Abstract
Research has indicated that introducing Aralia elata into larch plantations forms an agroforestry system which could provide economic benefits for local farmers and improve degraded soils. However, the impact of litter mixtures on soil chemical and microbial properties in this agroforestry system are unclear, which limits efficient management of the agroforestry system. A 365-d incubation experiment examined the effect of litter mixtures of different proportions of larch (L) and A. elata (A) on soil chemical and microbial properties. The results show that levels of mineral N, available P, microbial biomass carbon and nitrogen, cumulative C mineralization, and activities of hydrolases and oxidases increased with an increase of A. elata in the litter mixtures. Concentration of total soil carbon, nitrogen, and phosphorous did not change (except for total nitrogen). Compared with larch litter alone, levels of mineral N, available P, microbial biomass carbon and nitrogen, cumulative C mineralization, and the activities of hydrolases and oxidases increased by 7.6–433.5%. Most chemical and microbial properties were positively correlated with mixed litter proportions and the initial levels of N, P, K, Ca, Mg, Mn, Zn and Cu in the litter, while negatively correlated with the initial concentrations of C, Fe and lignin, C/N and lignin/N ratios. The results indicate that A. elata litter can improve degraded larch soil and the degree depends on the proportion of A. elata litter in the litter mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Northeast China, extensive areas of secondary forests have been replaced by larch (Larix olgensis A. Henry) plantations (2.6 × 106 ha) due to their inability to meet increasing timber demands since the 1950s (Mason and Zhu 2014; Gao et al. 2018). However, with the establishment of these plantations, soil degradation has occurred, such as soil acidification, decline of carbon (C), nitrogen (N), microbial biomass, and enzyme activities compared with adjacent secondary forests (Yang et al. 2010, 2012, 2013). This is attributed to the monoculture system leading to slower litter decomposition and ultimately resulting in soil degradation (Yang et al. 2013). In fact, larch litter decomposes more slowly than litter of other tree species, such as Manchurian ash (Fraxinus mandshurica Rupr.), white birch (Betula platyphylla Suk.), and David poplar (Populus davidiana Dode), thereby leading to the decline of nutrient recycling (Liu et al. 1998) which will not sustain the productivity of larch plantations. Therefore, accelerating the decomposition of litter is essential to resolve these problems.
Litter decomposition is a fundamental process which determines the rate of nutrient cycling and controls the level of C and nutrients (e.g. N and P entering the soil (Weedon et al. 2009)). Abiotic and biotic factors are the regulators of litter decomposition. For abiotic factors, the most important is the quality of the litter (Wieder et al. 2009). For example, decomposition of higher quality litter (e.g. low C/N and lignin/N ratios, higher N) is faster and thereby leads to higher nutrient availability (Mukhopadhyay and Joy 2010). Cabrera et al. (2005) found that plant litter materials with C/N ratios over 40 resulted in net N immobilization, while below 20 led to net N mineralization (Whitmore 1996). In addition, litter with high N levels contributed to the decomposition of water-soluble compounds and non-lignified cellulose; Mn and Ca also had a profound impact on litter decomposition rate (Keiluweit et al. 2015; Zhou et al. 2020). For biotic factors, microbes have been considered basic mediators in litter decomposition since they secrete various hydrolases [e.g. β-1,4-glucosidase (BG) and β-cellobiohydrolase (CB), β-1,4-N-acetyl-glucosaminidase (NAG), and acid phosphatase (AP)] and oxidases [e.g. polyphenol oxidase (PPO) and peroxidase (PER)] and change the composition of their community structure (Voříšková et al. 2011). In addition, species evenness (mixed proportions) in litter mixtures also affect litter quality, microbial biomass, and their activities, and thereby the litter decomposition rate (Li et al. 2013; Kuebbing and Bradford 2019). For example, litter mixtures with high proportions high quality litter lead to higher decomposition rates, carbon mineralization, microbial biomass, and activities (Mitchell et al. 2011; Kuebbing and Bradford 2019). Therefore, mixed litter proportions should not be neglected in the decomposition process.
Many studies of litter mixtures focus on mass loss, nutrient release and litter mixing effects (Makkonen et al. 2013). However, Jiang et al. (2013) indicated that soil properties (e.g. C, N and P cycling) are closely related to litter decomposition. Understanding litter decomposition on soil C, N, and P cycling is necessary for studying plant-soil interaction (Kuiters 1990). However, few studies have addressed the effect of litter decomposition on soil properties, especially for agroforestry systems. In addition, few results of the response of soil properties to litter mixtures decomposition came from relatively short duration experiments (e.g. six-week incubation, 65-d incubation, and 120-d incubation) or from equal mass mixed ratio (1:1) experiments (Yang and Zhu 2015; Chen et al. 2018; Zeng et al. 2021). However, as affected by forest structure, reforestation patterns, and planting duration, litter does not always strictly follow the equal mass mixed rules, especially in agroforestry systems which usually have a single tree species and perhaps inter-planted shrub(s) (Naeem et al. 2021). For example, Gao et al. (2022) found that litter mixed proportions changed significantly (Table S1) according to inter-planting years of Aralia elata (Miq.) Seem. in larch-A. elata agroforestry system (LAAS).
The LAAS, planting A. elata (a native broadleaved shrub or small tree with high economic values) into larch plantations, is becoming increasingly widespread in northeast China. The agroforestry system can improve degraded soils over 5 years, while achieving economic benefits (selling tender shoots or terminal buds of A. elata in spring with profits of approximately $ 2500–3500 ha−1 a−1) in the short-term simultaneously in the context of poverty alleviation and natural forest conservation (Gao et al. 2022). However, the impacts of the LAAS with different litter mixtures proportions on degraded soils remain unclear, especially over the long-term. Therefore, it is important to clarify the effect of litter mixtures of different proportions on soil properties in relatively long-term experiments in agroforestry systems.
In this study, soil chemical and microbial properties of different mixed proportions of larch and A. elata litter were compared over the 365-d incubation, which will provide evidence for our previous field study (a degraded larch soil was ameliorated after > 5 years since inter-planting), and references for the effects of other agroforestry systems on soil properties. It is hypothesized that soil chemical and microbial properties will improve with the increase of A. elata in the litter mixtures.
Materials and methods
Study site
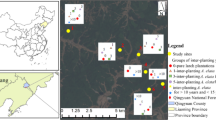
The study was carried out on the Qingyuan Forest CERN, National Observation and Research Station, Liaoning Province, China (41°51′ N, 124°54′ E, 500 − 1100 m a.s.l.). The region has a temperate, continental monsoon climate (warm and humid summers, cold and dry winters) with annual rainfall in 700–850 mm, with more than 80% falling in summer. Mean annual temperature ranges from 3.9 to 5.4 °C. A frost-free period lasts approximately 130 d. Soils are typical forest brown soil (Udalfs), with 25.6% of sand, 51.2% of silt, and 23.2% of clay. Historically, more than 70% of the primary forests in northeast China have been destroyed by anthropogenic activities (e.g. logging) or by extreme natural causes (e.g. snow and wind), and secondary forests were formed through natural regeneration of native broadleaved species. Since the 1950s, patches of secondary forests have been converted to larch plantations for timber production (2.6 × 106 ha) (Gao et al. 2018). The management strategies lasted for decades until they shifted from pursuing timber production to providing ecological services (e.g. improving soil quality), which resulted in economic losses to local foresters (Li and Zhou 2000). To obtain economic benefits in the short-term, A. elata (a local cash crop shrub) was inter-planted at 1 m × 1 m spacing, creating a larch-A. elata agroforestry system (LAAS). The LAAS used for this study was selected from six spatially separated sites in Qingyuan Forest. Each site consisted of paired stands of LAAS with as many inter-planting years of A. elata as logistically possible. Some LAAS stands shared the same larch plantation because of their closeness (details in Fig. 1 and Table S2) and adjacent larch plantations (400–1600 m), from similar ages and sizes of trees, geographical locations, and soil parent material (Table S2). In each paired stand, three replicates 20 m × 20 m plots, separated by > 80 m, were established to represent the characteristics of the stand, resulting in sixteen paired stands with 66 plots from 6 study sites (Fig. 1). Thickness of the litter layer ranged from 3.9 to 6.2 cm. The LAAS was managed according to standard practices, e.g. before and after the first 2 years of inter-planting, shrubs and herbaceous plants were removed to ensure the best survival of A. elata, and then clear-cut every 5 years to facilitate the harvest of tender shoots or terminal buds once or twice a year from late April to early May three years after inter-planting. The effects of harvesting terminal buds on A. elata input to the soil was negligible because of their low proportion in the whole plant biomass (approximately 2.2% for 3 years, 1.1% for 5 years, and 0.6% for > 10 years of inter-planting). The shrub layer of LAAS included A. elata and other naturally regenerated species such as Euonymus alatus (Thunb.) Sieb. and Lonicera japonica Thunb. The herbaceous layer of LAAS included Carex spp., Athyrium multidentatum (Döll) Ching, Sanicula chinensis Bunge, Rubus crataegifolius Bunge, Geranium wilfordii Maxim., and Rubia sylvatica (Maxim.) Nakai.
Soil and litter collection
Soil used for incubation was collected from 20 m × 20 m plots in 6 spatial sites with each site adjacent to LAAS (Table S2 and Fig. 1). After handpicking the litter layer and removing visible debris (e.g. plant roots and stones), the upper 10 cm the soil was randomly sampled at 9 points and pooled into one homogeneous sample of each plot. The pooled samples were passed through a 2-mm sieve and stored at 4 ℃ until the incubation experiment was set up.
Litter (larch and A. elata) for incubation was collected from one of the LAAS plots inter-planted with A. elata for 5 years, adjacent to larch plantations. After removing any diseased and insect-infested and rotten leaves, fresh leaves were collected in September–December 2019 at the time of natural abscission. The leaves were air-dried and cut into 1-cm pieces (only A. elata leaves were cut, larch leaves were not cut) and stored in paper bags until the incubation experiment started. The initial chemical properties of the litter are listed in Table 1.
Microcosm design and incubation
In the microcosm experiment, six litter mixtures were carried out with larch litter alone (10L:0A), A. elata litter alone (0L:10A), and litter mixtures of larch and A. elata (8L:2A, 6L:4A, 5L:5A, and 4L:6A), which represent the proportions of larch and A. elata in LAAS with different inter-planting years (1, 3, 5 and > 10 years). Soil without litter (CK) was used as a control.
Before incubation microcosms were set up, the soil was pre-incubated for a week at 25 ℃ and 60% water holding capacity to recover soil microbial activity. After pre-incubation, litter or litter mixtures mixed with predefined proportions were added on soil (20 g dry mass, with litter: soil = 1: 100) in 80 mm high × 67 mm diameter containers sealed with plastic wrap and incubated for 365 d at 25 ℃ under light shielding. The incubated microcosms also included a 10-mL centrifuge tube with 5 mL NaOH (0.5 M) used for measuring C mineralization. Each treatment had six replicates as the soil used for incubation was collected from six sites. During the incubation process, soil moisture was maintained by weighing the microcosms every 3 d and adding distilled water if necessary (Almagro et al. 2021).
The C mineralization was measured as CO2 evolution at 3, 7, 15, 30, 60, 90, 120, 180, 287, 328, 342 and 365 d of incubation. The incubated soil of each microcosm was sampled at the end of incubation. After sampling, soils from microcosms were mixed thoroughly after removing the litter, and stored at 4 °C until further analyses.
Litter and soil properties analysis
Total C and N of litter and soil were determined by dry combustion analysis (vario EL III elemental analyzer). Concentration of P, K, Ca, Mg, Fe, Mn, Zn and Cu of litter were determined by atomic absorption spectrophotometer (5100 ICP-OES) digested through concentrated HNO3–HClO4. The lignin concentration of litter was measured according to Wang et al. (2015).
Total P of soil was extracted by the H2SO4-HClO4 digestion method and measured by the ascorbic acid-molybdenum blue method (Olsen and Sommers 1982). Available P (Av-P) (extracted by 0.5 M NaHCO3, pH = 8.5, 0.5 h) of soil was determined using the method of John (1970). Soil mineral N (e.g. NH4+-N and NO3−-N) was determined by an auto analyzer (AutoAnalyzer III, Bran + Luebbe GmbH, Germany). Microbial biomass carbon (MBC), and microbial biomass nitrogen (MBN) were determined using the chloroform fumigation extraction method (Brookes et al. 1985; Vance et al. 1987). The evolved CO2 released in NaOH was measured by the method of back titration with 1 M HCl, which used phenolphthalein as an indicator (Yang and Zhu 2015).
Extracellular enzyme activities involved in C, N, and P cycling processes, including four hydrolases (e.g. BG, CB, NAG, and AP), and two oxidases (e.g. PPO and PER) were measured. Enzyme activities, in nmol h−1 g−1 were determined according to German et al. (2011).
Data analysis
Relative values (RV) were used to reveal differences in soil chemical and microbial properties among different litter mixtures. RV was calculated as follows:
where \(RV\) is the relative values of soil chemical and microbiological properties; \({P}_{i}\) is the soil chemical and microbiological properties of the different litter mixed proportions, \({K}_{0}\) is the soil chemical and microbiological properties of the controls.
One-way ANOVA followed by Tukey’s test evaluated the impact of litter mixtures on soil properties. Repeated ANOVA measurements tested the differences of CO2 evolution among litter mixtures. Pearson correlation analysis was used to examine relationships among litter mixtures, initial litter chemical properties and soil properties. Before analysis, the normality of variables was checked using the Shapiro–Wilk test, and the homogeneity of variances was examined using Levene’s test. All the statistical analyses were performed using IBM SPSS (version 26.0; IBM Corp., Armonk, NY, USA) with a significance level of P < 0.05.
Results
Initial chemical properties of litters
Most of the initial chemical properties of the two leaf litters used in this study varied significantly (P < 0.05) (Table1). Particularly, concentrations of N, P, K, Ca, Mg, Mn, Zn, and Cu in A. elata litter were 1.3–4.3 times that of larch litter. In contrast, concentrations of Fe and lignin, and ratios of C/N and lignin/N in larch litter were 1.6–6.3 times that of A. elata litter (Table 1).
Impacts of litter decomposition on soil chemical properties
After 365-d incubation, levels of soil total carbon, nitrogen and phosphorous of the larch litter alone (10L:0A) were significantly higher than that of the controls (P < 0.05), which increased by 2.6%, 6.1% and 5.3%, respectively. However, there were no significant differences among litter mixed proportions (P > 0.05) (Fig. 2), except for the A. elata litter alone (0L:10A); it was significantly higher that of other litter mixtures (P < 0.05).
Soil total carbon, nitrogen, and phosphorous after 365-d incubation for litter mixtures of larch (L) and A. elata (A) with different proportions. When the 95% confidence interval of TC, TN and TP spans 0, it means that there is no significant difference among the TC, TN and TP and CK (only soil without any litter added). When TC, TN and TP are significantly larger (smaller) than 0, it means that the TC, TN and TP of litter mixtures of larch (L) and A. elata (A) with different proportions are significantly higher (lower) than that of the CK. Bars indicated SE (n = 6)
Compared with the CK, concentrations of mineral N (Mi-N) and available P (Av-P) of all litter mixtures increased after 365 d of incubation, which increased with the increase of the litter proportion of A. elata in the litter mixtures (Fig. 3). For Mi-N, the descending order of the significant differences among different mixtures were: 0L:10A (226.2 mg kg−1) > 4L:6A and 5L:5A (133.7–147.8 mg kg−1) > 6L:4A and 8 L:2A (79.8–94.8 mg kg−1) > 10L:0A (37.8 mg kg−1) (P < 0.05) (Fig. 3a). For Av-P, the 0L:10A, 4L:6A and 5L:5A were all significantly higher than that of 8L:2A and 10L:0A (P < 0.05), while no significant differences were found among 4L:6A, 5L:5A and 0L:10A or 6L:4A (P > 0.05) (Fig. 3b). In addition, compared with the larch litter alone (10L:0A), levels of Mi-N and Av-P of litter mixtures (8L: 2A, 6L: 4A, 5L: 5A and 4L: 6A) increased by 7.7–20.2% and 7.6–20.9%, respectively (Fig. 3).
Soil mineral nitrogen and available phosphorous after 365-d incubation for litter mixtures of larch (L) and A. elata (A) with different proportions. When the 95% confidence interval of Mi-N and Av-P spans 0, it means that there is no significant difference among the Mi-N and Av-P and CK (only soil without any litter added). When Mi-N and Av-P are significantly larger (smaller) than 0, it means that the Mi-N and Av-P of litter mixtures of larch (L) and A. elata (A) with different proportions are significantly higher (lower) than that of the CK. Bars indicated SE (n = 6)
Impacts of litter decomposition on soil microbial properties
Microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) showed similar trends, increasing with the increase of the litter proportion of A. elata (Fig. 4a and b). For MBC, the descending order of the significant differences was: 0L: 10A and 4L: 6A > 5L: 5A, 4L: 6A and 8L: 2A > 10L: 0A (P < 0.05) (Fig. 4a). For MBN, the descending order was: 0L:10A > 4L: 6A and 5L: 5A > 4L: 6A and 8L: 2A > 10L: 0A (P < 0.05) (Fig. 4b). Notably, compared with the larch litter alone (10L:0A), concentrations of MBC and MBN of litter mixtures (8L: 2A, 6L: 4A, 5L: 5A and 4L: 6A) increased by 45.2–84.5% and 113.6–295.3%, respectively (Fig. 4a and b).
Soil microbial biomass C a and N b after 365-d incubation and the cumulative C mineralization c during decomposition process for litter mixtures of larch (L) and A. elata (A) with different proportions. When the 95% confidence interval of MBC, MBN and CO2-C spans 0, it means that there is no significant difference among the MBC, MBN and CO2–C and CK (only soil without any litter added). When MBC, MBN and CO2–C are significantly larger (smaller) than 0, it means that the MBC, MBN and CO2–C of litter mixtures of larch (L) and A. elata (A) with different proportions are significantly higher (lower) than that of the CK. Bars indicated SE (n = 6)
As decomposition proceeded, the cumulative C mineralization (C–CO2) of all litter mixtures gradually increased, all of which were significantly higher than the CK (P < 0.05) (Fig. 4c). During the 365 d of incubation, levels of C–CO2 of all litter mixtures significantly increased with the increase of A. elata in the litter mixtures (P < 0.05). Similarly, the descending order of the significant differences of levels of C–CO2 after 365-d incubation was: 0L: 10A and 4L: 6A > 5L: 5A and 4L: 6A > 8L: 2A and 10L: 0A (P < 0.05). Compared with the larch litter alone (10L: 0A), the concentration of C–CO2 of the mixtures (8L: 2A, 6L: 4A, 5L: 5A and 4L: 6A) increased by 37.7–92.9% (Fig. 4c).
Similarly, after 365-d incubation, extracellular enzyme activities of all litter mixtures were higher than that of the CK (P < 0.05), and increased with the increase of A. elata in the litter mixtures (Fig. 4). Compared with the larch litter alone (10L: 0A), activities of C cycle—related enzymes (e.g. BG and CB), N cycle related enzyme (e.g. NAG), P cycle related enzyme (e.g. AP) and oxidases (e.g. PPO and PER) of litter mixtures (8L: 2A, 6L: 4A, 5L: 5A and 4L: 6A) increased by 34.5–72.4%, 33.2–103.7%, 74.4–142.7% and 23.2–433.5%, respectively (Fig. 5).
Soil enzyme activities after 365-d incubation for litter mixtures of larch (L) and A. elata (A) decomposition process. When the 95% confidence interval of soil enzyme activities spans 0, it means that there is no significant difference among the soil enzyme activities and CK (only soil without any litter added). When soil enzyme activities are significantly larger (smaller) than 0, it means that the soil enzyme activities of litter mixtures of larch (L) and A. elata (A) with different proportions are significantly higher (lower) than that of the CK. Bars indicated SE (n = 6)
Relationships among litter initial chemical properties, litter mixtures, and soil biochemical properties
Most of soil chemical properties (Mi-N and Av-P) and microbial properties (MBC, MBN, BG, NAG, AP, and PPO) were significantly correlated with litter mixtures and initial chemical properties (P < 0.05 or P < 0.01) (Fig. 6). Most of the soil chemical and microbial properties were positively correlated with litter mixed proportions and the initial concentrations of N, P, K, Ca, Mg, Mn, Zn and Cu in the litter (P < 0.05), while they were negatively correlated with initial concentrations of carbon, Fe and lignin, carbon/nitrogen ratio and lignin/nitrogen ratio (P < 0.05).
The relationships among litter mixed proportions, litter initial chemical properties and soil biochemical properties (n = 6). Note: LMP, C, N, P, K, Ca, Mg, Fe, Mn, Zn, Cu, Lignin, C/N, and Lignin/N means litter mixed proportions, concentrations of carbon, nitrogen, phosphorus, potassium, calcium, magnesium, manganese, zinc, copper, lignin, carbon/nitrogen ratio, and lignin/nitrogen in initial leaf litter; respectively; TC, TN, TP, Mi-N, Av-P, C–CO2, MBC, MBN, BG, CB, NAG, AP, PPO and PER means total carbon, nitrogen, phosphorus, mineral N, available P, the cumulative C mineralization, microbial biomass carbon, microbial biomass nitrogen, β-glucosidase, β-cellobiohydrolase, N-acetyl-β-glucosaminidase, acid phosphatase, phenol oxidase and peroxidase, respectively. *, P < 0.05, **, P < 0.01
Discussion
Influence of litter decomposition on soil chemical properties
Of all the soil nutrients studied, including TC, TN, TP, Mi-N, and Av-P, differences were observed mainly in mineral N and available P, and not in TC, TN, and TP [except for the A. elata leaf litter alone (0L: 10A)]. The results were consistent with our previous field study (Gao et al. 2022) which indicated that levels of soil TC, TN, TP did not change, even in the humus layer and upper 10-cm depth when A. elata was inter-planted in larch plantations over 10 years, in which the total litterfall increased by 60.1 g m−2 compared with pure larch plantations (Table S1). Therefore, it is not surprising that there was no significant change in total soil nutrients with only 0.2 g litter added after 365-d incubation. Soil TC, TN, and TP increased by 2.6%, 6.1% and 5.2% in A. elata litter alone (0L: 10A), respectively. This may be attributed to: (1) the higher decomposition rate of A. elata, of which only 24% remained mass after 365-d incubation (Fig. S1); and (2) the higher nutrients released from the A. elata litter (levels of total T, N, and P were 29.2, 2.2 and 12.2 g kg−1), 3.9, 3.0, 3.7 times that of larch litter, respectively (Table 1).
After 365-d incubation, all litter mixtures showed net N mineralization, which was quite different from previous studies of the impacts of litter decomposition on soil properties. Contrary to our results, Yang and Zhu (2015) reported net N immobilization following the addition of five different leaf litters [Fraxinus chinensis subsp. rhynchophylla (Hance) E., Fraxinus mandshurica Rupr., Acer pictum subsp. mono (Maxim.) H. Ohashi, Quercus mongolica Fisch. ex Ledeb, Juglans mandshurica Maxim] in 6 weeks of incubation. Chen et al. (2018) also found net N immobilization following application of four different leaf litters [Juglans mandshurica Maxim, Carex moorcroftii Falc. ex Boott, Artemisia nanschanica Krasch., Leontopodium pusillum (Beauv.) Hand.—Mazz.] individually or combined randomly in a 120-d incubation study. The different results may be attributed to: (1) different sampling frequency and incubation time; and (2) the different species used in litter decomposition incubation. In fact, Chen et al. (2018) noted that the chemical diversity of litter and incubation time were important factors in litter decomposition. Additionally, Haynes (1986) reported that net N mineralization occurred only when the N concentration of litter was higher than 2% and the C/N ratio less than 20. The N concentration and C/N ratio were not measured in this study since remaining litter at the end of incubation was limited. However, the N concentration of the initial leaf litter ranged from 7.4 (larch leaf litter) to 29.2 g kg−1 (A. elata leaf litter), and the C/N ratios from 15.0 (A. elata litter) to 58.6 (larch litter) (Table 1). Zhang et al. (2019) concluded that N and P concentrations of the initial litter were positively related to decomposition rate, whereas the concentration of lignin and cellulose were negatively related. In addition, the rate of nutrient release (e.g. N and P) varied with litter mixed proportions, decomposition stages and microbial activities at different stages (Naeem et al. 2021). Therefore, it is speculated that soil N immobilization was a response to the requirements of growth and reproduction of microorganisms in earlier decomposition stages (Ilstedt and Singh 2005). However, N concentrations and C/N ratios reflected N mineralization after 365-d litter decomposition, leading to nutrients released and resulting in an increase in available nutrients (Zhou et al. 2016). The extent of the increases for mineral N and available P in different litter mixtures may be attributed to the litter mix proportions, initial chemical properties of the litter, and microbial activities (Ilstedt and Singh 2005; Zeng et al. 2021). Our correlation analysis among soil mineral N and available P, litter mix proportions and initial chemical properties of leaf litter also supports this (Fig. 6).
Influence of litter decomposition on soil microbial properties
Soil respiration reflects the activities and metabolism of microorganisms which utilize the easily decomposed compounds characterized by organic acids and labile C to meet growth and reproduction needs (Bhatnagar et al. 2018). For example, labile plant constituents were the dominant source of microbial products as they were utilized more efficiently by microbes (Cotrufo et al. 2013), and litter with high nitrogen content, low lignin/N and C/N ratios was associated with higher decomposition leading to rapid nutrient mobility for microorganisms (Prescott 2010). In our study, the N content of A. elata litter (29.2 g kg−1) was significantly higher than that of larch litter (7.4 g kg−1), and the lignin content of A. elata litter (225.0 g kg−1) significantly lower than that of larch litter (360.5 g kg−1). Lignin/N and C/N ratios of larch litter were 3.9 and 6.3 times that of A. elata litter, respectively (Table 1). This resulted in higher cumulative C mineralization in litter mixtures with higher proportions of A. elata. Additionally, correlation analysis also indicated that the cumulative C mineralization was positively related to the litter mix proportions and the initial levels of N, P, K, Ca, Mg, Mn, Zn, and Cu in litter, while it was negatively related to initial concentrations of C, Fe, lignin and C/N and lignin/N ratios (Fig. 6). Research has shown that litter decay may be dominated by microorganisms via binding to metal cations (e.g. K, Ca, Mg, Fe, and Mn) (Ganjegunte et al. 2005; Preston et al. 2009; Keiluweit et al. 2015; Wang et al. 2017; Sun et al. 2019). However, much research has focused mainly on the impact of C, N, P, and K, Ca, and Mg content on litter decomposition rate, rarely on the influence of trace elements (e.g. Mn, Fe, Zn, and Cu). Therefore, further studies on litter decomposition should give attention to trace elements. Garcia et al. (2005) reported that microbial respiration was positively correlated with microbial activities and soil nutrients, and our results also verified that the levels of mineral N, available P, microbial biomass carbon, microbial biomass nitrogen and enzyme activities increased with increase in the proportion of A. elata in the litter mixtures (Figs. 3, 4 and 5).
Microorganisms rapidly utilize nutrients stored in plant cells after litter addition, thus providing energy for themselves growth, reproduction and secretion enzymes for degradation of litter (Sall et al. 2003). Therefore, litter quality and quantity exert a strong influence on soil microbial characteristics (e.g. the microbial community, microbial biomass, and activities), for which a higher quality litter would supply more nutrients more rapidly for microorganisms than lower quality litter (Aka and Darici 2005; Teklay et al. 2007). This could explain why the mixtures of higher proportions of A. elata in litter had higher microbial biomass and enzyme activities in our study. In turn, this would stimulate nutrients to entering the soil more rapidly (Aggangan et al. 1999). Additionally, the physical properties of the litter also had an important influence on soil microbial properties (Joly et al. 2016; Xiao et al. 2019). Leaves of A. elata are thin and fragile, more conductive to growth and functions for microorganisms leading to higher biomass and activities. In contrast, leaves of larch are thick and tough to breakdown, resulting in lower microbial biomass and activities.
This study concerned the effects of litter mixtures with different proportions of larch and A. elata on soil biochemical properties at the end of incubation period (365 d). However, previous studies noted that the stage of decomposition was an important factor as the microbial biomass, enzyme activities and substrates were decomposition stage dependent (Hu et al. 2006; Weand et al. 2010; Wu et al. 2014). In earlier decomposition stages, hydrolase (e.g. cellulolytic enzymes) play an important role to degrade to liable compounds, while oxidase (e.g. polyphenol oxidase) becomes more important to decompose recalcitrant materials in later decomposition stages (Kourtev et al. 2002). This influences the different substrates derived from litter in different decomposition stages and ultimately leads to differences in nutrients entering the soil. Additionally, the litter mixture also affects soil biochemical properties through the release of nutrients (Gartner and Cardon 2004). As commonly known, additive-effects and non-additive effects (including synergistic and antagonistic effects) exist in mixed litter decomposition (Perez-Harguindeguy et al. 2008). Studies have reported that research related to litter decomposition showed significant mixing effects, which varied with the decomposition stage (Hector et al. 2000; Berglund et al. 2013; Chen et al. 2017). For example, Wu et al. (2014) noted that additive effects were observed in initial stages, while positive non-additive effects (synergistic) were in the mid stages, and negative non-additive effects (antagonistic) in later stages. Therefore, different results may occur at different sampling stages and care given when speculating from our results. The mixed effects of different stages should also be considered.
Although there are some limitations to laboratory microcosm experiments, they are also considered as an important component of a set of appropriate experimental methods to provide explanations and supplements for field studies due to their short experiment period and strong controllability (Fukami and Wardle 2005). In this study, the main interest was on the differences of the effect of litter mixtures of two coexisting species on soil biochemical properties in larch-A. elata agroforestry systems. Therefore, temperature and moisture were not considered, although these two factors are important in litter decomposition (Cornwell et al. 2006). The laboratory microcosms exactly met our requirements. However, it must be acknowledged that they were associated with additional restrained compared with natural field decomposition processes: (1) interactions between soil meso and macro fauna and leaf litter were not considered (Hättenschwiler et al. 2005); (2) the effects of mixing leaf litter, branches, stems, and roots were not considered (Cotrufo et al. 2010); and (3) other environment factors, such as dry and wet deposition, freezing, thawing, nitrogen fixation, and sedimentation were not considered (Bindraban et al. 2000). As such, our results could differ from field experiments which would be under conditions of the entire decomposer community and environment factors. However, trends of the impacts of the larch-A. elata agroforestry systems with different inter-planting years of A. elata associated with different litter proportions (Table S1), on soil biochemical properties in our prior field studies were similar to these microcosm experiments, which suggesting that our laboratory microcosm results may be field relevant (Fig. S2). Further, the improvement of soil chemical and microbiological properties between the laboratory incubation experiments and field experiments were compared, and the results showed that the extent of improvement of most chemical and microbial properties of the microcosm experiments were significantly higher than that of field experiments (Table S3). This suggests that the results from field studies may be more conservative than laboratory microcosm studies. Overall, while it is important to recognize that this study demonstrates a potential ecological function of leaf litter decomposition for improvement of soil quality or fertility in the absence of other important field variables, it is anticipated that our results might stimulate more studies. For example, research on the impact of litter decomposition on various soil properties and the underlying mechanisms of trace elements dynamics in litter might be examined. Sampling more frequently and the effects of litter mixtures should be given more attention, which could provide a reference for more comprehensive understanding of the continuous system of “plant-litter-soil”.
Conclusion
Though the improvements to some soil characteristics (increased total carbon, nitrogen and phosphorus levels) may not be immediately apparent after 365-d incubation experiment of litter mixtures of larch and A. elata, the A. elata litter can ameliorate degraded larch soil (such as enhancing mineral nitrogen, available phosphorus, microbial biomass and enzyme activities), and the extent of the improvement depends on the amount of A. elata litter in the litter mixtures, which is consistent with our hypothesis. However, caution must be made when speculating possible results of field experiments from our microcosm incubation experiments because improvement of soil chemical and microbiological properties of the microcosm experiments were significantly higher than those of field experiments. Therefore, it is recommended that field experiments, under the conditions of the wider decomposer community and numerous environment factors, should be the focus in future research.
Change history
18 October 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11676-022-01547-5
References
Aggangan RT, O’Connell AM, McGrath JF, Dell B (1999) The effects of Eucalyptus globulus Labill. leaf letter on C and N mineralization in soils from pasture and native forest. Soil Biol Biochem 31(11):1481–1487. https://doi.org/10.1016/S0038-0717(99)00052-8
Aka H, Darici C (2005) Carbon and nitrogen mineralization in carob soils with Kermes oak and Aleppo pine leaf litter. Eur J Soil Biol 41(1):31–38. https://doi.org/10.1016/j.ejsobi.2005.05.001
Almagro M, Ruiz-Navarro A, Díaz-Pereira E, Albaladejo J, Martínez-Mena M (2021) Plant residue chemical quality modulates the soil microbial response related to decomposition and soil organic carbon and nitrogen stabilization in a rainfed Mediterranean agroecosystem. Soil Biol Biochem 156:108198. https://doi.org/10.1016/j.soilbio.2021.108198
Berglund SL, Agren GI, Ekblad A (2013) Carbon and nitrogen transfer in leaf litter mixtures. Soil Biol Biochem 57:341–348. https://doi.org/10.1016/j.soilbio.2012.09.015
Bhatnagar JM, Peay KG, Treseder KK (2018) Litter chemistry influences decomposition through activity of specific microbial functional guilds. Ecol Monogr 88(3):429–444. https://doi.org/10.1002/ecm.1303
Bindraban PS, Stoorvogel JJ, Jansen DM, Vlaming J, Groot JJR (2000) Land quality indicators for sustainable land management: proposed method for yield gap and soil nutrient balance. Agr Ecosyst Environ 81(2):103–112. https://doi.org/10.1016/S0167-8809(00)00184-5
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil-nitrogen-a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Cabrera M, Kissel D, Vigil M (2005) Nitrogen mineralization from organic residues: research opportunities. J Environ Qual 34:75–79. https://doi.org/10.2134/JEQ2005.0075
Chen Y, Ma S, Sun J, Wang X, Cheng G, Lu X (2017) Chemical diversity and incubation time affect non-additive responses of soil carbon and nitrogen cycling to litter mixtures from an alpine steppe soil. Soil Biol Biochem 109:124–134. https://doi.org/10.1016/j.soilbio.2017.02.007
Chen Y, Ma S, Liu J, Cheng G, Lu X (2018) Soil C and N dynamics and their non-additive responses to litter mixture under different moisture conditions from an alpine steppe soil, Northern Tibet. Soil Biol Biochem 125:231–238. https://doi.org/10.1016/j.soilbio.2018.07.016
Cornwell WK, Schwilk DW, Ackerly DD (2006) A trait-based test for habitat filtering: convex hull volume. Ecology 87(6):1465–1471. https://doi.org/10.1890/0012-9658(2006)87[1465:ATTFHF]2.0.CO;2
Cotrufo MF, Galdo I, Piermatteo D (2010) Litter decomposition: concepts, methods and future perspectives. Soil Carbon Dyn: Integr Methodol. https://doi.org/10.1017/CBO9780511711794.006
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biol 19(4):988–995. https://doi.org/10.1111/gcb.12113
Fukami T, Wardle DA (2005) Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients. Proc R Soc b: Biol Sci 272(1577):2105–2115. https://doi.org/10.1098/rspb.2005.3277
Ganjegunte GK, Condron LM, Clinton PW, Davis MR (2005) Effects of mixing radiata pine needles and understory litters on decomposition and nutrients release. Biol Fert Soils 41(5):310–319. https://doi.org/10.1007/s00374-005-0851-x
Gao T, Zhu JJ, Yan QL, Deng SQ, Zheng X, Zhang JX, Shang GD (2018) Mapping growing stock volume and biomass carbon storage of larch plantations in Northeast China with L-band ALOS PALSAR backscatter mosaics. Int J Remote Sens 39(22):7978–7997. https://doi.org/10.1080/01431161.2018.1479793
Gao PZ, Zhu JJ, Yang K, Yan QL, Zhang JX, Yu LZ, Diao MM, Xu S (2022) Can larch-Aralia elata agroforestry systems improve the soil chemical and microbial properties of larch plantations? Agrofor Syst. https://doi.org/10.1007/s10457-022-00748-5
Garcia C, Roldan A, Hernandez T (2005) Ability of different plant species to promote microbiological processes in semiarid soil. Geoderma 124(1):193–202. https://doi.org/10.1016/j.geoderma.2004.04.013
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104(2):230–246. https://doi.org/10.1111/j.0030-1299.2004.12738.x
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Hättenschwiler S, Tiunov A, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218. https://doi.org/10.1146/annurev.ecolsys.36.112904.151932
Haynes RJ (1986) The decomposition process: mineralization, immobilization, humus formation, and degradation, chapter 2. Academic Press, Inc. pp 52–126
Hector A, Beale AJ, Minns A, Otway S, Lawton J (2000) Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos. https://doi.org/10.1034/j.1600-0706.2000.900217.x
Hu YL, Wang SL, Zeng DH (2006) Effects of single Chinese fir and mixed leaf litters on soil chemical, microbial properties and soil enzyme activities. Plant Soil 282(1):379–386. https://doi.org/10.1007/s11104-006-0004-5
Ilstedt U, Singh S (2005) Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol (ultisol) compared with organic compost. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2005.01.002
Jiang J, Li Y, Wang M, Zhou C, Cao G, Shi P, Song M (2013) Litter species traits, but not richness, contribute to carbon and nitrogen dynamics in an alpine meadow on the Tibetan Plateau. Plant Soil 373(1):931–941. https://doi.org/10.1007/s11104-013-1859-x
John MK (1970) Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci 109(4):214–0. https://doi.org/10.1097/00010694-197004000-00002
Joly F-X, Fromin N, Kiikkilä O, Hättenschwiler S (2016) Diversity of leaf litter leachates from temperate forest trees and its consequences for soil microbial activity. Biogeochemistry 129(3):373–388. https://doi.org/10.1007/s10533-016-0239-z
Keiluweit M, Nico P, Harmon Mark E, Mao J, Pett-Ridge J, Kleber M (2015) Long-term litter decomposition controlled by manganese redox cycling. Proc Natl Acad Sci 112(38):E5253–E5260. https://doi.org/10.1073/pnas.1508945112
Kourtev PS, Ehrenfeld JG, Huang WZ (2002) Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem 34(9):1207–1218. https://doi.org/10.1016/S0038-0717(02)00057-3
Kuebbing SE, Bradford MA (2019) The potential for mass ratio and trait divergence effects to explain idiosyncratic impacts of non-native invasive plants on carbon mineralization of decomposing leaf litter. Funct Ecol 33(6):1156–1171. https://doi.org/10.1111/1365-2435.13316
Kuiters AT (1990) Role of phenolic substances from decomposing forest litter in plant–soil interactions. Acta Botanica Neerlandica 39(4):329–348. https://doi.org/10.1111/j.1438-8677.1990.tb01412.x
Li CY, Zhou XF (2000) Status and future trends in plantation silviculture in China. Ambio 29(6):354–355. https://doi.org/10.1579/0044-7447-29.6.354
Li D, Peng S, Chen B (2013) The effects of leaf litter evenness on decomposition depend on which plant functional group is dominant. Plant Soil 365(1):255–266. https://doi.org/10.1007/s11104-012-1337-x
Liu SR, Li XM, Niu LM (1998) The degradation of soil fertility in pure larch plantations in the northeastern part of China. Ecol Eng 10(1):75–86. https://doi.org/10.1016/S0925-8574(97)10024-6
Makkonen M, Berg MP, van Logtestijn RSP, van Hal JR, Aerts R (2013) Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 122(7):987–997. https://doi.org/10.1111/j.1600-0706.2012.20750.x
Mason WL, Zhu JJ (2014) Silviculture of planted forests managed for multi-functional objectives: lessons from Chinese and British experiences. In: Trevor Fenning (ed) Challenges and opportunities for the world's forests in the 21st century. Springer, Dordrecht, Netherlands. pp 37–54
Mitchell J, Lockaby G, Brantley E (2011) Influence of Chinese privet (Ligustrum sinense) on decomposition and nutrient availability in riparian forests. Invasive Plant Sci Manag 4:437–447. https://doi.org/10.1614/IPSM-D-11-00020.1
Mukhopadhyay S, Joy VC (2010) Influence of leaf litter types on microbial functions and nutrient status of soil: ecological suitability of forest trees for afforestation in tropical laterite wastelands. Soil Biol Biochem 42(12):2306–2315. https://doi.org/10.1016/j.soilbio.2010.09.007
Naeem I, Asif T, Wu X, Hassan N, Yiming L, Wang H, Wang L, Wang D (2021) Species diversity induces idiosyncratic effects on litter decomposition in a degraded meadow steppe. Front Environ Sci 9:582409. https://doi.org/10.3389/fenvs.2021.582409
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (Eds). Methods of soil analysis. Part 2. Chemical and microbiological methods. Am Soc of Agron, Madison, pp 403–410
Pérez-Harguindeguy N, Blundo C, Gurvich D, Diaz S, Cuevas E (2008) More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through leaf chemistry. Plant Soil 303:151–159. https://doi.org/10.1007/s11104-007-9495-y
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochem 101(1–3):133–149. https://doi.org/10.1007/s10533-010-9439-0
Preston CM, Nault JR, Trofymow JA, Smyth C, Group CW (2009) Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 1. Elemental composition, tannins, phenolics, and proximate fractions. Ecosyst 12(7):1053–1077. https://doi.org/10.1007/s10021-009-9266-0
Sall SN, Masse D, Bernhard-Reversat F, Guisse A, Chotte J-L (2003) Microbial activities during the early stage of laboratory decomposition of tropical leaf litters: the effect of interactions between litter quality and exogenous inorganic nitrogen. Biol Fert Soils 39(2):103–111. https://doi.org/10.1007/s00374-003-0679-1
Sun T, Cui Y, Berg B, Zhang Q, Dong L, Wu Z, Zhang L (2019) A test of manganese effects on decomposition in forest and cropland sites. Soil Biol Biochem 129:178–183. https://doi.org/10.1016/j.soilbio.2018.11.018
Teklay T, Nordgren A, Nyberg G, Malmer A (2007) Carbon mineralization of leaves from four Ethiopian agroforestry species under laboratory and field conditions. Appl Soil Ecol 35(1):193–202. https://doi.org/10.1016/j.apsoil.2006.04.002
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Voříšková J, Dobiášová P, Šnajdr J, Vaněk D, Cajthaml T, Šantrůčková H, Baldrian P (2011) Chemical composition of litter affects the growth and enzyme production by the saprotrophic basidiomycete Hypholoma fasciculare. Fungal Ecol 4(6):417–426. https://doi.org/10.1016/j.funeco.2011.03.005
Wang C, Peng QD, Yang XC, Wang SM (2015) Researches on lignocellulose components of different black locust clones for producing bioethanol. J Cent South Univ For Technol 35(124–127):138. https://doi.org/10.14067/j.cnki.1673-923x.2015.06.023 (in Chinese)
Wang Y, Wang H, He J-S, Feng X (2017) Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nat Commun 8(1):15972. https://doi.org/10.1038/ncomms15972
Weand MP, Arthur MA, Lovett GM, McCulley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem 42(12):2161–2173. https://doi.org/10.1016/j.soilbio.2010.08.012
Weedon JT, Cornwell WK, Cornelissen JHC, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12(1):45–56. https://doi.org/10.1111/j.1461-0248.2008.01259.x
Whitmore AP (1996) Modelling the release and loss of nitrogen after vegetable crops. Njas-Wageningen J Life Sci 44:73–86. https://doi.org/10.1016/j.biosystemseng.2014.12.004
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90(12):3333–3341. https://doi.org/10.1890/08-2294.1
Wu F, Peng C, Yang W, Zhang J, Han Y, Mao T (2014) Admixture of alder (Alnus formosana) litter can improve the decomposition of eucalyptus (Eucalyptus grandis) litter. Soil Biol Biochem 73:115–121. https://doi.org/10.1016/j.soilbio.2014.02.018
Xiao W, Chen HYH, Kumar P, Chen C, Guan Q (2019) Multiple interactions between tree composition and diversity and microbial diversity underly litter decomposition. Geoderma 341:161–171. https://doi.org/10.1016/j.geoderma.2019.01.045
Yang K, Zhu JJ (2015) Impact of tree litter decomposition on soil biochemical properties obtained from a temperate secondary forest in Northeast China. J Soils Sediments 15(1):13–23. https://doi.org/10.1007/s11368-014-0975-4
Yang K, Zhu JJ, Zhang M, Yan QL, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol-Uk 3(3):175–182. https://doi.org/10.1093/jpe/rtq022
Yang K, Zhu JJ, Yan QL, Zhang JX (2012) Soil enzyme activities as potential indicators of soluble organic nitrogen pools in forest ecosystems of Northeast China. Ann Forest Sci 69(7):795–803. https://doi.org/10.1007/s13595-012-0198-z
Yang K, Shi W, Zhu JJ (2013) The impact of secondary forests conversion into larch plantations on soil chemical and microbiological properties. Plant Soil 368(1–2):535–546. https://doi.org/10.1007/s11104-012-1535-6
Zeng Q, Chen Z, Tan W (2021) Plant litter quality regulates soil eco-enzymatic stoichiometry and microbial nutrient limitation in a citrus orchard. Plant Soil 466(1):179–191. https://doi.org/10.1007/s11104-021-05025-5
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28(10):1469–1486. https://doi.org/10.1111/geb.12966
Zhou L, Cai L, He Z, Wang R, Wu P, Ma X (2016) Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ Sci Pollut Res Int 23(23):24135–24150. https://doi.org/10.1007/s11356-016-7624-y
Zhou S, Butenschoen O, Barantal S, Handa IT, Makkonen M, Vos V, Aerts R, Berg MP, McKie B, Van Ruijven J, Hättenschwiler S, Scheu S, Pérez-Harguindeguy N (2020) Decomposition of leaf litter mixtures across biomes: the role of litter identity, diversity and soil fauna. J Ecol 108(6):2283–2297. https://doi.org/10.1111/1365-2745.13452
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This project was supported financially by the National Natural Science Foundation of China (U1808201).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yu Lei
The original version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, P., Zhu, J., Yan, Q. et al. The amelioration of degraded larch (Larix olgensis) soil depends on the proportion of Aralia elata litter in larch-A. elata agroforestry systems. J. For. Res. 34, 1065–1076 (2023). https://doi.org/10.1007/s11676-022-01526-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-022-01526-w