Abstract
We studied the effect of tree species on nitrification in five young plantations and an old native beech coppice forest at the Breuil experimental site in central France. The potential net nitrification (PNN) of soil was high in beech, Corsican pine, and Douglas fir plantations (high nitrifying stands denoted H) and low in spruce and Nordmann fir plantations as well as in native forest stands (low nitrifying stands denoted L). We hypothesized that tree species would stimulate or inhibit nitrification in transplanted soil cores within a few years after the cores were transplanted between stands. We first initiated a transplant experiment where soil cores were exchanged between all stands. The PNN remained high in soil cores from H transferred to H and low in soil cores from L transferred to L. The PNN increased considerably after 16 months in soil cores transferred from L to H, whereas the transfer of soil cores from H to L decreased the PNN only slightly after 28 months. In a second transplant experiment, forest floor material was exchanged between the Douglas fir (H) and the native forest (L) stand. Six months later, the forest floor from the native forest had increased the PNN of the Douglas fir soil considerably, whereas the forest floor from Douglas fir did not affect the PNN of the soil in the native forest stand. It was concluded that beech, Corsican pine, and Douglas fir rapidly stimulate soil nitrification by either activation of suppressed nitrifier communities and/or colonization by new nitrifier communities. Conversely, the slow and irregular reduction of nitrification in spruce, Nordmann fir, and native forest was probably due to the low and heterogeneously distributed flux of inhibiting substances per volume of soil. Our experiments suggest that the inhibition of nitrification is not tightly connected to forest floor leachates, but that the forest floor both reflects and maintains the major ongoing processes. In the long term, humus build up and the production of inhibiting substances may completely block the nitrification activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most ecosystems, nitrogen (N) mineralization and nitrification supply mineral N to plants. Nitrification determines the form of N present in the soil and, therefore, how N is absorbed or dispersed into the environment. The assessment of nitrification is of critical importance to ecologists because nitrate (NO3 −) availability strongly influences plant community composition (Andrianarisoa and others 2009) and because the leaching of NO3 − may cause soil acidification and groundwater pollution (Aber and others 1989).
Nitrification is sensitive to environmental physical conditions (soil temperature and moisture) and varies depending on soil types (Andrianarisoa and others 2009), soil pH (Herbauts 1974; Le Tacon 1976; Falkengren-Grerup and others 1998; Li and others 2007; Pietri and Brookes 2008), litter lignin/N ratio (Van Cleve and others 1993; Scott and Binkley 1997; Joshi and others 2003), soil C/N (Persson and others 2000; Goodale and Aber 2001; Joshi and others 2003), and vegetation (Schaffers and Sykora 2000; Falkengren-Grerup and Schottelndreier 2004). Many studies have shown that tree species may strongly influence soil nitrification (Gower and Son 1992; Son and Lee 1997; Berendse 1998; Priha and others 1999; Augusto and Ranger 2001; Brierley and others 2001; Templer and others 2002; Moukoumi and others 2006; Russell and others 2006; Zeller and others 2007). For example, net nitrification is higher under beech than under Norway spruce stands (Wedraogo and others 1993; Jussy and others 2004; Zhong and Makeschin 2004, 2006).
Tree species may control nitrification by several processes acting at different levels ranging from the canopy to the root–soil interface in the ecosystem. Tree species and forest management create specific microclimate conditions by influencing light (Ranger and others 2004), rainfall interception, and evapotranspiration, which, in turn, may alter soil temperature (Barg and Edmonds 1999), moisture (Augusto and Ranger 2001), and the composition of understory vegetation (Augusto and others 2003). Dry deposition of N compounds is higher on evergreen species which have a high leaf area index (Lovett and Lindberg 1986). For instance, Schrijver and others (2007) showed that the flux of mineral N in throughfall below conifer forests was larger than that below deciduous forests in Europe and North America.
Leaf litter quantity and quality, characterized by the lignin/N and C/N ratios, vary among tree species (Prescott and Preston 1994; Nugroho and others 2006) and influence the rate of litter decomposition, N mineralization, and nitrification in forests (Scott and Binkley 1997; Persson and others 2000). As a result, tree species strongly influence humus types. Nitrification is higher in the mull humus type than in the moder humus type (Bottner and others 1998). Polyphenolic compounds (Northup and others 1995; Paavolainen and others 1998), phenols, tannins (Kraus and others 2003; Kraus and others 2004; Smolander and others 2005; Kanerva and others 2006), and terpenoids (White 1986) in leaf litter and humus or released by roots (Subbarao and others 2006, 2007) may directly reduce or inhibit nitrification.
On the other hand, tree roots may exert a considerable influence on soil microbial communities and N transformation through the depletion of nutrients and water in the rhizosphere and the secretion of protons, enzymes, and carbon compounds from root surfaces. It has been demonstrated that N mineralization and microbial biomass were higher in root-associated soil compared to bulk soil (Norton and Firestone 1996; Reydellet and others 1997; Priha and others 1999; Whalen and others 2001; Colin-Belgrand and others 2003). Root exudates may contain compounds that stimulate or inhibit microbial activity. In addition, when mineral N availability is low, tree roots compete for ammonium (NH4 +) with nitrifiers. The partitioning of mineral N between plants and microorganisms is influenced by the availability of carbon (C) and N in the rhizosphere (Schimel and others 1989), by the plant and microbial preferences for NH4 + over NO3 − (Jackson and others 1989; Harrison and others 2007) and by differences in ionic mobility.
The objective of this paper was to investigate how rapidly and to what extent tree species control nitrification. At the Breuil experimental site (Central France) in 1976, monospecific plantations of five species were established on the same soil type. At this site, Moukoumi and others (2006) and Zeller and others (2007) repeatedly showed that soil potential net nitrification (PNN) was high in Fagus sylvatica L. (hereafter referred to as beech), Pinus nigra Arn. ssp laricio Poiret var Corsicana (hereafter referred to as Corsican pine), and Pseudotsuga menziesii Franco (hereafter referred to as Douglas fir) plantations and low in Picea abies Karst. (hereafter referred to as spruce), Abies nordmanniana Spach. plantations (hereafter referred to as Nordmann fir), and in an old coppice with standard stands (hereafter referred to as native forest). We hypothesized that tree species would rapidly stimulate or inhibit nitrification in foreign soil cores. Here, we report the results of soil core and forest floor transplant experiments designed to determine the following: (1) how rapidly soil nitrification is stimulated or inhibited by different tree species; and (2) whether the forest floor plays a driving role in the control of nitrification.
Materials and Methods
Site Description
The study site is located in the Breuil-Chenue Experimental Forest in the Morvan highlands (Central France, 47°18′10″N and 4°44′44″E; Figure 1A) at an elevation of 650 m. Detailed information about the site, the experimental design, and the analytical techniques are reported in Ranger and others (2004). The climate is continental with a mean annual temperature of 9°C and mean annual precipitation of 1280 mm. Snow covers the soil for about 1 month each winter. Average open field wet N deposition, monitored for 6 years, was 15.2 kg N ha−1 y−1 (NO3 −: 3.9 kg ha−1 y−1; NH4 +: 7.5 kg ha−1 y−1, and DON: 3.8 kg ha−1 y−1) (Ranger and others 2004). Dissolved organic carbon deposition was 40 kg C ha−1 y−1. The native forest was an old coppice (now 150 years old) dominated by Fagus sylvatica L. with sparse Quercus sessiliflora Smith. In 1976, a 10-ha flat area was clear-cut; bole wood and large branches were harvested. The area was planted in rows with monospecific plantations (1000 m2) of beech, Corsican pine, Douglas fir, Nordmann fir, and spruce in a block design to compare the growth of these different tree species (Figure 1B). A part of the native forest was held in reserve as a reference plot (Bonneau and others 1977). The humus in the native forest is a dysmoder (a moder with an Oa layer well differentiated). The soil is classified as a Typic Dystrochrept (USDA 1999) and developed from granite. The soil presents a thin, discontinuous E horizon and a 2- to 3-cm thick, dark-brown Bh layer. This sequence is often reported to be a consequence of surface micropodzolisation (Ranger and others 2004). The texture of the soil is sandy-loam (60% sand and <20% clay) (Calvaruso and others 2009). The soil is acidic (pH 4–4.5). This experimental site was selected for the homogeneity of its soil. Twenty-four years after the plantation, Ranger and others (2004) confirmed soil homogeneity by a systematic soil sampling (n = 16 profiles per stand) and analysis of all relevant soil chemical parameters. They performed an ANOVA for soil parameters and observed significant differences for the C and N content among stands but only in the upper horizon (the forest floor and A horizon). These layers are strongly influenced by tree species inputs through the processes of litterfall, litter decomposition, and soil organic matter formation.
Schematic representation of the soil core exchange experiment at the Breuil experimental site: A location of the Breuil experimental site; B spatial location of the different stands within the site; and C position of soil cores within stands (for example, Douglas fir plantation). In each line, soil cores from different stands were randomly distributed among the 60 holes.
Stand Characteristics (Table 1)
In 2000, trees were equipped with rings to measure the diameter at breast height (DBH). Mean DBH in 2007 was highest in native forest (51.2 cm). Among plantations, mean DBH was highest in Douglas fir (28.2 cm) and lowest in beech (8.6 cm). The O horizon, formed within 30 years, was mull in beech, Corsican pine, and Douglas fir stands and moder with an Oe (~1 cm thick) and Oa (1–3.5 cm thick) layer in Nordmann fir and spruce (Ranger and others 2004; Moukoumi 2006). The activity of epigeic earthworms in the Oa horizon of the native forest and in the upper A horizon of native forest, Douglas fir, beech, and spruce (1–2 cm) was deduced from the observation of thin sections (Moukoumi 2006). This activity was more pronounced in beech and less pronounced in spruce plantations. Nevertheless, during coring operations (420 soil cores), we never observed any earthworms.
In each stand, fluxes of organic C and N were monitored monthly from November 2001 to December 2006. Litterfall was collected by five baskets (60 × 60 cm), and C and N contents were determined by dry combustion (elemental analyzer, Carlo Erba NA 1500, Italy). Throughfall was collected using four replicates of 2-m long polyethylene gutters. Solutions were stored underground, collected monthly and analyzed. NO3 − and NH4 + contents were determined using continuous flow colorimetry (TRAACS, Bran and Luebbe). Litterfall N (leaves + branches + fruit and buds) was very high in native forest (65 kg N ha−1 y−1), high in Douglas fir (54.6 kg N ha−1 y−1) and spruce (52.7 kg N ha−1 y−1) and low in the other stands (42–45 kg N ha−1 y−1). The average C/N ratio of litterfall was higher in native forest, spruce, Nordmann fir, and Corsican pine (46–49) than in beech and Douglas fir (35–40) (Table 1). The C/N value of litterfall was probably overestimated in Corsican pine because the soil was covered by patches of Deschampsia flexuosa, Rubus fructicosus, and Pteridium aquilinum; litterfall from these species is comparatively richer in N; below the other stands, the understory vegetation was very sparse. The biomass and litter production of the understory vegetation were not measured precisely and are therefore not reported here. The mean annual throughfall N fluxes as N–NO3 − and N–NH4 + were significantly higher in Douglas fir compared to all other stands (Table 1). In the A horizon, the contents of C and N were determined by dry combustion, as described above for litterfall (Ranger and others 2004). They were the highest in beech and the lowest in spruce. The mineral soil C/N ranged from 18 to 20 and was not significantly different among stands (Table 1).
In beech, spruce, and Douglas fir plantations, temperature probes connected to a central data logger were installed at 130 cm above the soil and at 15 cm belowground to measure air and soil temperature. For the period between February 2006 and June 2008, mean air temperature was not significantly different between stands. The mean of soil temperature at the 15-cm depth was not significantly different between Douglas fir (9°C) and spruce (8.8°C) stands but was higher in beech (10.8°C).
Soil moisture was determined by drying an aliquot of soil sample at 105°C. In June 2007, the soil moisture was significantly higher in beech (41.8%) but was not significantly different among other stands. In May 2008, soil moisture was higher in beech (46%) and native forest (41.5%), lower in spruce (31.1%) and Nordmann fir (33.4%) and intermediate in Douglas fir (37.7%) and Corsican pine (36.2%).
Experimental Design and Soil Sampling
Experiment 1: Soil Core Exchange
In February 2006, 60 intact soil cores (diameter 8 cm, depth 15 cm), including the forest floor layer, were collected with an auger along two parallel lines situated at 0.5 m from a tree row (Figure 1C) in beech, Corsican pine, Douglas fir, Nordmann fir, and spruce. In the native forest, lines were placed at 0.5 m from a beech dominant tree. Soil cores were wrapped in mesh bags (mesh size 2 mm). Ten cores were put back into their original place (hereafter referred to as “disturbed soil cores”) and 50 were equitably distributed into the assigned holes in the core field of the five other stands (hereafter referred to as “transferred soil cores”). In each stand, half (n = 30) of the soil cores were collected after 16 months (June 2007) and the remaining after 28 months (May 2008). In addition, both in June 2007 and May 2008, five soil cores were taken from each stand in an undisturbed area (0.5 m away from the rows) to serve as a reference value (hereafter referred to as “undisturbed soil cores”).
On the sampling day, before removing soil cores from their specific locations in the core field, the residual forest floor material from the original stand and the newly fallen litter from the host stand were collected. Soil cores were sieved to 4 mm or less and fine (<2 mm) and coarse (>2 mm) roots (live or dead) were removed. The forest floor and mineral soil samples were stored for 2 days at 4°C in a cold room before analyses.
Experiment 2: Forest Floor Exchange
In March 2007, an exchange of O horizons was performed between Douglas fir and native forest (with very different nitrification rates). In each stand, an area of 1.5 × 1.8 m was selected and subdivided into 18 equal parts of 0.3 m2. Three treatments were randomly assigned to the 18 rectangles (six repetitions of each) within each sub-plot: (1) exchange, where the forest floor (O layer) was carefully collected and transferred to the other stand; (2) disturbance, where the forest floor was scraped, raised and put back at its original place; and (3) removal, where the forest floor was removed.
In Douglas fir, the forest floor was collected by hand because it was composed of fresh and partly decomposed needles. In native forest, fresh litter was first collected by hand, and then packets (15 × 15 cm) of intact forest floor (Oe and Oa) were carefully cut with a knife. Thereafter, the forest floor (without litter) was reconstructed on a rectangular plate and transferred to the Douglas fir plantation (exchange), returned to its original place (disturbance) or removed (removal).
After 6 months, three soil cores were collected from each experimental rectangle with an auger (Ø 8 cm, depth 15 cm). In addition, nine soil cores were taken in each stand under the undisturbed forest floor to serve as control cores. The forest floor was separated from the underlying mineral soil. Samples were sieved to 4 mm or less, and fine (<2 mm) and coarse (>2 mm) roots (live or dead) were removed. Samples were transported to the laboratory and kept for 2 days at 4°C before analyses.
Soil and Forest Floor Analyses
Aliquots of the forest floor and mineral soil samples were oven dried at 65 and 105°C, respectively, for 48 h to determine the moisture. For each soil core, the total amount of fresh forest floor and 20 g of wet mineral soil were shaken in 1 M KCl (300 ml and 100 ml, respectively) for 1 h and then filtered. The nitrate–N and ammonium–N concentrations of extracts (hereafter referred to as [NO3 −] and [NH4 +], respectively) were measured using continuous-flow colorimetry (TRAACS, Bran and Luebbe). The potential net N mineralization (PNM) and PNN were measured only in mineral soil samples. Aliquots of mineral soil (200 g) at sampling moisture (close to field capacity) were put into jars with airtight lids and incubated at 20°C in the dark for 42 days. The jars were opened for a few minutes twice a week. Inorganic N (NH4 + and NO3 −) was extracted at the beginning and at the end of the incubation. Potential net N mineralization was the amount of total inorganic N accumulated during the incubation period, and PNN was the amount of NO3 − formed from the nitrification of the NH4 + already present in the soil core before the incubation as well as that mineralized during the incubation. Both were calculated as mg N kg−1 soil d−1. All concentrations and rates are presented on a dry weight basis.
Statistical Analyses
Soil Core Exchange Experiment
For “undisturbed soil cores”, a one-way ANOVA followed by a Tukey’s test was performed to compare [NH4 +], [NO3 −], PNM, and PNN between stands for each sampling date. Within each stand, mean values of [NH4 +], [NO3 −], PNM, and PNN were compared between the two sampling dates with a Student’s t-test.
“Disturbed” and “transferred” soil cores were identified in relation to their origin (“source stands”) and to their destination (“receptor stands”). The following tests were applied to [NO3 −] and PNN: (1) within each “receptor stand”, a one-way ANOVA followed by a Tukey’s test was used to compare mean values of each “source stand” for each sampling date; (2) for a given “source stand”, a one-way ANOVA was used to compare mean values of each “receptor stand” for each sampling date. For all data, the relationships between variables were examined using a Pearson correlation analysis.
The rate of increase or decrease of PNN between undisturbed soil cores and transferred soil cores collected 16 or 28 months after transplant was calculated for the different groups, and significance was tested with a paired Student’s t-test.
Forest Floor Exchange Experiment
For each stand, means of [NH4 +], [NO3 −], PNM, and PNN were compared among treatments using a one-way ANOVA followed by a Tukey’s test. All statistical data analysis was performed using SAS software version 9.00 (SAS 2002, SAS Institute Inc., Cary, NC, USA).
Results
Nitrate and Ammonium Concentrations in Undisturbed Soil Cores
Forest Floor
In June 2007, the forest floor [NO3 −] was low compared to [NH4 +] in all stands, but it was significantly higher in Corsican pine (22.2 mg N kg−1), beech (10.9 mg N kg−1), and Douglas fir (13.8 mg N kg−1) than in spruce (0.6 mg N kg−1). Forest floor [NO3 −] was below detection in Nordmann fir and native forest. In May 2008, forest floor [NO3 −] was higher in Corsican pine (49.8 mg N kg−1), beech (30.3 mg N kg−1), and Douglas fir (83.7 mg N kg−1) than in native forest (5.55 mg N kg−1), Nordmann fir (5.53 mg N kg−1), and spruce (3 mg N kg−1). Forest floor [NO3 −] was higher in 2008 than in 2007 except for beech (Figure 2A). Forest floor [NH4 +] was highly variable between replicates, but it was greater than [NO3 −] in both sampling dates. In 2007, forest floor [NH4 +] was greatest in Douglas fir (149.3 mg N kg−1), lowest in native forest (36.0 mg N kg−1) and intermediate in other plantations. In May 2008, the forest floor [NH4 +] did not differ among stands. Forest floor [NH4 +] was greater in May 2008 than in June 2007 except in beech and Corsican pine (Figure 2A).
Nitrate (mg N–NO3 − kg−1) and ammonium (mg N–NH4 + kg−1) concentration in A forest floor and B mineral soil (0–15 cm) of six different stands for two sampling dates at the Breuil experimental site. Histograms are means (n = 5) and error bars are standard deviations. Symbol * indicates a significant difference (P < 0.05) between two sampling dates for a given stand; ** for P < 0.01; *** for P < 0.001; and ns for not significant. Tables under each graphic summarize the results of Tukey’s tests between each stand for a given sampling date. Means with same letters are not significantly different.
Mineral Soils (0–15 cm)
For both sampling dates, the [NO3 −] in mineral soil was at least 3-fold higher in beech, Corsican pine, and Douglas fir compared to native forest, Nordmann fir, and spruce (Figure 2B). The [NH4 +] in mineral soil was highly variable between stands. In June 2007, [NH4 +] in the mineral soil was highest in spruce (3.9 mg N kg−1 soil), lowest in beech (1.4 mg N kg−1 soil) and intermediate in the other stands. In May 2008, [NH4 +] in the mineral soil was highest in Douglas fir (4.1 mg N kg−1 soil) and spruce (4.0 mg N kg−1 soil) and lowest in Corsican pine (1.5 mg N kg−1 soil). [NH4 +] in the mineral soil was not significantly different for the two sampling dates in all stands except Corsican pine (Figure 2B).
Potential Net N Mineralization and Nitrification in Undisturbed Soil Cores
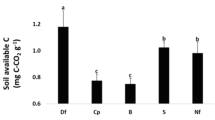
In June 2007, PNM was highest in beech (0.8 mg N kg−1 d−1) and lowest in Nordmann fir (0.3 mg N kg−1 d−1). In May 2008, PNM was not significantly different between stands. Potential net N mineralization was significantly higher in May 2008 than in June 2007 in all plantations except beech and Corsican pine (Figure 3A). PNN was high in beech, Corsican pine, and Douglas fir and low in native forest, Nordmann fir, and spruce on both the sampling dates. PNN for Douglas fir was higher in June 2007 than in May 2008, but PNN was not significantly different between the two sampling dates (Figure 3B).
Potential net N A mineralization (PNM, mg N kg−1 soil d−1) and B nitrification (PNN, mg N–NO3 kg−1 soil d−1) in different stands at the Breuil experimental site (Morvan, France). Histograms are means and error bars are standard deviations. Symbol * indicates a significant difference (P < 0.05) between two sampling dates for a given stand; ** for P < 0.01; *** for P < 0.001; and ns for not significant. Tables following each graphic summarize the results of Tukey’s tests between each stand for a given sampling date. Means with same letters are not significantly different.
Beech, Corsican pine, and Douglas fir plantations are referred to as “High nitrifying stands”, or H, and native forest, Nordmann fir, and spruce stands are referred to as “Low nitrifying stands”, or L. All H stands had mull type humus whereas all L stands had moder type humus (Table 1). No significant relationship was found between PNN and soil C/N. Considering all stands, there was no significant relationship between PNN and litterfall C/N (r = −0.7; P = 0.08). However, as mentioned above, the C/N value of litterfall in Corsican pine is probably overestimated when considering only dead needles because patches of herbaceous species covered the soil. When the Corsican pine plantation was excluded, the relationship became significant (r = −0.9; P = 0.02) (Figure 4). Soil [NO3 −] and PNN were not related to mean DBH, growth or to N deposition in throughfall. For instance, [NO3 −] and PNN were very high in both beech and Douglas fir, whereas growth and throughfall [NO3 −] were low in beech and high in Douglas fir.
Relationship between litterfall C/N ratio and PNN (mg N–NO3 kg−1 soil d−1) at the Breuil experimental site. The dotted line is the linear regression (R 2 = 0.86, P = 0.02) established when excluding data from the Corsican pine plantation. Values are means (n = 4 for litterfall C/N and n = 10 for PNN) and error bars are standard deviations (for clarity, only the second part is presented).
Results of the Soil Core Exchange Experiment
For a synthetic description of the main results, we gathered the experimental trajectories into four groups of transfers: HH, HL, LH, and LL (Figure 5). HH indicates that soil cores from high nitrifying (H) stands were transferred to other high nitrifying stands. LH indicates that soil cores from low nitrifying (L) stands were transferred to H. The same pattern was employed for HL and LL groups.
Synthetic scheme of the experimental transfer. H means “High nitrifying stands” and L means “Low nitrifying stands”. The group HH means soil cores from “High nitrifying stands” transferred to “High nitrifying stands”; LH means soil cores from “Low nitrifying stands” transferred to “High nitrifying stands”. The same pattern was employed for HL and LL groups.
Nitrate and Ammonium Concentration (Mineral Soil 0–15 cm)
Nitrate concentrations of “disturbed soil cores” (soil cores raised and put back in their original place) were not significantly different from the undisturbed soil at the same date (Figure 6; superscript letters). In June 2007, 16 months after the start of the experiment, [NO3 −] was greater than 3 mg N kg−1 in most soil cores of the HH group (Figure 6A). In the HL and LH groups, the [NO3 −] in soil cores was variable but often was less than 3 mg kg−1. Finally, [NO3 −] was less than 1 mg N kg−1 in soil cores of the LL group. Mean [NH4 +] was ordered as follows: LH (7.9 mg N kg−1 soil) > LL (4.1 mg N kg−1 soil) = HH (2.9 mg N kg−1 soil) > HL (2.1 mg N kg−1 soil) (data not shown). In May 2008, soil cores transferred to beech and Corsican pine had high values of [NO3 −] independently of the “source stand”. Among the soil cores transferred into Douglas fir, those originating from H had high [NO3 −], whereas those originating from L had variable [NO3 −]. As in 2007, soil cores in the HL group had variable [NO3 −], whereas soil cores in the LL group had low [NO3 −]. Mean [NH4 +] showed the following order: LH (7 mg N kg−1 soil) > LL (3 mg N kg−1 soil) = HH (2.9 mg N kg−1 soil) = HL (2.7 mg N kg−1 soil) (data not shown).
Nitrate concentration (mg N–NO3 kg−1 soil) in mineral soil samples (0–15 cm) A 16 months and B 28 months after soil core transplant. Values are means (n = 5). Letters in superscripts are Tukey’s groups for the comparisons of means within each line, and letters in brackets are Tukey’s groups for the comparisons of means within each column. Means with the same letters are not significantly different.
Potential Net N Nitrification and Mineralization
The PNN varied between 0.1 and 1.4 mg N–NO3 kg−1 d−1 (Figure 7). There was no significant effect of disturbance on PNN for the two sampling dates. In 2007, 16 months after the start of the experiment, the PNN in most soil cores in the HH, HL, and LH groups was significantly higher than in soil cores belonging to the LL group. The PNN in transfer groups HH and HL were not significantly different from the PNN in undisturbed soil cores in H. Moreover, the PNN in the LL group was not different from that in undisturbed soil cores in L. The PNN in transfer group LH increased 7 fold after 16 months compared with undisturbed soil cores from L. From 2007 to 2008, the PNN of soil did not change for groups HH and LL. However, the LH group increased by 66% (P < 0.001) and the HL group decreased by 17% (not significantly).
Potential net nitrification (mg N–NO3 kg−1 soil d−1) of mineral soil samples (0–15 cm) measured in the laboratory (20°C, 42 days) after A 16 months and B 28 months of soil core transplantation. Values are means (n = 5). Letters in superscripts are Tukey’s groups for comparisons of means within each line, and letters in brackets are Tukey’s groups for comparisons of means within each column. Means with the same letters are not significantly different.
The PNN was positively correlated with the [NO3 −] in the forest floor (r = 0.4, P < 0.0001 in 2007 and r = 0.5, P < 0.0001 in 2008; n = 180) and in the mineral soil (r = 0.6, P < 0.0001 in 2007 and r = 0.4, P < 0.0001 in 2008; n = 180). PNM did not differ significantly among transfer groups and ranged from 1.1 to 0.8 mg N kg−1 d−1 in 2007. In 2008, PNM was significantly greater in LH (1.3 mg N kg−1 d−1) and HL (1.3 mg N kg−1 d−1) than in LL (0.9 mg N kg−1 d−1) and HH (1.0 mg N kg−1 d−1). PNM was positively correlated to PNN (r = 0.4, P < 0.0001 for both sampling dates; n = 180; Figure 8). In the HH group, the relationship between PNM and PNN was always significant (Figure 8). Because mineralized N was fully nitrified, the slope was close to 1. In the LL group, the relationship between PNM and PNN was not significant for both dates of sampling. PNM was very variable whereas PNN remained very low. In the LH, within 16 months, the slope of the linear regression between PNN and PNM became significant, and the intercept increased strongly compared to LL (from 0.01 to 0.3), although data points were scattered between about 30% and 100% of nitrification. Within 28 months, the slope of the linear regression did not change, but the variability in PNN increased strongly with percent nitrification values over 100%. These values indicate the nitrification of a pool of NH4 + already present in the soil before the incubation. In the HL group, within 16 months, the slope of the relationship between PNN and PNM decreased slightly compared to HH, but the variability in PNN increased strongly with percent nitrification values as low as 40%. After 28 months, the slope of the linear regression decreased, and data points were extremely scattered from almost 100% nitrification to less than 10% nitrification (Figure 8).
Relationship between potential net N mineralization (mg kg−1 soil d−1) and potential net nitrification (mg kg−1 soil d−1) from the soil core exchange experiment at the Breuil experimental site: A soil cores collected in June 2007 (16 months after the onset of the experiment) and B soil cores collected in May 2008 (28 months after the onset of the experiment). L designation indicates “Low nitrifying stands”; H for “High nitrifying stands”; HH for the group of soil cores from “H” transferred to “H”; LL for the group of soil cores from “L” transferred to “L”. The same pattern was employed for HL and LH groups. Linear regression equations between PNN and PNM for different groups and their significance are presented in the tables following each graphic.
Results of the Forest Floor Exchange Experiment
Nitrate Concentration
Nitrate concentration was high in the undisturbed forest floor of Douglas fir (35.8 mg N kg−1) and low in the undisturbed forest floor of the native forest (3.4 mg N kg−1). Six months after disturbance of the forest floor, there was no effect of this disturbance on the [NO3 −] in the forest floor of either stand. Nitrate concentration in the forest floor of Douglas fir did not significantly decrease when it was transferred into native forest (from 35.8 to 21.7 mg N kg−1 d.m.). Nitrate concentration in the forest floor of the native forest increased significantly (from 3.4 to 56.5 mg N–NO3 kg−1) when it was transferred to Douglas fir (Figure 9A).
Results of the reciprocal exchange of forest floor samples between the Douglas fir plantation and the native forest stand 6 months after the onset of the experiment: A [NO3 −] and [NH4 +] of the forest floor; B [NO3 −] and [NH4 +] of mineral soil (0–15 cm); and C potential net N mineralization (PNM) and nitrification (PNN) of mineral soil (0–15 cm). Different treatments are presented on the x-axis: “removed” when the forest floor was removed; “undisturbed” when the forest floor was not disturbed; “disturbed” when the forest floor was raised and put back in its original place; and “replaced” when the forest floor was removed and replaced by the forest floor from the other plantation. Histograms are means (n = 18) and bars are standard deviations. Means with the same letters were not significantly different.
In Douglas fir, disturbance of the forest floor had no significant effect on the [NO3 −] of the underlying mineral soil. Removing the forest floor did not significantly decrease [NO3 −] in the mineral soil (2.5 mg N kg−1 soil). Replacing the forest floor of Douglas fir by that of the native forest significantly increased the [NO3 −] (from 5 to 15 mg N kg−1 soil, P < 0.001; Figure 9B) in the underlying mineral soil. In native forest, disturbance of the forest floor, removal of the forest floor, or exchange of the forest floor by the Douglas fir forest floor did not significantly change the [NO3 −] of the underlying mineral soil (0.66, 0.75, and 1.04 mg N kg−1 soil, respectively).
Ammonium Concentration
In the forest floor, [NH4 +] of the undisturbed forest floor was not significantly different between Douglas fir (66.4 mg N kg−1) and native forest (82.5 mg N kg−1). Disturbance had no effect on the [NH4 +] in either Douglas fir or native forest. [NH4 +] in the forest floor from the native forest placed below Douglas fir was 1.8 times higher than in the original forest floor in the native forest (P < 0.01). [NH4 +] in the Douglas fir forest floor transferred into the native forest or returned to the Douglas fir was not changed. There was no significant change in the [NH4 +] of the mineral soil layer for all treatments in Douglas fir and native forest stands.
Potential Net N Mineralization and Nitrification (Mineral Soil 0–15 cm Depth)
In the undisturbed soils, PNM was not significantly different between Douglas fir (0.55 mg N kg−1 soil d−1) and native forest (0.5 mg N kg−1 soil d−1) stands. In contrast, as seen above, PNN was higher in Douglas fir (0.56 mg N kg−1 soil d−1) than in the native forest (0.11 mg N kg−1 soil d−1). In the Douglas fir plantation, the PNM in the mineral soil below the forest floor from the native forest was significantly greater than that below the undisturbed forest floor. In the native forest, the PNM in the mineral soil below the forest floor from Douglas fir was not significantly different from that below the undisturbed forest floor. In both stands, replacing the original forest floor by the forest floor from the other stand increased PNN (1.5 fold in Douglas fir and 2 fold in native forest; P < 0.01; Figure 9C).
Discussion
Tree Species Affect NO3 − Cycling in Soil
At Breuil experimental site, our results confirmed the observations made by Moukoumi and others (2006) and Zeller and others (2007): there were two groups of stands with regards to NO3 − production and availability. In beech, Corsican pine, and Douglas fir, [NO3 −] and PNN were high and in Nordmann fir and spruce as well as in native forest, [NO3 −] and PNN were low. The [NO3 −] in the mineral soil at a given moment results from the balance between sources (atmospheric deposition of N and soil nitrification) and sinks (plant uptake, microbial immobilization and NO3 − leaching). The relationship between soil [NO3 −] in the field and PNN measured in the laboratory suggests that in this ecosystem, soil [NO3 −] was controlled by the same microbial processes active during the laboratory experiment and not by N deposition, uptake, and leaching.
Tree species may influence soil nitrification by changing the microclimate, the organic matter quality (Wedraogo and others 1993; Persson and others 2000; Goodale and Aber 2001; Joshi and others 2003; Lovett and others 2004), and properties of the root–soil interface in forest ecosystems. At Breuil, neither air temperature or soil moisture differed between H and L groups (Ranger and others 2004). The C/N ratio of litterfall and, as a result, the humus type may be the best indicator of PNN. The chemical and biochemical compositions of leaves were close in beech and native forest, but litterfall C/N was higher in the native forest because of a large amount of woody material (Ranger and others 2004). In beech and Douglas fir, PNN was high and litterfall C/N was low, but the pattern was opposite in spruce and Nordmann fir. Excluding the pine plantation in which the ground vegetation may have modified the litterfall C/N, the relationship between litterfall C/N and PNN was strong (r = −0.9; P = 0.02). A high litterfall C/N may slow litter decomposition and promote the formation of moder type humus with a high C/N. This organic matter accumulation may promote NH4 + immobilization and increase the production of nitrification inhibitor compounds. Particularly in spruce, organic compounds leached from needles and the humus layer inhibit soil nitrification (Brierley and others 2001; Suominen and others 2001; Smolander and others 2005). Zeller and others (2007) found no autotrophic nitrification activity in native forest although gross mineralization was high; in contrast, significant autotrophic nitrification activity was found in beech. Because the beech plantation was established in a clear-cut of an old beech stand (native forest), we believe that nitrifiers were present in the soil of the native forest, but suppressed, as they are presently in the spruce and fir plantations. The autotrophic nitrifier population was suppressed during the aging of the beech stand and the formation of the moder humus.
Forest Floor Control of Soil Nitrification
Forest floor [NO3 −] in native forest was very low compared to that in Douglas fir (Figure 9A) whereas the [NH4 +] was the same. After incubation in the laboratory, Trum (2004) extracted large amounts of NO3 − from Oi layers in Douglas fir at the same site and thereby showed active nitrification; in contrast, no NO3 − was extracted from the other stands. The transplantation of the forest floor of native forest in the Douglas fir soil strongly increased soil PNN. This may be because the NH4 + produced by the large amount of forest floor from the native forest could be transformed into NO3 − once inoculated with nitrifiers from Douglas fir roots or soil. Nevertheless, this means that if nitrification inhibitor compounds were produced in the humus of the native forest, then either their life span is short or they were produced by roots in the humus.
Removal of the forest floor in the native forest did not increase nitrification, which shows that the low nitrification in the native forest soil was not tightly connected to forest floor leachates, at least in the medium term. On the other hand, the addition of nitrifiers through the transplant of forest floor from Douglas fir onto the native forest soil was not sufficient to increase the nitrifying activity in native forest soil. Hence, the addition or removal of the forest floor was not sufficient to transform H soil into L or vice versa.
Finally, nitrification increased dramatically within 16 months in soil cores of the native forest, that is, the old beech coppice, that were transferred into the beech plantation; this occurred even though they were covered by their original forest floor. Because nothing except root colonization was changed, this suggests control by the roots.
Hierarchy and Timing of Nitrification Control
No change was observed in the [NO3 −] or in the PNN of soil cores in the HH and LL groups compared to undisturbed soil cores. This result confirmed that after 16 months, the disturbance caused by root cutting had no prolonged effect on nitrification. PNN was strongly enhanced when soil cores from L were transferred to H, but data points were extremely scattered between 30% and full nitrification. PNN was not significantly reduced after 28 months in soil cores from H transferred to L. However, the slope of the linear regression between PNN and PNM decreased slightly after 16 months and strongly after 28 months. Additionally, data points were extremely scattered from almost full nitrification to less than 10% nitrification, which illustrates the spatial variability of inhibition. Hence, inhibition of nitrification was much slower than stimulation, and both processes were spatially uneven. In response to a reciprocal transfer of soil cores in Oregon, Bottomley and others (2004) also showed that soils from a low nitrifying stand transferred to a high nitrifying stand nitrified at the same level as those of the high nitrifying stand. This stimulation was accompanied by an increase (10–100 fold) of ammonia-oxidizing bacteria populations to values that were similar to those measured in the high nitrification stand.
Two processes might explain the increase of nitrification in LH soil cores
-
(1)
Colonization of soil cores by active microorganisms implying different nitrifying communities between H and L stands. Two mechanisms of bacterial colonization have been described by Wertz and others (2007): (i) transport of bacteria in the water flow from the newly fallen litter of highly nitrifying stands and (ii) bacterial growth following root growth in the cores.
-
(2)
Activation of existing nitrifiers by release from inhibition or by release from competition for NH4 +. PNM and NH4 + availability were relatively unaffected by the soil transfer, suggesting that competition for NH4 + was not the main process slowing nitrification. Release from inhibition requires a reduction in the supply of inhibitor substances from needle leaching or roots. Again, because the forest floor from the original L stand was not removed, this suggests that root activity primarily controlled nitrification.
The low inhibition of nitrification in soil cores from H transferred to L may be because inhibiting substances transported in water or excreted by roots had little effect on nitrifier activities within the short period of time; inhibiting substances were probably not present in sufficient concentrations to quickly reduce the nitrifier population and inhibit nitrification in the whole of the soil core volume. It has been shown that at low concentrations (<100 mg/kg), inhibition substances, such as phenolic acids, may serve as a carbon source for some heterotrophic bacteria (Blum and Shafer 1988). White (1994) affirmed that, regardless of the mode of inhibition, the nature and the relative concentration of inhibiting substances are the primary factors controlling the magnitude of inhibition. In addition, the comparison between old and young beech suggests that nitrification inhibition is a process that builds up slowly over the long term.
Conclusions
Within 16 months, nitrification increased considerably in soils from low nitrification stands transferred into high nitrification stands. This rapid change may first be attributed to the progressive colonization of soil cores by nitrifiers, especially in the case of the native forest, and to the activation of existing nitrifiers by release from inhibition or from competition for NH4 +. Contrastingly, the reduction of nitrification in soils transferred from high nitrifying stands to low nitrifying stands was slow and extremely variable, and this may be due to the insufficient amount of inhibiting substances produced during the short period of our experiment.
Controls of nitrification by tree species were probably multifactorial and acted at different levels. Our experiments suggest that the inhibition of nitrification is not tightly connected to forest floor leachates, but that the forest floor both reflects and maintains the major ongoing processes. Hence, we believe that roots exerted the major short and medium term control on nitrification, and nitrification was probably controlled through root turnover, root litter production, and root exudates, which may vary with stand age. In the long term, humus build up and the production of inhibiting substances may completely block the nitrification activity.
Further work on root turnover may highlight the role of root litter production and root colonization on soil nitrification.
References
Aber JD, Nadelhoffer KJ, Steudler P, Mellilo JM. 1989. Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–86.
Andrianarisoa KS, Zeller B, Dupouey JL, Dambrine E. 2009. Comparing indicators of N status in 50 beech forests (Fagus sylvatica) in Northeastern France. For Ecol Manag 257:2241–53.
Augusto L, Ranger J. 2001. Impact of tree species on soil solutions in acidic conditions. Ann For Sci 58:47–58.
Augusto L, Dupouey JL, Ranger J. 2003. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann For Sci 60:823–31.
Barg AK, Edmonds RL. 1999. Influence of partial cutting on site microclimate soil nitrogen dynamics, and microbial biomass in Douglas-fir stands in western Washington. Can J For Res 29:705–13.
Berendse F. 1998. Effect of dominant plant species on soils during succession in nutrient-poor ecosystems. Biogeochemistry 42:73–88.
Blum U, Shafer SR. 1988. Microbial populations and phenolic acids in soil. Soil Biol Biochem 30:1077–89.
Bonneau M, Brethes A, Lacaze JF, Lelong F, Levy G, Nys C, Souchier B. 1977. Modifications de la fertilité des sols sous boisements artificiels de résineux purs. C.R. Fin d’Etude D.G.R.S.T., p 88.
Bottner P, Austrui J, Cortez J, Billes G, Couteaux MM. 1998. Decomposition of 14C and 15N labelled plant material, under controlled conditions, in coniferous forest soils from a North-South climatic sequence in Western Europe. Soil Biol Biochem 30:597–610.
Bottomley PJ, Taylor AE, Boule SA, McMahon SK, Rich JJ, Cromack K, Myrold DD. 2004. Responses of nitrification and ammonia-oxidizing bacteria to reciprocal transfers of soil between adjacent coniferous forest and meadow vegetation in the Cascade Mountains of Oregon. Microb Ecol 48:500–8.
Brierley EDR, Wood M, Shaw PJA. 2001. Influence of tree species and ground vegetation on nitrification in an acid forest soil. Plant Soil 229:97–104.
Calvaruso C, Mareschal L, Turpault MP, Leclerc E. 2009. Rapid clay weathering in the rhizosphere of Norway spruce and oak in an acid forest ecosystem. Soil Sci Soc Am J 73:331–8.
Colin-Belgrand M, Dambrine E, Bienaimé S, Nys C, Turpault MP. 2003. Influence of tree roots on nitrogen mineralization. Scand J For Res 18:260–8.
Falkengren-Grerup U, Brunet J, Diekmann M. 1998. Nitrogen mineralization in deciduous forest soils in south Sweden in gradients of soil acidity and deposition. Environ Pollut 102:415–20.
Falkengren-Grerup U, Schottelndreier M. 2004. Vascular plants as indicators of nitrogen enrichment in soils. Plant Ecol 172:51–62.
Goodale CL, Aber JD. 2001. The long-term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecol Appl 11:253–67.
Gower ST, Son Y. 1992. Differences in soil and leaf litterfall nitrogen dynamics for five forest plantations. Soil Sci Soc Am J 56:1959–66.
Harrison KA, Bol R, Bardgett RD. 2007. Preferences for different nitrogen forms by coexisting plant species and soil microbes. Ecology 88:989–99.
Herbauts J. 1974. Evaluation de la disponibilité potentielle en azote minéral dans différents types forestiers lorrains. Nancy: Université de Nancy I. p 66.
Jackson LE, Schimel JP, Firestone MK. 1989. Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem 21:409–15.
Joshi AB, Vann DR, Johnson AH, Miller EK. 2003. Nitrogen availability and forest productivity along a climosequence on Whiteface Mountain, New York. Can J For Res 33:1880–91.
Jussy JH, Colin-Belgrand M, Dambrine E, Ranger J, Zeller B, Bienaime S. 2004. N deposition, N transformation and N leaching in acid forest soils. Biogeochemistry 69:241–62.
Kanerva S, Kitunen V, Kiikkilä O, Loponen J, Smolander A. 2006. Response of soil C and N transformations to tannin fractions originating from Scots pine and Norway spruce needles. Soil Biol Biochem 38:1364–74.
Kraus TEC, Dahlgren RA, Zasoski RJ. 2003. Tannins in nutrient dynamics of forest ecosystems, a review. Plant Soil 256:41–66.
Kraus TEC, Zasoski RJ, Dahlgren RA, Horwath WT, Preston CM. 2004. Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species. Soil Biol Biochem 36:309–21.
Le Tacon F. 1976. La présence de calcaire dans le sol influence sur le comportement de l’Epicéa commun (Picea excelsa Link.) et du pin noir d’Autriche (Pinus nigra nigricans HOST.). Nancy : Institut National Polytechnique de Lorraine. p 214.
Li XG, Rengel Z, Mapfumo E, Singh B. 2007. Increase in pH stimulates mineralization of native organic carbon and nitrogen in naturally salt-affected sandy soils. Plant Soil 290:269–82.
Lovett GM, Lindberg SE. 1986. Dry deposition of nitrate to a deciduous forest. Biogeochemistry 2:137–48.
Lovett GM, Weathers KC, Arthur MA, Schultz JC. 2004. Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry 67:289–308.
Moukoumi J. 2006. Effet des essences forestières sur la biodégradation des matières organiques: impact sur la dynamique et le cycle du carbone de l’azote et des éléments minéraux. Nancy: Université Henri Poincaré. p 255.
Moukoumi J, Munier-Lamy C, Berthelin J, Ranger J. 2006. Effect of tree species substitution on organic matter biodegradability and mineral nutrient availability in a temperate topsoil. Ann For Sci 63:763–71.
Northup RR, Yu ZS, Dahlgren RA, Vogt KA. 1995. Polyphenol control of nitrogen release from pine litter. Nature 377:227–9.
Norton JM, Firestone MK. 1996. N dynamics in the rhizosphere of Pinus ponderosa seedlings. Soil Biol Biochem 28:351–62.
Nugroho RA, Röling WFM, Laverman AM, Verhoef HA. 2006. Net nitrification rate and presence of Nitrosospira cluster 2 in acid coniferous forest soils appear to be tree species specific. Soil Biol Biochem 38:1166–71.
Paavolainen L, Kitunen V, Smolander A. 1998. Inhibition of nitrification in forest soil by monoterpenes. Plant Soil 205:147–54.
Persson T, Rudebeck A, Jussy JH, Colin-Belgrand M, Priemé A, Dambrine E, Karlsson PS, Sjöberg RM. 2000. Soil nitrogen turnover—mineralisation, nitrification and denitrification in European forest soils. In: Schulze ED, Ed. Carbon and nitrogen cycling in European forest ecosystems. Berlin: Springer. p 297–331.
Pietri JCA, Brookes PC. 2008. Nitrogen mineralization along a pH gradient of a silty loam UK soil. Soil Biol Biochem 40:797–802.
Prescott CE, Preston CM. 1994. Nitrogen mineralization and decomposition in forest floors in adjacent plantations of western red cedar western hemlock and Douglas-fir. Can J For Res 24:2424–31.
Priha O, Hallantie T, Smolander A. 1999. Comparing microbial biomass, denitrification enzyme activity and numbers of nitrifiers in the rhizospheres of Pinus sylvestris, Picea abies and Betula pendula seedlings by microscale methods. Biol Fertil Soils 30:14–19.
Ranger J, Andreux F, Bienaimé S, Berthelin J, Bonnaud P, Boudot JP, Bréchet C, Buée M, Calmet JP, Chaussod R, Gelhaye D, Gelhaye L, Gerard F, Jaffrain J, Lejon D, Le Tacon F, Lévêque J, Maurice JP, Merlet D, Moukoumi J, Munier-Lamy C, Nourisson G, Pollier B, Ranjard L, Simonsson M, Turpault MP, Vairelles D, Zeller B. 2004. Effet des substitutions d’essence sur le fonctionnement organo-minéral de l’écosystème forestier, sur les communautés microbiennes et sur la diversité des communautés fongiques mycorhiziennes et saprophytes (cas du dispositif expérimental de Breuil - Morvan). INRA, Nancy. p 201.
Reydellet I, Laurent F, Olivier R, Siband P, Ganry F. 1997. Quantification par méthode isotopique de l’effet de la rhizosphère sur la minéralisation de l’azote (cas d’un sol ferrugineux tropical). Agronomie 320:843–7.
Russell AE, Raich JW, Valverde-Barrantes OJ, Fisher RF. 2006. Tree species effects on soil properties in experimental plantations in tropical moist forest. Soil Sci Soc Am J 71:1389–97.
Schaffers AP, Sykora KV. 2000. Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. J Veg Sci 11:225–44.
Schimel JP, Jackson LE, Firestone MK. 1989. Spatial and temporal effects on plant-microbial competition for inorganic nitrogen in a California annual grassland. Soil Biol Biochem 21:1059–66.
Schrijver AD, Geudens G, Augusto L, Staelens J, Mertens J, Wuyts K, Gielis L, Verheyen K. 2007. The effect of forest type on throughfall deposition and seepage flux: a review. Oecologia 153:663–74.
Scott NA, Binkley D. 1997. Foliage litter quality and annual net N mineralization: comparison across North American forest sites. Oecologia 111:151–9.
Smolander A, Loponen J, Suominen K, Kitunen V. 2005. Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol Biochem 37:1309–18.
Son Y, Lee IK. 1997. Soil nitrogen mineralization in adjacent stands of larch, pine and oak in Central Korea. Ann For Sci 54:1–8.
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL. 2006. A bioluminescence assay to detect nitrification inhibitors released from plant roots; case study with Brachiaria humidicola. Plant Soil 288:101–12.
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL. 2007. Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant Soil 294:5–18.
Suominen K, Kitunen V, Kotiaho T, Ketola R, Smolander A. 2001. Concentrations of volatile monoterpenes in forest soil microair under Picea abies, Pinus sylvestris, and Betula pendula. In: Weber J, Jamroz E, Drozd J, Karczewska A, Eds. Biochemical processes and cycling of elements in the environment. Wroclaw, Poland: Polish society for humic substances. p 371–2.
Templer P, Findlay S, Lovett G. 2002. Soil microbial biomass and nitrogen transformations among five tree species of the Catskill Mountains, New York, USA. Soil Biol Biochem 35:607–13.
Trum F. 2004. Impact de l’essence sur la composition chimique des litières et de leurs percolats en conditions contrôlées. Belgium: Faculté d’ingénierie biologique agronomique et environnementale, Université Catholique de Louvain. p 143.
USDA. 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. 2nd edn. Washington (DC): U.S. Gov. Print. Office.
Van Cleve K, Yarie J, Erickson R, Dyrness CT. 1993. Nitrogen mineralization and nitrification in successional ecosystems on the Tanana River floodplain, interior Alaska. Can J For Res 23:970–78.
Wedraogo FX, Belgy G, Berthelin J. 1993. Seasonal nitrification measurements with different species of forest litters applied to granite sand filled lysimeters in the field. Biol Fertil Soils 15:28–34.
Wertz S, Czarnes F, Bartoli F, Renault P, Commeaux C, Guillaumaud N, Clays-Josserand A. 2007. Early-stage bacterial colonization between a sterilized remoulded soil clod and natural soil aggregates of the same soil. Soil Biol Biochem 39:3127–37.
Whalen JK, Bottomley PJ, Myrold DD. 2001. Short-term nitrogen transformations in bulk and root-associated soils under ryegrass. Soil Biol Biochem 33:1937–45.
White C. 1986. Volatile and water-soluble inhibitors of nitrogen mineralization and nitrification in a ponderosa pine ecosystem. Biol Fertil Soils 2:97–104.
White C. 1994. Monoterpenes: their effects on ecosystem nutrient cycle. J Chem Ecol 20:1381–406.
Zeller B, Recous S, Kunze M, Moukoumi J, Colin-Belgrand M, Bienaime S, Ranger J, Dambrine E. 2007. Influence of tree species on gross and net N transformations in forest soils. Ann For Sci 64:151–8.
Zhong Z, Makeschin F. 2004. Comparison of soil nitrogen dynamics under beech, Norway spruce and Scots pine in central Germany. Eur J Forest Res 123:29–37.
Zhong Z, Makeschin F. 2006. Differences of soil microbial biomass and nitrogen transformation under two forest types in central Germany. Plant Soil 283:287–97.
Acknowledgements
We acknowledge “Conseil Regional Lorraine” and “Office National des Forêts” for the PhD grant. We sincerely thank Benoit Poillier, Gilles Nourrisson, Serge Didier, Pascal Bonnaud and Dominique Gelhaye for their assistance in the field and laboratory. We sincerely thank all the students of UR “Biogéochimie des Ecosystèmes Forestiers” of INRA Nancy for technical support during the field campaign. We are grateful to Linda Pardo from the USDA Forest Service in Burlington, and to Gregory van der Heijden for thoughtful reviews of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
Kasaina Sitraka Andrianarisoa conceived of or designed study, performed research, analyzed data and wrote the paper. Bernd Zeller conceived of or designed study, performed research. Frank Poly conceived of or designed study. Henri Siegenfuhr performed research, analyzed data. Severine Bienaimé performed research. Jacques Ranger conceived of or designed study. Etienne Dambrine conceived of or designed study, analyzed data, and wrote the paper.
Rights and permissions
About this article
Cite this article
Andrianarisoa, K.S., Zeller, B., Poly, F. et al. Control of Nitrification by Tree Species in a Common-Garden Experiment. Ecosystems 13, 1171–1187 (2010). https://doi.org/10.1007/s10021-010-9390-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-010-9390-x