al. 2019). However, the effects of this practice on soil properties remain uncertain because both positive and negative effects have been reported. For example, Chen et al. (2019) found that inter-planting rubber (Hevea brasiliensis (Willd. ex A. Juss.) Muell. Arg.) with cacao (Theobroma cacao L.) significantly improved soil physical properties and soil nutrients (C, N, P, Ca, and Mg) compared with pure rubber plantations. In contrast, Liu et al. (2016) reported that inter-planting larch with Panax ginseng (Panax ginseng C. A. Meyer) significantly decreased the soil carbon stock compared with pure larch plantations. The contrasting results may be caused by differences in forest structures, reforestation patterns, and planting duration. Indeed, previous studies have shown that the effects of agroforestry on soil properties with different inter-planting years are not consistent either. For instance, Kalita et al. (2020) found that the total C stock increased with the inter-planting year of tea agroforestry systems. In contrast, Arevalo-Gardini et al. (2015) reported a decrease in the concentration of soil organic matter and other nutrients (P, K, Fe, Mg, and Al) with the inter-planting years of cacao. Therefore, it is crucial to consider the inter-planting year effects when assessing the influence of agroforestry on soil properties.
Some new practices, which associate LP with other shrubs of additional economic value, have been carried out in Northeast China in the context of poverty alleviation and natural forest conservation projects. For example, Aralia elata (Miq.) Seem., a native broadleaved shrub or small tree with high economic value, has been widely interplanted with LP. A. elata produces tender shoots or terminal buds in spring that can be harvested by local foresters and sold as vegetables, resulting in a high economic value of approximately $2500–3500 ha− 1 year− 1. However, the impacts of this agroforestry practice on the degraded soil of larch plantations remain unclear. Therefore, it is essential to explore whether the larch-A. elata agroforestry system (LAAS) could improve the degraded soil of larch plantations. And if so, at which years since inter-planting year(s)? In this study, we compared a set of chemical and microbial properties at four soil depths (humus, 0–10 cm, 10–20 cm, and 20–30 cm) in paired stands of larch plantations and adjacent LAAS with different inter-planting years (1, 3, 5, and > 10 years). This study aimed to (1) test the differences of soil chemical and microbial properties between the LP and adjacent LAAS, based on which to determine the years since inter-planting that resulted in the improvement of soil properties, and (2) determine the main factors that affect soil chemical and microbial properties.
Materials and methods
Study site
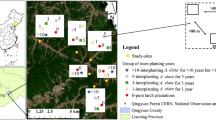
Our study was conducted at Qingyuan Forest CERN, National Observation and Research Station, Liaoning Province, China (41°51′N, 124°54′E, 500–1100 m above sea level). The region has a temperate continental monsoon climate, with warm and humid summers and cold and dry winters. The mean annual air temperature is 4.3 °C (with a minimum in January and a maximum in July) and annual precipitation is 758 mm (with more than 80% falling during the summer) during 2010–2021. The frost-free days last approximately 130 days, with an early frost in October and a late frost in April (Zhu et al. 2007). The soil is classified as typical forest brown soil (Udalfs), with 25.6% sand, 51.2% silt, and 23.2% clay, according to the US soil taxonomy of the second edition.
In Northeast China, more than 70% of primary forests (the mixed broadleaved Korean pine forests) have been destroyed by human beings (i.e., logging) or extreme natural causes (i.e., snow and wind disasters) and formed into secondary forests through the natural regeneration of native broadleaved tree species. Since the 1950s, secondary forests have been gradually converted into LP, which covers an area of 2.6 × 106 ha due to the growing demand for timber (Wang et al. 2011; Gao et al. 2018). The forest management strategies of LP have shifted from pursuing timber production to providing ecological services (i.e., improving soil quality), leading to local foresters’ economic losses (Li and Zhou 2000). The tree-based agroforestry, inter-planting A. elata (a local “cash” shrub) with LP, is becoming increasingly prevalent because this practice can provide economic benefits in the short term. The shrub layer of LAAS mainly included A. elata and other natural regenerated species, such as Euonymus alatus (Thunb.) Sieb and Lonicera japonica Thunb. The herbaceous layer mainly included Carex spp., Athyrium multidentatum (Döll) Ching, Sanicula chinensis Bunge, Rubus crataegifolius Bunge, Geranium wilfordii Maxim., and Rubia sylvatica (Maxim.) Nakai.
Study design
We selected six spatially separated study sites in Qingyuan Forest. On each site, we selected LAAS with different inter-planting years of A. elata (1, 3, 5, and > 10 years) as treatment groups and an adjacent (ranged from 400 to 1600 m) LP as control groups. Each study site contained as many inter-planting years of A. elata as logistically possible (details in Fig. 1 and Table S1), leading to four spatially random paired stands for each inter-planting year to ensure the reliability of the research results. Some experimental groups shared the same control group because of their closeness to the same control. In order to represent the general characteristics of the stand, three repetitive plots (20 m × 20 m) separated from each other by > 80 m were established in each paired stand. In total, sixteen paired stands with 66 plots were selected from six study sites (Fig. 1). Moreover, we selected the paired stands based on similar ages and sizes of larch trees, geographical conditions, and soil parent material (Table S1). The thickness of the litter layer on the forest floor varied from 3.9 to 6.2 cm. The root depth of larch tree was distributed om 0–60 cm soil layer (Wang et al. 2014). A. elata individuals were interplanted under larch trees at a density of 1 m × 1 m, based on local planting records. In order to obtain the basal area value of the LAAS, the basal diameter, height, age, and composition of the understory were surveyed in five randomly selected subplots of 5 m × 5 m within each selected plot (detailed information in Tables S1 and S2). The LAAS was managed according to standard practices. For example, shrubs and herbaceous plants were generally clear-cut before and after the first two years of inter-planting practice to ensure the best survival of A. elata, and then they were clear-cut approximately every 5 years to facilitate the harvest of tender shoots or terminal buds. The terminal buds of A. elata (as the vegetable) were harvested once or twice a year in spring (from late April to early May) after three years of inter-planting. Notably, the biomass of harvested terminal buds accounted for a small proportion of the whole plant biomass (approximately 2.20% for 3 years, 1.10% for 5 years, and 0.58% for > 10 years of inter-planting, respectively).
Litterfall, forest floor, and mineral soil sampling
Litterfall was collected monthly from September to November 2019. Five litter traps were randomly installed in each plot. Each trap (1 m × 1 m) consisted of 1 mm mesh nylon netting (fixed on four polyvinyl chloride pipes with nylon cable). Each trap was raised 50 cm above the ground, except for the inter-planting with A. elata for 1 year (approximately 20 cm above the ground) due to the height limit of A. elata. The collected litterfall on each occasion was oven-dried at 55 °C (Vesterdal et al. 2008) to a constant weight, pooled to one composite sample by months and plots, and subsequently hand-sorted into two fractions: foliar and non-foliar litterfall (other debris except for leaves such as branches and bark). Foliar litterfall was subsequently separated into three parts: larch leaves, A. elata leaves, and non-A. elata leaves. These hand-sorted fractions were all weighed after each sampling occasion and pooled to one composite sample by the same type of foliar or non-foliar in all collecting events to gauge the proportion of each foliar type (Table S2).
Forest floor and mineral soils were randomly sampled at nine points within each plot during the growing season of 2019, before the onset of foliar litterfall for deciduous species. The forest floor, which refers to the litter layer and humus (including combined fermentation layer and humified layer), were sampled on an area basis using a 25 × 25 cm wooden frame. Notably, to detect sensitive soil changes in LAAS, humus was regarded as a layer of soil in our study.
The mineral soil was collected according to three predefined fixed depth layers: 0–10 cm, 10–20 cm, and 20–30 cm using soil cores (5 cm diameter × 10 cm depth) at the same point, where the forest floor and humus had been sampled. Sampling was carefully done to avoid contamination, and the 0 cm line corresponded to the boundary between the humus and the underlying mineral soil. The nine subsamples (forest floor, humus, and soils from each depth) in each plot were mixed into a composite sample according to the same type.
All samples were transported to the laboratory, and visible residues (macrofauna, stones, and large roots) were subsequently removed by hand for forest floor and humus or by a 2 mm sieve for soils of each depth. The forest floor samples were dried at 55 °C to a constant weight and ground for chemical analysis (Vesterdal et al. 2008; Chen et al. 2019; Guo et al. 2019). The soil (humus, 0–10 cm, 10–20 cm, and 20–30 cm) samples were divided into two sets of subsamples. One set of soil samples was air-dried for further analysis of chemical properties except for inorganic N. Another set of soil samples was stored at 4 °C to determine inorganic N and microbial properties (microbial biomass carbon, nitrogen and phosphorus (P), and enzyme activities) (Yang et al. 2012; German et al. 2011).
Laboratory analysis
Soil chemical properties
Total C and N were examined by a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). Total P was extracted by the H2SO4-HClO4 digestion method and measured by the ascorbic acid-molybdenum blue method (Olsen and Sommers 1982). The available P (Av–P) (extracted by 0.5 M NaHCO3, pH = 8.5, 0.5 h) was determined using the method of John (1970). Mineral N (Mi–N) (i.e., NH4+–N and NO3−–N), extracted with 2 M KCl, was analyzed using an auto-analyzer (Auto Analyzer III, Bran + Luebbe GmbH, Germany). The pH was measured in a 1/2.5 soil-water slurry.
Soil microbial properties
Microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), and microbial biomass phosphorus (MBP) were determined using the chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987). MBC, MBN, and MBP concentrations were calculated by dividing the value of differences between fumigated and unfumigated samples with a conversion factor of 0.45 (Wu et al. 1990), 0.54 (Brookes et al. 1985), and 0.40, respectively.
We measured the extracellular enzyme activities involved in C, N, and P cycling processes, including four hydrolases [i.e., β-glucosidase (BG), β-cellobiohydrolase (CB), N-acetyl-β-glucosaminidase (NAG), and acid phosphatase (AP)], and two oxidases [i.e., phenol oxidase (PPO) and peroxidase (PER)]. Enzyme activities (represented in units of nmol h− 1 g − 1) were determined according to the protocol described by German et al. (2011).
Statistical analyses
Relative values (RV) were used in the following data analysis to reveal the effects of inter-planting with A. elata and its temporal effects on soil properties. RV was calculated as follows (Zhou et al. 2013):
where \(RV\) is the relative value of the given variables (i.e., the chemical or microbial properties of soil), \({LA}_{i}\) is the value of the given variable in the LAAS, and \({LP}_{i}\) is the value of the corresponding variable in the paired LP. The values of the variables \({LA}_{i}\) and \({LP}_{i}\) are the mean values of the three repetitive plots in each paired stand.
A linear mixed model (LMM) was used to analyze the differences in soil chemical and microbial properties between LAAS and LP, with the forest types and inter-planting years as the fixed factors and the study sites as the random factor. To distinguish significant differences at p < 0.05 level, the statistical significance of fixed factors was tested by an analysis of variance (ANOVA), followed by a Tukey’s test. The relationships between soil properties (chemical and microbial) and inter-planting years, forest types, properties of basal area, litterfall, and forest floor were tested using Pearson correlation analysis. Before analysis, the normality of variables was checked using the Shapiro–Wilk test, and the homogeneity of variances was examined using Levene’s test. All the statistical analyses were performed using IBM SPSS (version 26.0; IBM Corp., Armonk, NY, USA).
Results
Soil chemical properties
Compared with LP, LAAS did not affect total C, N and P, and the stoichiometric C:N, C:P and N:P ratios in either inter-planting years or soil depths (p > 0.05; Table S3). In contrast, mineral N (i.e., NH4+–N and NO3−–N), available P, and pH changed significantly in LAAS (p < 0.05), and the effects varied with both inter-planting years and soil depths (Fig. 2). LMM analysis indicated that compared with LP, inter-planting with A. elata for 3, 5, and > 10 years significantly increased mineral N by 13%, 13%, and 34% at humus layer, and by 19%, 32%, and 31% at 0–10 cm soil depth, respectively (p < 0.05), whereas inter-planting with A. elata for 1 year significantly decreased mineral N by 8% at both humus layer and 0–10 cm soil depth (p < 0.05; Fig. 2A). In addition, inter-planting with A. elata for 5 and > 10 years significantly increased available P by 24% and 43% at humus layer, and by 26% and 33% at 0–10 cm soil depth, respectively (p < 0.05; Fig. 2B). Inter-planting with A. elata for all years significantly increased the pH by 5–8% at the humus layer and by 3–4% at 0–10 cm soil depth compared with LP, respectively (p < 0.05). Notably, compared with LP, no differences in mineral N (except for > 10 years at 10–20 cm) (Fig. 2A), available P (Fig. 2B), and pH (Fig. 2C) could be detected at either 10–20 cm or 20–30 cm soil depths in LAAS (p > 0.05).
Soil mineral N (i.e., NH4+–N and NO3−–N), available P and pH (relative values) of different inter-planting years of A. elata at each soil depth. Bars represent standard errors of the means (n = 4). Different letters indicate significant differences between different inter-planting years, at p < 0.05
One-way ANOVA indicated that at humus layer and 0–10 cm soil depth, mineral N and available P of inter-planting with A. elata for > 10 years were significantly higher than those of 1 year (p < 0.05). Moreover, at 10–20 cm soil depth, mineral N of inter-planting with A. elata for > 10 years was significantly greater than that of 3 years (p < 0.05).
Soil microbial properties
LAAS significantly changed MBC, MBN, and MBP compared with LP, and the effects were different with inter-planting years and soil depths (p < 0.05; Fig. 3). At the humus layer, inter-planting with A. elata for > 10 years significantly increased MBC, MBN, and MBP by 67%, 70%, and 32%, respectively (p < 0.05). In contrast, inter-planting with A. elata for 1 and 3 years decreased MBC, MBN, and MBP by 11–68%. At 0–10 cm soil depth, inter-planting with A. elata for > 10 and 5 years significantly increased MBC, MBN, and MBP by 16–69% (p < 0.05). Interestingly, for the inter-planting with A. elata for 1 and 3 years, the changes of MBC, MBN, and MBP were inconsistent (positive, neutral, and negative). At 10–20 cm soil depth, inter-planting with A. elata for > 10 years significantly increased MBC, MBN, and MBP by 17%, 22%, and 39%, respectively (p < 0.05).
One-way ANOVA showed that at humus layer, MBC, MBN, and MBP of inter-planting with A. elata for > 10 years were significantly higher than those of 1, 3, and 5 years (except for MBN in 5 years) (p < 0.05; Fig. 3). At 0–10 cm soil depth, MBN of inter-planting with A. elata for 5 years was significantly greater than that of 1 and 3 years (p < 0.05; Fig. 3B). At 10–20 cm soil depth, MBP of inter-planting with A. elata for > 10 years was significantly higher than that of 1 and 3 years (p < 0.05; Fig. 3C).
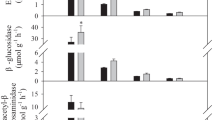
LAAS significantly modified enzyme activities compared with LP (p < 0.05), and the effects varied with inter-planting years, soil depths, and individual enzymes (Figs. 4 and 5). At humus layer and 0–10 cm soil depth, in particular, inter-planting with A. elata for > 10, 5, and 3 years significantly increased BG, CB, NAG, and AP (except for 3 years) by 4–34%, 6–34%, 7–22% and 4–19%, respectively (p < 0.05; Fig. 4). Similarly, inter-planting with A. elata for > 10 and 5 years significantly increased PPO and PER by 17–97% and 15–110%, respectively (p < 0.05; Fig. 5). However, inter-planting with A. elata for 1 year showed a reverse trend, in which all hydrolases (except for CB at humus layer and AP at 0–10 cm soil depth) and oxidases decreased significantly compared with LP (p < 0.05). At 10–20 cm, inter-planting with A. elata for > 10 and 5 years significantly increased four hydrolases (BG, CB, NAG, and AP) by 7–54%, and inter-planting with A. elata for > 10 years significantly increased PER by 25% (p < 0.05). At 20–30 cm, inter-planting with A. elata for > 10 years significantly increased AP, PPO, and PER by 5%, 51%, and 125%, respectively (p < 0.05).
Soil BG (β-glucosidase), CB (β-cellobiohydrolase), NAG (N-acetyl-β-glucosaminidase), and AP (acid phosphatase) (relative values) at different inter-planting years of A. elata at each soil depth. Bars represent standard errors of the means (n = 4). Different letters indicate significant differences between different inter-planting years, at p < 0.05
One-way ANOVA showed that at humus layer and 0–10 cm soil depth, four hydrolases (BG, CB, NAG, and AP) and two oxidases (PPO and PER) of inter-planting with A. elata for 5 and > 10 years were significantly higher than those of 1 year (except for PPO at 0–10 cm soil depth) (p < 0.05; Figs. 4 and 5). At 20–30 cm soil depth, PER of inter-planting with A. elata for > 10 years was significantly higher than those of 1 year (p < 0.05; Fig. 5B).
Relationships of soil properties with properties of basal area, litterfall, and forest floor
Soil TP was significantly related to forest type, basal area and foliar litterfall of non-A. elata leaves (p < 0.05). Furthermore, soil C/N, pH, mineral N, available P, MBP, BG, CB, NAG, AP, PPO, and PER were significantly related to inter-planting years and total basal area (except for soil C/N), basal area of A. elata, total litterfall (except for MBP and PER), foliar litterfall (except for soil C/N, MBP, NAG, and PER), foliar litterfall ratio of larch and A. elata (except for soil C/N), and C/N ratio of forest floor (except for MBP, CB, and NAG) (p < 0.05 or p < 0.001). Additionally, soil C/N and pH were also significantly related to forest type, basal area of non-A. elata, foliar litterfall ratio of non-A. elata leaves, and C concentration of the forest floor (p < 0.05 or p < 0.001). MBN was significantly related to the total basal area and foliar litterfall ratio of larch (p < 0.05 or p < 0.001). Notably, the correlation of the basal area or the foliar litter ratio with soil properties showed opposite trends between A. elata (positive) and non-A. elata (negative) species.
Pearson correlation coefficients for the properties of soil, inter-planting years, forest types, properties of basal area, litterfall, and forest floor
TC, TN, and TP: total carbon, nitrogen, and phosphorus; MBC, MBN, and MBP: microbial biomass carbon nitrogen and phosphorus; BG: β-glucosidase; CB: β-cellobiohydrolase; NAG: N-acetyl-β-glucosaminidase; AP: acid phosphatase; PPO: phenol oxidase; PER: peroxidase; IPY: inter-planting years; FT: forest types; TBA: total basal area; ABA: A. elata basal area; NABA: non-A. elata basal area; TL: total litterfall; FL: foliar litterfall; FLRL: foliar litterfall ratio of larch; FLRA: foliar litterfall ratio of A. elata; FLRNA: foliar litterfall ratio of non-A. elata; FLC and FLN: C and N concentration of forest floor; FL C/N: C and N ratio of forest floor. *, p < 0.05, **, p < 0.01, n = 8 (2 soil depths × 4 replicates)
Tables Schedule
Discussion
Effects of inter-planting with A. elata on soil chemical properties
Among all the studied chemical properties, significant changes were mainly detected in soil mineral N, available P, and pH, not in total soil C, N, and P and stoichiometry of C: N: P in LAAS.
In general, soil chemical properties are mainly affected by the nutrient balance of the input and output variables. In this study, nutrient inputs were mainly from (1) organic matter, which was mainly derived from the leaves and roots of larch trees and other shrubs in LP, or the leaves and roots of larch trees, other shrubs, and the introduced species of A. elata in LAAS; (2) other sources, such as nitrogen fixation and sedimentation, and wet and dry deposition. Nutrient outputs included (1) removed products, which refers to larch logging in LP, or larch logging, other clear-cut shrubs (to ensure the survival ratio and the harvest convenience of tender shoots of A. elata), and the harvested tender shoots of A. elata in LAAS; (2) other forms, such as gaseous loss and leaching (Rao et al. 2021). In our study, we chose paired adjacent LP and LAAS sites. Therefore, the nitrogen fixation and sedimentation and wet and dry deposition can be expected to be similar. Furthermore, all the studied larch trees (Table S1) belonged to the same forest farm, and they had experienced the same management. Therefore, the changes in soil nutrients of LAAS possibly resulted from the imbalance between input and output, which was caused by the introduction of A. elata and other mechanisms, such as changes in microbial community composition and biomass, microclimate, and soil physical structure (i.e., bulk density, porosity, and aggregates). In terms of nutrient inputs, LAAS was associated with a higher volume of stand biomass that resulted in large root turnover (data not shown) and litterfall. The total litterfall in LAAS of > 10 inter-planting years (205.9 g m− 2) was higher than that of LP (145.8 g m− 2) (Table S2). In addition, the quality of leaf litter also affects soil nutrients. In LAAS, the quality of leaf litter changed significantly. For example, the foliar litter (172.1 g m− 2) in LAAS of > 10 inter-planting years was higher than that of LP (137.1 g m− 2), and the foliar litter ratio of larch and A. elata (73:27) and the C/N ratio of forest floor (13.89) in LAAS of > 10 inter-planting years changed compared with LP (85:15 and16.15) (Table S2). Generally, a higher proportion of foliar litter of A. elata (a broadleaved shrub or small tree) indicates a higher number of broad leaves. Compared with coniferous leaves, broad leaves have a faster decomposition rate and higher nutrient release rate, resulting in greater soil available nutrient supply (Guo et al. 2019). In addition, our correlation analysis determined that the changes in soil nutrients were mainly attributable to A. elata rather than other shrubs (Fig. 6). Regarding nutrient outputs, according to Rao et al. (2021), LAAS may be associated with lower gas loss and splash erosion (we did not mention splash erosion because it does not occur in our study sites). Therefore, although there was clear-cutting of other shrubs and harvesting of the terminal buds of A. elata in LAAS of over 5 inter-planting years, the total input was still higher than the output, leading to higher soil nutrients in LAAS than in LP. However, the mineral N of inter-planting with A. elata for 1 year in LAAS may be associated with lower input, which resulted from clear-cutting before inter-planting with A. elata.
Compared with LP, the total soil C, N, and P and their stoichiometry of C, N, and P did not change in LAAS for all inter-planting years. This finding was consistent with other studies in which no differences of soil organic carbon and total nitrogen were observed in rubber-based agroforestry compared with pure rubber plantations (Liu et al. 2018). This may be explained by (1) the nutrient balance of the input and output. In our study, no significant differences were obtained in input variables [i.e., the total litterfall, foliar litterfall and C and N concentration of forest floor between LP and LAAS (Table S2)] on the bases of similar output; (2) the total C, N, and P are less sensitive than the soil available nutrients (i.e., mineral N and available P) and microbial biomass and enzyme activities (Muñoz-Rojas 2018).
Notably, the effects of LAAS on soil properties mainly occurred at the humus layer and 0–10 cm soil depth and rarely occurred at deeper layers (10–20 cm and 20–30 cm). The possible reason for this may be as follows: (1) aboveground litter was the major source of organic matter in topsoil, while root biomass or exudation of live fine roots or decomposition of dead roots were the major source of organic matter in subsoil (Liebmann et al. 2020); (2) according to our field investigation and root digging, the roots of A. elata were mainly distributed at the 0–30 cm soil layer, and the activities (i.e., decomposition rate and exudation) of fine roots were higher at the upper soil layer compared with deeper soil layer (Matamala et al. 2004); and (3) a relatively long period, even for decades, is needed for organic matter or root exudation to migrate down (Rumpel and Kögel-Knabner 2011). Given that the longest inter-planting year was more than 10 years but less than 15 years in our study, it was not surprised that no changes in deeper soil layer in LAAS was observed.
Effects of inter-planting with A. elata on soil microbial properties
Compared with LP, inter-planting with A. elata for 5 and > 10 years significantly increased soil MBC, MBN, and MBP at humus layer and 0–10 cm soil layer (p < 0.05). Similar results were obtained by Guo et al. (2019), who suggested that the MBC and MBN in ginkgo agroforestry systems were significantly higher compared with pure ginkgo plantations. The quality and quantity of substrate input affect microbial biomass via organic matter (i.e., litter and fine roots) (Guo et al. 2019), which was also proven by the significant correlations of MBN and MBP with the basal area, foliar litterfall ratio, or inter-planting years (Fig. 6). Interestingly, inter-planting with A. elata for 1 and 3 years significantly decreased soil MBC, MBN, and MBP at humus layer and soil MBN at 0–10 cm soil depth (p < 0.05). This may be explained by (1) the input decrease, which resulted from the removal of understory before inter-planting with A. elata, and (2) the decrease of litter quantity (the litterfall derived from A. elata (inter-planting 1 year) was less than native cleared shrubs in LAAS) and quality (higher foliar litter proportion of larch in LAAS of 1 year) (Table S2).
It is well known that soil enzymes participate in the biochemistry of decomposition and nutrient cycling. β-glucosidase (BG) and β-cellobiohydrolase (CB) are directly related to the C cycle by depolymerizing multiple organic molecules into simple units. N-acetyl-β-glucosaminidase (NAG) and acid phosphatase (AP) are associated with N and P cycles, respectively. Phenol oxidase (PPO) and peroxidase (PER) can promote the degradation of materials containing phenols and carbohydrates (i.e., cellulose and lignin). Compared with LP, the higher enzyme activities (BG, CB, NAG, AP, PPO and PER, except for 3 years for AP, PPO and PER) in LAAS (inter-planting for 3, 5, and > 10 years) indicated that LAAS was associated with greater efficiency of C, N, and P cycles and higher ability of decomposition for recalcitrant multiple organic compounds. Moreover, the studied enzymes of BG, CB, NAG, AP, and PPO were significantly related to inter-planting years, specific properties of basal area, litterfall, foliar litterfall ratio, and forest floor. A similar result was reported by Feng et al. (2019), who suggested that litter production and planting years could affect soil enzyme activities. Furthermore, fine root mortality, soil available nutrients, and microbial biomass also contribute to soil enzyme activities (Miguel et al. 2020). In contrast, all studied enzyme activities (except for CB at humus layer) in LAAS of 1 year were significantly lower than those of LP (p < 0.05), and the reason for this may be similar to microbial biomass in LAAS of 1 year.
Notably, most of soil chemical (C/N, mineral N, available P and pH) and microbial (MBP, BG, CB, NAG, AP, PPO and PER) properties were positively significant related to inter-planting years (IPY), total basal area (TBA) and A. elata basal area (ABA), total litterfall (TL), foliar litterfall (FL) and foliar litter ratio of A.elata (FLRA). This may be explained by (1) these soil chemical properties were more sensitive to environment changes (Muñoz-Rojas 2018); (2) the influences of these factors on soil properties were higher than other studied factors, such as non-A. elata basal area (NABA), foliar litterfall ratio of non-A. elata (FLRNA), C and N concentrations of forest floor (FLC and FLN). In contrast, most of soil chemical (C/N, mineral N, available P and pH) and microbial (MBP, BG, CB, NAG, AP, PPO and PER) properties were poorly related to the factors of NABA, FLRNA, FLC and FLN, which may be because there were no significant (p > 0.05) differences of the properties of NABA, FLRNA, FLC, and FLN among different inter-planting years of A. elata (Table S2). In addition, most of soil chemical (C/N, mineral N, available P and pH) and microbial (MBP, BG, CB, NAG, AP, PPO and PER) properties were negatively related to foliar litter ratio of larch (FLRL), which may be associated with that the foliar litter ratio of larch and A. elata was completely opposite based on the changeless of total litterfall and foliar litterfall (p > 0.05) (Table S2).
Conclusions
Inter-planting with A.elata could improve the degraded soil (i.e., mineral N, available P, pH, microbial biomass and enzyme activities) of larch plantations, especially after > 5 years since the inter-planting. Therefore, the LAAS model, which can improve soil quality and provide economic benefits simultaneously in the short term, is recommended for widespread popularization in the sustainable management of larch plantations.
References
Arévalo-Gardini E, Canto M, Alegre J et al (2015) Changes in soil physical and chemical properties in long term improved natural and traditional agroforestry management systems of cacao genotypes in Peruvian Amazon. PLoS ONE 10:1–29. https://doi.org/10.1371/journal.pone.0132147
Brookes P, Landman A, Pruden G et al (1985) Chloroform fumigation and the release of soil-nitrogen - a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Chen C, Liu W, Wu J et al (2019) Can intercropping with the cash crop help improve the soil physico-chemical properties of rubber plantations? Geoderma 335:149–160. https://doi.org/10.1016/j.geoderma.2018.08.023
Diao M, Yang K, Zhu J et al (2020) Native broad-leaved tree species play key roles on maintaining soil chemical and microbial properties in a temperate secondary forest, Northeast China. For Ecol Manage 462:117971. https://doi.org/10.1016/j.foreco.2020.117971
Feng C, Ma Y, Jin X et al (2019) Soil enzyme activities increase following restoration of degraded subtropical forests. Geoderma 351:180–187. https://doi.org/10.1016/j.geoderma.2019.05.006
Gao T, Zhu J, Yan Q et al (2018) Mapping growing stock volume and biomass carbon storage of larch plantations in Northeast China with L-band ALOS PALSAR backscatter mosaics. Int J Remote Sens 39(22):7978–7997. https://doi.org/10.1080/01431161.2018.1479793
German DP, Weintraub MN, Grandy AS et al (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Guo J, Wang G, Wu Y et al (2019) Leaf litter and crop residue decomposition in ginkgo agroforestry systems in eastern China: Soil fauna diversity and abundance, microbial biomass and nutrient release. J For Res 30:1895–1902. https://doi.org/10.1007/s11676-018-0758-7
John MK (1970) Colorimetric Determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci 109(4):214–220. https://doi.org/10.1021/i560102a037
Kalita RM, Das AK, Sileshi GW et al (2020) Ecosystem carbon stocks in different aged tea agroforestry systems: implications for regional ecosystem management. Trop Ecol 61(2):203–214. https://doi.org/10.1007/s42965-020-00084-8
Li C, Zhou X (2000) Status and future trends in plantation silviculture in China. Ambio 29(6):354–355. https://doi.org/10.1579/0044-7447-29.6.354
Liebmann P, Wordell-Dietrich P, Kalbitz K et al (2020) Relevance of aboveground litter for soil organic matter formation - a soil profile perspective. Biogeosciences 17:3099–3113. https://doi.org/10.5194/bg-17-3099-2020
Liu S, Li X, Niu L (1998) The degradation of soil fertility in pure larch plantations in the northeastern part of China. Ecol Eng 10:75–86. https://doi.org/10.1016/S0925-8574(97)10024-6
Liu W, Liu M, Li W et al (2016) Characteristics of plant diversity and carbon stock under the Larix spp.-Panax ginseng agroforestry system. Scientia Silvae Sinicae 52(09):124–132 (in Chinese). https://doi.org/10.11707/j.1001-7488.20160915
Liu CA, Nie Y, Zhang YM et al (2018) Introduction of a leguminous shrub to a rubber plantation changed the soil carbon and nitrogen fractions and ameliorated soil environments. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-35762-0
Mason WL, Zhu JJ (2014) Silviculture of planted forests managed for multi-functional objectives: lessons from Chinese and British experiences. In: Fenning T (ed) Challenges and opportunities for the world’s forests in the 21st century. Forestry sciences, vol 81. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7076-8_3
Matamala R, Gonzalez-Meler MA, Jastrow JD et al (2004) Response to comment on “Impacts of fine root turnover on forest Npp and soil C sequestration potential”. Science 302(5649):1385–1387. https://doi.org/10.1126/science.1099340
Miguel DL, da Silva EMR, da Silva CF et al (2020) Soil microbiological properties and enzyme activity in agroforestry systems compared with monoculture, natural regeneration, and native caatinga. Biosci J 36:1–16. https://doi.org/10.1126/science.1099340
Muñoz-Rojas M (2018) Soil quality indicators: critical tools in ecosystem restoration. Curr Opin Environ Sci Heal 5:47–52. https://doi.org/10.1016/j.coesh.2018.04.007
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. Chemical and Microbiological Methods, Am.Soc. Agron, Madison, pp 403–410
Rao X, Liu CA, Tang JW et al (2021) Rubber-leguminous shrub systems stimulate soil N2O but reduce CO2 and CH4 emissions. For Ecol Manage 480:118665. https://doi.org/10.1016/j.foreco.2020.118665
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158. https://doi.org/10.1007/s11104-010-0391-5
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19(6):703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Vesterdal L, Schmidt I, Callesen I et al (2008) Carbon and nitrogen in forest floor and mineral soil under six common european tree species. For Ecol Manage 255(1):35–48. https://doi.org/10.1016/j.foreco.2007.08.015
Wang W, Qiu L, Zu Y et al (2011) Changes in soil organic carbon, nitrogen, pH and bulk density with the development of larch (Larix Gmelinii) plantations in China. Glob Change Biol 17(8):2657–2676. https://doi.org/10.1111/j.1365-2486.2011.02447.x
Wang W, Wang H, Zu Y (2014) Temporal changes in SOM, N, P, K, and their stoichiometric ratios during reforestation in China and interactions with soil depths: Importance of deep-layer soil and management implications. For Ecol Manage 325:8–17. https://doi.org/10.1016/j.foreco.2014.03.023
Wu J, Joergensen RG, Pommerening B et al (1990) Measurement of soil microbial biomass C by fumigation extraction - an automated procedure. Soil Biol Biochem 22(8):1167–1169. https://doi.org/10.1016/0038-0717(90)90046-3
Yang K, Zhu J, Yan Q, Zhang J (2012) Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann For Sci 69:795–803. https://doi.org/10.1007/s13595-012-0198-z
Yang K, Shi W, Zhu J (2013) The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 368:535–546. https://doi.org/10.1007/s11104-012-1535-6
Zhou D, Zhao S, Liu S et al (2013) A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 10:3691–3703. https://doi.org/10.5194/bgd-10-787-2013
Zhu J, Mao Z, Hu L et al (2007) Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. J For Res 12(6):403–416. https://doi.org/10.1007/s10310-007-0033-9
Zhu J, Yang K, Yan Q et al (2010) Feasibility of implementing thinning in even-aged Larix olgensis plantations to develop uneven-aged larch-broadleaved mixed forests. J For Res 15(1):71–80. https://doi.org/10.1007/s10310-009-0152-6
Acknowledgements
We are grateful to G. Geoff Wang for his help on the revision of the manuscript. We are grateful to the two anonymous reviewers and the associate editor for their constructive comments that greatly helped improve the manuscript. This work was financially supported by the National Natural Science Foundation of China (U1808201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, P., Zhu, J., Yang, K. et al. Can larch-Aralia elata agroforestry systems improve the soil chemical and microbial properties of larch plantations?. Agroforest Syst 96, 885–896 (2022). https://doi.org/10.1007/s10457-022-00748-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-022-00748-5