Abstract
The introduction of trees in cropland may be a way to improve the mineral nitrogen (N) use efficiency since tree roots can intercept N leached below the crop rooting zone and recycle it as organic N. The aim of this study was to determine soil mineral N (SMN) and total N (STN) contents after 14 years of hybrid walnut tree growth in an agroforestry system. Soil cores were collected and analyses in mid-autumn 2009, in intercropped agroforestry (AF), pure tree (FC) and sole crop control (CC) plots. The SMN was significantly reduced in AF compared to CC (64, 58 and 51 % of reduction at 0.2, 1 and 2 m depth respectively). In the top 1 m of soil, the stock of SMN was 77.7 kg N ha−1 in CC versus 32.8 kg N ha−1 in AF. Trees in AF developed deeper fine roots than in FC, likely involved in the reduction of SMN when compared to CC. Despite this quantitative reduction, trees also progressively modified the form of mineral N in soil by decreasing the percentage of nitrate (NO3 −) in SMN, particularly in FC compared to CC, while AF was intermediate. The STN was not significantly different between AF and CC; but was higher in FC in the top soil, probably due to weeds and superficial tree root biomasses. Our results suggest that the introduction of hybrid walnut trees into cropland may be an efficient practice to reduce the potentially leachable N by winter rainfall.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to increase plant primary production to satisfy human food needs, the use of nitrogen (N) fertilizer in arable systems increased considerably worldwide from 5 × 106 Mg in 1950 to 9 × 107 Mg in 2000 (Vance 2001). The residual mineral N remaining in soil due to inefficient recovery by crops (Smil 1999) may be transferred into surface and underground waters through runoff and leaching. Agroforestry systems are defined as mixtures of trees and crops (Dupraz and Liagre 2008) and can be expected to modify mineral N fluxes in soils. Some common agroforestry tree species have deep roots (Jose et al. 2001) and may play a complementary role to crops in mineral N use efficiency by intercepting the mineral N leached below the crop rooting zone (Jose et al. 2000; Udawatta et al. 2002; Allen et al. 2004; Palma et al. 2007). In some situations, trees adapt their root system development because of water and nutrient competition and the phenological lag with crops. For instance, when deciduous trees open their buds in spring, winter crops planted in autumn (e.g. Triticum turgidum L. subsp. durum) have already taken up some water and mineral N from their own rooting zone, forcing the trees to develop deeper roots (Mulia and Dupraz 2006). Alternatively, when trees develop fine feeder roots in the topsoil, these roots can be pruned at early stages of the tree development to force tree roots to grow into deeper soil layers.
The mineral N captured by trees in deep soil layers is recycled, resulting in a more conservative N budget in agroforestry systems. Part of this captured N is stored in the biomass of trees, while part of it is returned to the soil as organic matter (OM) resulting from the decomposition of dead leaves, dead roots and pruned branches. For instance, over a 10-year period following the conversion of sugar cane fields into cocoa plantations, soil organic matter content (SOM) increased by 21 % under pruned leguminous Erythrina poeppigiana and by 9 % under unpruned non-leguminous Cordia alliodora (Beer et al. 1990). This additional supply of OM is likely to increase with the age of the stand and may occur in the topsoil as well as in the deeper soil horizons.
It is commonly assumed that N mineralization is very low in deep soil layers (Persson and Wiren 1995) mainly due to the low OM content and to the recalcitrance of the SOM. The partial relocation of the tree rooting zone into deep horizons may favor the release of labile C compounds known to be the limiting factor for soil N mineralization at this depth (Fontaine et al. 2007). In addition, deep tree roots increase OM input in deep horizons and may change the pattern of soil potential net N mineralization (PNM) at this depth. Babbar and Zak (1994) found higher rates of N mineralization in Costa Rica coffee plantations shaded by E. poeppigiana than in unshaded plantations. However, Harmand et al. (2007) showed that the inclusion of Eucalyptus in a coffee plantation did not affect the PNM of soil 7 years after planting.
Hybrid walnut trees (Juglans regia × nigra) are often used in temperate alley cropping systems for their economic value and high quality hardwood. It has been shown that they develop deep roots when intercropped with winter crops such as durum wheat (Mulia and Dupraz 2006) but their impact on mineral N interception at depth and on the SOM has not been investigated. The aim of this study was to determine the soil mineral N (SMN) and total N (STN) after 14 years of hybrid walnut tree (J. regia × nigra cv. NG23) growth in a temperate cropping system. We hypothesized that hybrid walnuts would reduce the SMN in autumn as trees are the main N sink in the soil since the crop harvest in summer. This is of relevance, as high rainfall in autumn and winter usually induces N leaching into water bodies. We also hypothesized that after 14 years under trees, the SOM in soil layers would increase due to cumulative litter from the tree leaves and roots since planting.
Materials and methods
Experimental site
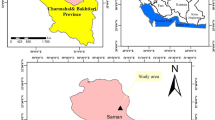
The experiment was carried out at the Restinclières farm in southern France (43°42′N, 3°51′E, elevation 61 m). The climate is sub-humid Mediterranean with an average annual temperature of 14.5 °C and an average annual rainfall of 880 mm (years 1996–2008). The soil is a deep calcic alluvial fluvisol (WRB 2006) with a silt loam texture and a mean pH of 8.4 in the 0.0–0.2 m layer and pH 8.6 below (Table 1). The clay content increased from 19.5 % in the upper layer to 25 % at 2 m soil depth. The CaCO3 content was 46 % in the 0.0–0.2 m soil layer and increased with depth. The mean C/N ratio was 12.2 and did not vary significantly throughout the soil profile (Table 1). Beyond 2 m depth, redoximorphic features such as mottles and bleaching formed by the oxidation and reduction of iron and/or manganese have been observed on the soil profile, indicating that the soil is subject to an alternation of unsaturated and saturated conditions.
In February 1995, 1 year old hybrid walnut trees (Juglans nigra × regia type NG23) were planted at a density of 192 trees ha−1 (13 × 4 m) in north–south oriented rows. Since then, they were intercropped with the following crop rotation: durum wheat for 2 or 4 years and rapeseed in 1998, 2001 and 2006. A strip of 1 m wide along the tree lines was not cultivated. Weeds were left to grow in this strip and were cut down annually. Half the trees were removed in February 2004, keeping only the biggest (96 trees ha−1). This plantation will be called the agroforestry plot or AF. At the same period of AF establishment, an uncropped hybrid walnut plot with a density of 204 trees ha−1 (7 × 7 m) to be referred to as a forestry control plot or FC and a sole crop control plot or CC, were also set up. The CC plot was situated in the headland at the edge of AF (Fig. 1a) and was conducted with exactly the same crop rotation. In order to avoid any possible influence of trees due to the proximity between the two plots, the CC plot was sited at least 10 m from the closest trees in AF. Both AF and CC received 150 kg N ha−1 year−1 of N fertilizer as NH4 +NO3 − but no N fertilizer was applied in FC. The three plots were bordered on their western side by the Lez river and the direction of the water flow was parallel to the tree rows (Fig. 1a).
Schematic representation of the soil coring location: (a) location of the Restinclières experimental site and spatial representation of the different plots, (b) position of soil cores within forestry and (c) agroforestry plots. AF, FC and CC mean agroforestry, forestry control and crop control plot, respectively. Black or white symbols representing trees are not to scale
In 2009, mean tree height and diameter at breast height (DBH) were 10.0 m and 0.20 m respectively in AF. Soil tillage consisted of a summer superficial stubble plowing after the crop harvest and either a soil preparation by plowing (20 cm depth) before durum wheat sowing in November or a shallow tillage when the crop changed. During the first 5 years, the farmers also sub-soiled to 30 cm depth in summer to encourage water infiltration. Sub-soiling during the first years and soil plowings pruned tree roots in the top 10–20 cm of soil in the alleys (Mulia and Dupraz 2006).
In FC, the mean tree height and DBH were 8.4 m and 0.13 m respectively. Soil was disked twice a year in order to control weeds. In 2002, 4 and 2 piezometer tubes were installed in AF and FC respectively in order to monitor the groundwater table fluctuations. Since then, the groundwater level was monitored at 15 day intervals using a water level indicator (Hydrotechnik GmbH—D-Oberguenzburg). The daily mean groundwater level was calculated from data collected from 2003 to 2009 and is presented in Fig. 2. It reached a minimum at the end of August (−3.0 m), after which it increased and reached a maximum at the end of January (−1.4 m). The maximum depth of the groundwater recorded for the 7 years of monitoring was 4.05 m in August 2006 and the minimum was at the soil surface, recorded at the end of January 2009.
Before the plantation of the trees in 1995, the soil homogeneity was tested for texture, bulk density and mineral nitrogen content (down to 0.3 m depth) throughout the experimental site (Dufour et al. 2013). As tree growth variability is also an integrated indicator of soil heterogeneity, Dupraz et al. (2000) confirmed that the soil homogeneity did not outweigh tree growth variability in our experimental plot. Therefore, the present day differences on soil variables observed between treatments can be considered as due to plot management (AF vs. FC vs. CC), as recently confirmed by Cardinael et al. (2015) for soil C content.
Soil sampling and analysis
Soils were sampled in October 2009. Two walnut trees of roughly average size were chosen in AF and in FC. Under each tree, soil cores were taken at 2, 4, 6 and 7.2 m from the tree trunk in AF and at 1.75 and 3.5 m in FC (Fig. 1b, c). Soil cores (8.5 cm diameter and 2 m depth) were collected with a micro caterpillar driller equipped with an auger (n = 10 and n = 8 in AF and FC, respectively). In CC, 3 soil cores were randomly taken at least 3 m away from each other.
The soil cores were cut into 5 layers at 0.2, 0.4, 0.6, 1 and 2 m depth, then sieved at 4 mm and stored at 6 °C for 18 days before analysis. All dead and living roots (coarse and fine) were removed manually from the samples.
The soil water content was determined by oven drying a subsample of soil at 105 °C for 48 h. Soil pH was measured in deionized water with a glass electrode by diluting 5 g of air-dried and sieved soil (<2 mm) in 25 g of water (ISO norm 10390). The soil total N (STN) and organic C contents were measured by dry combustion of a finely ground subsample of soil according to the ISO norms 13878 and 10694 respectively. The SOM was estimated through multiplying the organic C value by the Van Bemmelen factor of 1.724 (Rosell et al. 2001), based on the assumption that the OM contains 58 % of organic C. The nitrate (NO3–N) and ammonium (NH4–N) content of soil were determined by shaking 20 g of soil with 100 ml of a 0.5 M K2SO4 solution for 1 h and then filtering. The NO3–N and NH4–N concentrations of extracts were measured using continuous flow colorimetry (San++, Skalar). The SMN was the sum of NO3–N and NH4–N presented as mg N per kg of dry soil. The soil C/N values were calculated as the ratio between the soil organic C (g kg−1) and organic N content (g kg−1) itself resulting from the difference between STN and SMN.
The soil potential net N nitrification (PNN) and mineralization (PNM) were determined by 51 days of laboratory incubation. A subsample (200 g) of each soil sample was put into plastic containers with airtight lids at 25 °C in the dark. All samples were adjusted to pF 2.5 using the pedotransfer function developed by Wösten et al. (1999). Containers were opened for a few minutes twice a week to avoid anaerobic conditions during the incubation. Mineral N (NH4 + and NO3 −) was extracted at the beginning and every 17 days until the end of the incubation. The PNM was defined as the daily amount of mineral N produced during the incubation period, expressed as mg N kg−1 soil day−1. It was calculated as the slope of the linear regression between the amount of mineral N measured at different dates from the beginning of incubation and the number of days for which the cores had been incubated. The PNN was calculated in the same way as PNM but it concerned only nitrate N.
The SMN, measured at the beginning of incubation (\({\text{NO}}_{{3_{incub} }}\) and \({\text{NH}}_{{4_{incub} }}\)), did not fit the SMN at the sampling date (\({\text{NO}}_{{3_{sampling} }}\) and \({\text{NH}}_{{4_{sampling} }}\)) due to possible N mineralization during the 18 days in the cold room (6 °C). To estimate the SMN at the sampling date, duplicates of some samples were incubated in the cold room and the mineral N production pattern was characterized after 45 days. The SMN at the sampling date was estimated by the following linear regressions: \({\text{NO}}_{{3_{sampling} }} = {\text{NO}}_{{3_{incub} }} - 0.324\) for the 0–0.6 m soil layer and \({\text{NO}}_{{3_{sampling} }} = {\text{ NO}}_{{3_{incub} }} {-} \, 0. 1 4 4\) for the 0.6–2.0 m soil layer. No significant net NH4 + production was observed during the stay in the cold room.
Finally, the SOM, STN and SMN were calculated on a surface area basis (kg N ha−1) down to 2 m depth using soil bulk density measured in May 2008 by a gamma radiation probe. The percentage of mineral N reduction (MNR) caused by the introduction of trees into cropland was calculated with the equation proposed by Allen et al. (2004):
where MNCC is the cumulative stock of mineral N (nitrate and ammonium; kg N ha−1) throughout the soil profile in CC and MNAF in AF.
Tree fine root biomass determination
The tree fine root biomass was assessed using the “core-break” method (Mulia and Dupraz 2006) with the same soil cores collected as described above. Each 2 m soil core was divided into 20 cm long sub-cores with a knife. Each sub-core was then broken by hand, near to the middle, and the number of living fine roots (diameter < 2 mm) visible on both horizontal surfaces was counted (Nb root ). The tree fine root biomass was calculated according the equation proposed by Van Noordwijk et al. (2000):
where X is a calibration factor depending on tree species (X = 0.61 for hybrid walnut trees; Mulia and Dupraz 2006); r and h are the radius and the height of the soil core in m respectively (r = 0.0425 m; h = 0.2 m); SRL is the specific root length of hybrid walnut (SRL = 9.9 m g−1; Mulia et al. 2010).
Leaf litter biomass measurement and nitrogen content
In order to assess the dead leaf biomass and nitrogen supply annually from leaf litter fall, two trees with a DBH close to the average were selected in AF and FC in September 2009. Each tree was wrapped in a mesh bag (mesh size < 4 mm), closed at the bottom at tree breast height until the end of leaf fall. The leaves accumulated in the mesh bag were collected every week, oven dried at 60 °C for 48 h and weighed. The weight of fallen leaves per hectare was calculated by multiplying the total amount of leaf litter measured in each selected tree by the total number of trees. The N content of the leaf litter was determined by dry combustion of finely ground samples.
Statistical analysis
For each soil layer, a Kruskal–Wallis test (Kruskal and Wallis 1952) followed by a posthoc analysis using Dunn’s test (Dunn 1964) was performed to compare means of STN, SMN, SOM, PNN, PNM and tree fine root biomass between AF, FC and CC. A generalized linear model was realized between all the measured variables and the coefficients of determination (R2) were calculated. Throughout the text, a symbol *** was used to indicate a significant R2 at p < 0.001 level, a symbol ** for p < 0.01 level and a symbol * for p < 0.05 level. All statistical tests were made with the R software version 2.5.1 (http://www.r-project.org/).
Results
Tree root distribution in agroforestry and forestry plots
Despite the proximity of CC with AF, neither fine nor coarse tree roots were recorded in soil cores collected in CC. Over the whole soil profile, the mean cumulative amount of tree fine root biomass was significantly higher in FC than in AF. In FC, the tree fine root biomass decreased from 0.43 Mg ha−1 in the 0.0–0.2 m soil layer to 0.01 Mg ha−1 at 2 m depth. It was uniformly distributed from the tree trunk to the middle of alley regardless of the soil layer (Table 3b; Fig. 5a). In contrast in AF, the tree fine root biomass was very low in the 0.0–0.2 m soil layer, increased to 0.2 Mg ha−1 in the 0.2–0.4 m and remained relatively constant until the 2 m depth (Fig. 3d). The tree fine root biomass was higher in FC than in AF above 1.4 m depth but was lower below. Almost 71 % of the tree fine root biomass was present in the 0–1 m layer in FC, versus 42 % in AF. A linear regression analysis showed that the land use (AF or FC) explained 56 % of the variation in the tree fine root biomass in the 0.0–0.2 m layer. It was reduced to 37 % at 1 m depth and 6 % at 2 m depth. The tree fine root biomass was negatively correlated with SMN (Fig. 5b), but not at all with PNM or PNN, throughout the whole soil profile. Especially in AF, when considering a profile of 1 m depth, the tree fine root biomass decreased significantly with the distance from the tree trunk (Fig. 5a; R2 = 0.11*). The cumulative amount of fine root biomass was not significantly different with distance from tree trunk except in the first 0.0–0.4 m layer where it was higher close to the tree trunk (Table 3b).
Soil mineral N (SMN) (a), total N (STN) (b), potential net N nitrification (PNN) (c), and tree fine root biomass (d) distribution throughout the soil profile in agroforestry (AF), forestry control (FC) and crop control (CC) plots at the Restinclières experimental site. Symbols are means (n = 10, 8 and 3 at AF, FC and CC respectively) and error bars are SEs. For a given layer, tables beside each graphic represent the results of Dunn’s test. Means followed by the same letters are not significantly different at p < 0.05
Soil mineral N content (SMN)
In the upper 0.0–0.2 m soil layer, the SMN was higher in CC than in AF and FC (Fig. 3a). The percentage of nitrate in the SMN was 97 % in CC, 88 % in AF and 55 % in FC (Table 2). In the 0–1 m soil layer, the mean SMN was also significantly higher in CC than in AF and FC. The cumulative stock of SMN in this layer was 77.7, 32.8 and 16.4 kg ha−1 in CC, AF and FC respectively (Table 2). It represented 0.5, 0.2 and 0.1 % of the STN in CC, AF and FC respectively. Throughout the soil profile (0–2 m depth), the cumulative stock of SMN remained significantly higher in CC than in AF and FC (Table 2). The percentage of mineral N reduction (MNR) induced by the introduction of hybrid walnut trees in cropland, calculated for 0.2, 1 and 2 m depth, was 64, 58 and 51 % respectively.
In AF, the cumulative stock of SMN in the 0–1 m layer was 22, 18, 35 and 67 kg N ha−1 at 2, 4, 6 and 7.2 m from the tree trunk respectively (Table 3a). Indeed, the SMN was positively correlated with the distance from the tree trunk (R2 = 0.32***; Fig. 4). However, these differences were observed neither in the upper 0.0–0.2 m nor beyond 1 m depth (Table 3a). Over a 2 m soil profile, there was a small but significant negative relationship between the SMN and tree fine root biomass in AF (R2 = 0.05*; Fig. 5b).
Relationship between the soil mineral N (SMN, mg kg−1 dry soil) and the distance from tree trunk (m) in agroforestry (AF) and forestry (FC) plots throughout soil profile down to 2 m depth. Solid or broken lines represent linear regression between the two variables. A significant R2 is indicated by a symbol triple asterisk with p < 0.001. A R2 without any symbol is not significant
Relationship between tree fine root biomass (Mg ha−1) and (a) distance from tree trunk and, (b) soil mineral N content (mg kg−1 dry soil) throughout soil profile down to 2 m depth in agroforestry (AF) and forestry (FC) plots. Solid or broken lines represent linear regression between variables. A significant R2 with p < 0.05 or p < 0.01 is indicated by one or double asterisk, respectively. R2 values without any symbol are not significant
In FC, the cumulative stock of SMN in the 0–1 m layer was not significantly different between soil cores taken in the middle and close to tree trunk (Table 3a). Over the entire profile, SMN was significantly higher in the middle of the alley than close to the tree trunk. No significant relationship was observed between SMN and the distance from tree trunk at 0–1 m depth whereas SMN increased significantly from the tree trunk to the middle of alley in the 1–2 m depth (Fig. 4; R2 = 0.83***) in FC. However, values of SMN in this layer were at the same magnitude than values measured in AF at the same depth. Throughout the entire soil profile, no significant relationship was observed between the SMN and tree fine root biomass (Fig. 5b).
Soil total N (STN) and organic matter content (SOM)
The average STN was 1.2 g N kg−1 in the 0.0–0.2 m layer and decreased with depth. There was no significant difference in STN between FC, AF and CC throughout the soil profile except in the 0.0–0.2 and 0.5–1.0 m layer (Fig. 3b). When expressed in Mg ha−1, the stock of STN was significantly higher in FC (4.1 Mg ha−1) than in AF and CC plots (3.5 Mg ha−1) (Table 2) in the upper 0.0–0.2 m soil layer. However in a profile of 2 m depth the mean stock of STN was 25.3 Mg ha−1 and no significant difference was observed between plots. Linear regression analysis showed that the STN was negatively correlated with soil pH (R2 = 0.34***) throughout the soil profile. The soil pH was lower in 0.0–0.2 m than in the deeper layers but was not significantly different between AF, CC and FC regardless of soil depth.
The SOM was on average 25.5 g kg−1 in the top 0.0–0.2 m layer and decreased with depth. The average stock of SOM was high in FC and was not significantly different between AF and CC in the upper 0.0–0.2 m soil layer (Table 2). This trend was observed over the 1 m soil profile. However, in a profile of 2 m depth there was no significant difference in SOM between the three plots. The SOM was positively correlated to the STN throughout the soil profile (R2 = 0.4***).
Soil potential net N nitrification (PNN) and mineralization (PNM)
The PNN in the upper 0.0–0.2 m soil layer was slightly higher in FC than in other plots. It decreased with depth and reached its minimum value in all plots at 2 m depth (Fig. 3c). In the 0.2–0.6 m layer, it was significantly higher in CC than in FC. It was positively correlated to the STN (R2 = 0.6***) and to the SMN (R2 = 0.05***) and negatively correlated to the soil pH (R2 = 0.27***). There was no significant relationship between the PNN and the soil C/N ratio. The PNM was positively correlated to the PNN (R2 = 0.99***), to the STN (R2 = 0.62***) and to the SMN (R2 = 0.04**), and negatively to the soil pH (R2 = 0.27***). In the top 0.0–0.2 m layer, the linear regression showed that the land use (AF, FC or CC) explained 25 % of the PNM variation. In order to characterize the recalcitrance of the soil OM throughout the soil profile, the PNM was expressed per unit of STN in each layer. The mean PNM was the highest in the 0.0–0.2 m soil layer (19 µg N g−1 SMN day−1) and then decreased with depth.
Organic matter supply from tree fine roots and leaf litter
The height and the diameter at breast height (DBH) of the trees selected for leaf litter biomass measurement were 11.5 and 0.2 m respectively in AF and 9.1 and 0.16 m respectively in FC. The total amount of collected leaves was 16.5 kg tree−1 in AF and 7.7 kg tree−1 in FC. Considering the total number of trees per ha, the leaf litter fall input for the 2009 growing year was 1.6 Mg ha−1 in AF and FC. For the upper 0.0–0.2 m soil layer, it represented 2 and 1.8 % of the stock of SOM in AF and FC respectively. The leaf litter N content was 1.12 % (±0.4) and 1.41 % (±0.1) in AF and FC respectively; hence the amount of N input from dead leaves was 18 kg N ha−1 in AF and 22 kg N ha−1 in FC.
Assuming a fine root turnover of about 1 year−1, the tree fine roots brought 0.69 Mg ha−1 of OM into the 0–1 m depth in AF against 1.81 Mg ha−1 in FC in 2009. It represented 0.2 and 0.6 % of the stock of SOM in AF and FC respectively in this layer. Between 1 and 2 m depth, 0.95 Mg ha−1 of OM (or 0.4 % of the stock of SOM) was supplied to the soil from tree fine roots in AF against 0.73 Mg ha−1 (or 0.3 % of the stock of SOM) in FC. Assuming that roots contained 50 % carbon with a C/N ratio of 26 (Jose et al. 2001), the tree fine roots in AF returned 1.3 and 4.7 kg N ha−1 into 0–0.2 m and 0–1 m soil layers, respectively.
Discussion
Walnut hybrid trees reduced the soil mineral N content in the upper soil layer
Our results clearly show that the introduction of hybrid walnut trees in cropland reduced by 64 and 58 % the SMN (mainly in NO3 − form) in the top 0.2 and 1 m soil depths respectively, with respect to the CC treatment. In addition, particularly in AF, the SMN progressively decreased from the middle of the alley to the tree trunk especially at 0–1 m depth whereas in FC, no significant correlation was observed between SMN and the distance from tree trunk (Table 3a; Fig. 4). This reduction of the SMN in AF may be attributed to (i) the tree root activity or (ii) to differences in mineral N production between plots or (iii) to both processes. As the soil PNM was not different between CC and AF (Table 2), the root effect is the most likely. The root effect may be not only due to N uptake but also to microbial N immobilization fed by exudation of labile carbon compounds. Our root counting clearly revealed that the tree fine root system, which was shallower in FC, became deeper in AF (Fig. 3d). According to Mulia and Dupraz (2006), the relocation of the tree rooting zone in deeper layers was induced partially by competition with the crop roots. These deeper fine roots likely contributed to reducing the mineral nitrogen content in the crop system even though the relationship that we observed between tree fine roots and SMN in AF was weak (Fig. 5b). As tree fine root biomass and growth were reported to vary with many parameters such as tree species (Finer et al. 2007), tree age (Idol et al. 2000), soil temperature (Lee et al. 2007), soil water conditions (Meier and Leuschner 2008), site productivity (Keyes and Grier 1981) and soil fertility (Fujimaki et al. 2004), the weak relationship that we observed between SMN and tree fine roots biomass was not surprising. According to Hendricks et al. (2006), there is no general response of tree roots biomass and growth to increased N availability.
The reduction of mineral N content in AF relative to CC, mainly in NO3 − form in autumn by the trees, may limit the potential risk of pollution of water bodies by nitrate. Many studies have shown that the presence of trees in arable systems decreases N leaching (Jose et al. 2000; Udawatta et al. 2002; Allen et al. 2004; Palma et al. 2007). In temperate regions, N leaching losses usually increase in seasons with large amounts of drainage, e.g. during the autumn–winter period when evapotranspiration is low and the soil moisture content is high. The mineral N not taken up by the crop, plus the additional N mineralized during the autumn, are prone to leaching at the beginning of winter (Di et al. 1999). Cambardella et al. (1999) and Drury et al. (1996) showed that most N leaching occurs when there is no vegetation growing in the soil i.e. November–May. At our experimental site, a daily autumn/winter rainfall of more than 50, 75 and 100 mm occurs every year, every 3 years out of four, and every 3 years respectively (data not shown). Such high rain events induce heavy flushes of mineral N into water bodies. When this happened in January 2009, the water table reached the soil surface and water flowed into the Lez river bordering the plots (Fig. 1a). Much of the nitrate N in the soil at the time of such an event would enter water bodies and pollute them. Reducing the amount of mineral N in cropped soils is therefore a clear advantage of agroforestry systems by decreasing the potentially leachable N at the beginning of the drainage period. This effect is likely to increase with the age and the size of the trees, and agroforestry process-based models (e.g. Van Noordwijk and Lusiana 1998; Mulia et al. 2010; Talbot et al. 2014) could be used to predict the long-term reduction of N leaching by an agroforestry system.
As well as reducing the amount of SMN, trees also decreased the percentage of NO3 − in soil mineral N (Table 2) particularly in FC compared to CC, while AF was intermediate. In CC, due to agricultural management practices (e.g. soil tillage, compaction, application of pesticides and chemical fertilizers), the soil microbial communities may be modified and less diverse compared to FC (Jeffery et al. 2010). For example, the high input of mineral N from fertilizers may favor the bacterial-pathways of OM decomposition dominated by labile substrates and opportunistic, bacterial-feeding fauna (Jeffery et al. 2010) including nitrifying communities (Shen et al. 2008). As a result, ammonium N is quickly transformed to nitrate N. Contrariwise, the presence of ammonium in FC soil means either a higher soil N ammonification or a lower soil N nitrification or a high microbial immobilization of nitrate favored by labile C compounds released by tree roots. Because of its positive charge, ammonium is less susceptible to leaching due to adsorption on negatively charged soil particles. Therefore, not only is the amount of mineral N present in soil reduced by the presence of trees but a larger proportion of this mineral N is in the form of less mobile ammonium. Both processes are known to decrease the amount of potentially leachable N to groundwater.
Finally, the N taken up by trees in cropland is returned back into the soil in a conservative manner. For instance, in the upper 1 m soil layer, SMN was 77.7 kg N ha−1 in CC, compared with 32.8 kg N ha−1 in AF (Table 2), a difference of 45 kg N ha−1 not used by the crop in CC but captured by trees in AF. At the end of leaf fall, 18 kg N ha−1 (see “Organic matter supply from tree fine roots and leaf litter” section) or 40 % went back into the soil as leaf litter in organic form and may be beneficial for the next crop after N mineralization. The tree fine root biomass provided 4.7 kg N ha−1 (see “Organic matter supply from tree fine roots and leaf litter” section) or 10 % of the captured N in the 0.0–1.0 m soil layer. Finally, the last part of the captured N was sequestered in the deep soil horizons by tree fine root biomass and/or was immobilized in the tree trunk, branches and coarse roots.
Soil total N content did not change after 14 years of hybrid walnut presence in cropland
After 14 years, hybrid walnut trees did not increase the STN throughout the soil profile in this particular experimental site (Table 2). This is not surprising because the stock of N in the upper 0.0–0.2 m layer was 3.5 Mg ha−1 while the maximum amount of N supplied annually by the leaf litter and tree fine root biomass was 18 kg N ha−1 (i.e. 0.5 %) and 1.3 kg N ha−1 (i.e. 0.04 %) respectively in the 0.0–0.2 m soil layer in AF. Considering a soil profile down to 2 m depth, the maximum annual supply of N from tree leaves and fine roots represented 0.2 % of the stock of STN, which was lower than the confidence interval of mean STN (±0.23 Mg ha−1 or 0.9 %). However in the pure stand walnut plantation, STN was significantly higher than in the agroforestry and sole crop plots, especially in the upper soil layers (Table 2). In forestry systems, the shallower fine root system of hybrid walnut (Jose et al. 2000; Mulia and Dupraz 2006) and the presence of weeds, which were turned over and buried each year but never harvested, may have increased the OM content of the upper soil layer.
Soil total N content as driver of soil potential net N mineralization
The PNM throughout the soil profile was related to the land use system (AF, FC or CC) only in the top 0.0–0.2 m layer. It was strongly correlated to the PNN and to the STN throughout the soil profile. The relationship between the land use systems and the PNM in the upper soil horizons may be explained by the higher STN in FC. Unlike Babbar and Zak (1994), we did not observe a difference in PNM between AF and CC. Harmand et al. (2007) also did not find a significant effect of the tree presence on PNM in a 7-year agroforestry coffee plantation. The relationship between PNM and STN has often been reported elsewhere. In this study, as the soil pH and C/N varied very little, the main variables that really differed in the different soil layers and might influence the PNM were the soil total N content, the root activities and the water saturation. Wang et al. (2001) also showed that the main factor that controls N mineralization is the soil organic N content. In the upper horizon, not only was the STN high but also its recalcitrance was low compared to deeper soil layers, two conditions favoring high N mineralization.
The relationship between PNM and PNN has already been observed by many authors on different sites (Owen et al. 2003; Andrianarisoa et al. 2010). In our experimental site, this positive relationship indicates that almost all the mineralized N was in NO3 − form. This result was not surprising because, nitrification has often been shown to be maximal at high pH (Andrianarisoa et al. 2009; Falkengren-Grerup et al. 1998) and at low C/N (Janssen 1996) as at our experimental site.
Conclusions
Our results show that the presence of 14-year-old hybrid walnut trees in cropland can help to reduce the residual mineral N unused by the crop in arable systems. The deep agroforestry tree root system allows the capture of N leached below the crop rooting zone and thus limits the environmental problems related to the mobility of nitrate in soil and its polluting effect. Part of the recovered N is returned to the soil as organic N in dead leaves and fine root biomass. Another part is immobilized in woody parts of trees and sequestered in deep soil layers by coarse roots.
Despite the supply of OM from dead fine root biomass, no significant change in soil total N content was observed throughout soil profile. Fourteen years of hybrid walnut growth in cropland may not be enough to record a significant change in SOM. Further measurements should be made in the future to see if the changes in SOM that we may expect will occur after longer periods of agroforestry. Changes in SOM were only observed in the uppermost layer of the pure forestry plot, where the weeds had never been harvested since the trees were planted and where the tree root system was concentrated near the soil surface, providing large amounts of dead fine roots.
Unfortunately, some measurements of the different N fluxes, such as microbial N immobilization, denitrification, volatilization and nitrate leaching, were omitted from our study and this prevented us from establishing an exhaustive N balance for the comparison between agroforestry and monoculture systems. However, our findings may help to improve numeric and dynamic models of N cycling in agroforestry systems.
References
Allen SC, Jose S, Nair PKR, Brecke BJ, Nkedi-Kizza P, Ramsey CL (2004) Safety-net role of tree roots: evidence from a pecan (Carya illinoensis K. Koch)-cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. For Ecol Manag 192:395–407. doi:10.1016/j.foreco.2004.02.009
Andrianarisoa KS, Zeller B, Dupouey JL, Dambrine E (2009) Comparing indicators of N status of 50 beech forests (Fagus sylvatica) in Northeastern France. For Ecol Manage 257:2241–2253. doi:10.1016/j.foreco.2009.02.037
Andrianarisoa KS, Zeller B, Ranger J, Bienaimé S, Dambrine E (2010) Control of nitrification by tree species in a common garden experiment. Ecosystems 13:1171–1187. doi:10.1007/s10021-010-9390-x
Babbar LI, Zak DR (1994) Nitrogen cycling in coffee agroecosystems: net N mineralization and nitrification in the presence and absence of shade trees. Agric Ecosyst Environ 48:107–113. doi:10.1016/0167-8809(94)90081-7
Beer JW, Bonneman A, Chavez W, Fassbender HW, Imbach AC, Martel I (1990) Modeling agroforestry systems of cacao with Cordia alliodora and Erythrina poeppigiana in Costa Rica. V. Productivity indices, organic matter models and sustainability over ten years. Agrofor Syst 12:229–249. doi:10.1007/BF00137286
Cambardella CA, Moorman TB, Jaynes DB, Hatfield JL, Parkin TB, Simpkins WW et al (1999) Water quality in Walnut Creek watershed: nitrate-nitrogen in soils, subsurface drainage water, and shallow groundwater. J Environ Qual 28:25–34. doi:10.2134/jeq1999.00472425002800010003x
Cardinael R, Chevallier T, Barthès BG, Saby NPA, Parent T, Dupraz C, Bernoux M, Chenu C (2015) Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon. A case study in a Mediterranean context. Geoderma 259–260:288–299. doi:10.1016/j.geoderma.2015.06.015
Di HJ, Cameron KC, Moore S, Smith NP (1999) Contributions to nitrogen leaching and pasture uptake by autumn-applied dairy effluent and ammonium fertilizer labeled with 15 N isotope. Plant Soil 210:189–198. doi:10.1023/A:1004677902049
Drury CF, Tan CS, Gaynor JD, Oloya TO, Welacky TW (1996) Influence of controlled drainage—sub-irrigation on surface and tile drainage nitrate loss. J Environ Qual 25:317–324. doi:10.2134/jeq1996.00472425002500020016x
Dufour L, Metay A, Talbot G, Dupraz C (2013) Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J Agro Crop Sci 199:217–227. doi:10.1111/jac.12008
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Dupraz C, Liagre F (2008) Agroforesterie: des arbres et des cultures. Edition France Agricole, Paris
Dupraz C, Auclair D, Barthélémy D, Caraglio Y, Sabatier S, Bariteau M, Kreiter S, Tixier Garcin M-S, Maillet J (2000) Programme Intégré de Recherches en Agroforesterie à ResTinclières (PIRAT). INRA Montpellier. http://www.agroof.net/PIRAT/Doc/rapports/pirat1999.pdf. Accessed 31 Aug 2015
Falkengren-Grerup U, Brunet J, Diekmann M (1998) Nitrogen mineralization in deciduous forest soils in south Sweden in gradients of soil acidity and deposition. Environ Pollut 102:415–420. doi:10.1016/S0269-7491(98)80062-6
Finer L, Helmisaari H-S, Lõhmus K, Majdi H, Brunner I, Børja I, Eldhuset T, Godbold D, Grebenc T, Konôpka B, Kraigher H, Möttönen MR, Ohashi M, Oleksyn J, Ostonen I, Uri V, Vanguelova E (2007) Variation in fine root biomass of three European tree species: beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.) and Scots pine (Pinus sylvestris L.). Plant Biosyst 141:394–405. doi:10.1080/11263500701625897
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–281. doi:10.1038/nature06275
Fujimaki R, Tateno R, Hirobe M, Tokuchi N, Takeda H (2004) Fine root mass in relation to soil N supply in a cool temperate forest. Ecol Res 19:559–562. doi:10.1111/j.1440-1703.2004.00669.x
Harmand JM, Avila H, Dambrine E, Skiba U, de Miguel S, Renderos RV, Oliver R, Jimenez F, Beer J (2007) Nitrogen dynamics, soil nitrate retention and nitrate water contamination in a Coffea arabica–Eucalyptus deglupta agroforestry system in Southern Costa Rica. Biogeochemistry 85:125–139. doi:10.1007/s10533-007-9120-4
Hendricks JJ, Hendrick RL, Wilson CA, Mitchel RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57. doi:10.1111/j.1365-2745.2005.01067.x
Idol TW, Pope PE, Ponder F Jr (2000) Fine root dynamics across a chronosequence of upland temperate deciduous forests. For Ecol Manag 127:153–167. doi:10.1016/S0378-1127(99)00127-9
Janssen BH (1996) Nitrogen mineralization in relation to C: N ratio and decomposability of organic materials. Plant Soil 181:39–45. doi:10.1007/BF00011290
Jeffery S, Gardi C, Jones A, Montanarella L, Marmo L, Miko L, Ritz K, Peres G, Römbke J, van der Putten WH (eds) (2010) European atlas of soil biodiversity. European Commission, Publications Office of the European Union, Luxembourg
Jose S, Gillespie AR, Seifert JR, Mengel DB, Pope PE (2000) Defining competition vectors in a temperate alley cropping system in the midwestern USA: 3. Competition for nitrogen and litter decomposition dynamics. Agrofor Syst 48:61–77. doi:10.1023/A:1006241406462
Jose S, Gillespie AR, Seifert JR, Pope PE (2001) Comparison of minirhizotron and soil core methods for quantifying root biomass in a temperate alley cropping system. Agrofor Syst 52:161–168. doi:10.1023/A:1010667921970
Keyes MR, Grier CC (1981) Above- and belowground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can J For Res 11:599–605. doi:10.1139/x81-082
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Lee EH, Tingey DT, Beedlow PA, Johnson MG, Burdick CA (2007) Relating fine root biomass to soil and climate conditions in the Pacific Northwest. For Ecol Manag 242:195–208. doi:10.1016/j.foreco.2007.01.033
Meier IC, Leuschner C (2008) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14:2081–2095. doi:10.1111/j.1365-2486.2008.01634.x
Mulia R, Dupraz C (2006) Unusual fine root distributions of two deciduous tree species in southern France: what consequences for modelling of tree root dynamics? Plant Soil 281:71–85. doi:10.1007/s11104-005-3770-6
Mulia R, Dupraz C, Van Noordwijk M (2010) Reconciling root plasticity and architectural ground rules in tree root growth models with voxel automata. Plant Soil 337:77–92. doi:10.1007/s11104-010-0502-3
Owen JS, Wang MK, Wang CH, King HB, Sun HL (2003) Net N mineralization and nitrification rates in a forested ecosystem in northeastern Taiwan. For Ecol Manag 176:519–530. doi:10.1016/S0378-1127(02)00225-6
Palma JHN, Graves AR, Bunce RGH, Burgess PJ, Filippi R, De Keesman KJ, Van Keulen H, Liagre F, Mayus M, Moreno G, Reisner Y, Herzog F (2007) Modelling environmental benefits of silvoarable agroforestry in Europe. Agric Ecosyst Environ 119:320–334. doi:10.1016/j.agee.2006.07.021
Persson T, Wiren A (1995) Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant Soil 168(169):55–65. doi:10.1007/BF00029313
Rosell RA, Gasparoni JC, Galantini JA (2001) Soil organic matter evaluation. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Assessment methods for soil carbon. CRC Press, Lewis Publishers, Boca Raton, pp 311–322
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611. doi:10.1111/j.1462-2920.2008.01578.x
Smil V (1999) Nitrogen in crop production. Global Biogeochem Cy 13:647–662. doi:10.1029/1999GB900015
Talbot G, Roux S, Graves A, Dupraz C, Marrou H, Wery J (2014) Relative yield decomposition: a method for understanding the behaviour of complex crop models. Environ Model Softw 51:136–148. doi:10.1016/j.envsoft.2013.09.017
Udawatta RP, Krstansky JJ, Henderson GS, Garrett HE (2002) Agroforestry practices, runoff, and nutrient loss: a paired watershed comparison. J Environ Qual 31:1214–1225. doi:10.2134/jeq2002.1214
Van Noordwijk M, Lusiana B (1998) WaNuLCAS, a model of water, nutrient and light capture in agroforestry systems. Agrofor Syst 43:217–242. doi:10.1023/A:1026417120254
Van Noordwijk M, Brouwer G, Meijboom F, Do Rosario G, Oliveira M, Bengough AG (2000) Trench profile techniques and core break methods. In: Smit AL, Bengough AG, Engels C, Van Noordwijk M, Pellerin S, Van De Geijn SC (eds) Roots methods. Springer, Berlin, pp 212–231
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition: plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397. doi:10.1104/pp.010331
Wang WJ, Smith CJ, Chalk PM, Chen DL (2001) Evaluating chemical and physical indices of nitrogen mineralization capacity with an unequivocal reference. Soil Sci Soc Am J 65:368–376. doi:10.2136/sssaj2001.652368x
Wösten JHM, Lilly A, Nesmes A, Le Bas C (1999) Development and use of a database of hydraulic properties of European soils. Geoderma 90:169–185. doi:10.1016/S0016-7061(98)00132-3
WRB IWG (2006) World reference base for soil resources, 2nd edn. World Soil Resources Reports No. 103. FAO, Rome
Acknowledgments
We acknowledge “L’Agence de l’eau: Rhône Méditerranée Corse” for the financial support of this study. The Restinclières walnut experimental plot was established and maintained thanks to a grant by the PIRAT program funded by the “Département de l’Hérault”, (the owner of the Restinclières estate). We sincerely thank J-F. Bourdoncle, P Parra and C Enard for conducting the difficult field soil coring activities. We also thank the two anonymous reviewers for their meaningful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrianarisoa, K.S., Dufour, L., Bienaimé, S. et al. The introduction of hybrid walnut trees (Juglans nigra × regia cv. NG23) into cropland reduces soil mineral N content in autumn in southern France. Agroforest Syst 90, 193–205 (2016). https://doi.org/10.1007/s10457-015-9845-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9845-3