Abstract
The recent decline of Mediterranean oak woodlands in SW Iberian Peninsula is related to insect pests which affect both cork oak (Quercus suber) and holm oak (Quercus rotundifolia). We identified twenty-six bird species as potential regular predators of twenty major pests by reviewing the diet of breeding, wintering and resident species in this ecosystem. Foraging guilds are strongly associated with predation at distinct stages of the pests’ life-cycle: ground-foragers prey on overwintering pupae and larvae of seed-borers, tree-foragers prey on eggs, larvae and pupae of defoliating and wood-boring pests, and aerial-sweepers prey on airborne imagines. Bird predation can cover the complete life-cycle of pest species because different species may be complementary due to a dissimilar exploitation of foraging niches and periods. Small generalist tree-foraging passerines are important pest predators given their high densities and widespread distribution in Mediterranean oak woodlands, but management practices can have a significant negative effect in their populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mediterranean oak woodlands extend over 6.3 million hectares in SW Iberian Peninsula, and are known as montados in Portugal and dehesas in Spain (Joffre et al. 1999; Fig. 1). This ecosystem is characterized by a scattered tree cover dominated by two evergreen Mediterranean oaks, cork oak (Quercus suber) and holm oak (Quercus rotundifolia), and has been structured by a systematic combination of agricultural, pastoral, and forestry uses (Marañón 1988; Pinto-Correia 1993; Plieninger and Wilbrand 2001). Traditional management has been developed for extensive livestock rearing, originally Iberian pigs but nowadays also sheep and cattle, which feed on acorns, from autumn to early spring, and herbs during the rest of the year (Joffre et al. 1988; Pinto-Correia and Mascarenhas 1999). Poor or non-agricultural land is mostly cultivated to prevent shrub invasion of grassland and to supply fodder and grain for livestock; harvesting is a secondary goal (Gómez Guttierez and Pérez Fernández 1996; Olea et al. 2005; Costa et al. 2009). Forestry management is aimed at the exploitation of cork, the most economically significant product of this system (Carvalho Mendes and Graça 2009; Ribeiro et al. 2010) and at enhancing crown coverage per tree for acorn production, as well as other side-products such as firewood.
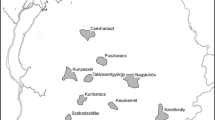
Distribution of cork oak (light grey) and holm oak (dark grey) and species coexistence areas (black) (adapted from Costa et al. 1998)
A sustainable human usage of Mediterranean oak woodlands conceivably occurred since the Middle Age, however, in the second half of the 19th century the decline and mortality of cork and holm oaks were first recorded for trees of distinct ages (Almeida 1898; Câmara-Pestana 1898). Most of the described symptoms can still be observed in present days: trunk cankers, wounds, resinous exudates from the bark, reduced branch growth, epicormic shooting, necrosis in the root cortex, defoliation and transparency of the crown, chlorosis, dieback and finally death (Branco and Ramos 2009). Several authors linked this slow decaying process to the incidence of pathogenic fungi, mainly Phytophthora cinnamomi and Biscogniauxia mediterranea, in trees periodically exposed to soil drought and dampness (Natividade 1945; Azevedo 1958; Barbosa 1958; Torres 1985; Brasier 1993; Cobos et al. 1993; Tuset et al. 1996; Vannini et al. 1996; Gallego et al. 1999; Luque et al. 1999, 2000; Santos 2003; Martín et al. 2005; Henriques et al. 2012; Serrano et al. 2012). Furthermore, insect pests which until recently did not markedly affect these ecosystems, except for sporadic outbreaks of defoliator insects (Neves 1950), boosted the physiological instability of trees and hampered the regeneration of cork and holm oaks.
Severe tree defoliations, caused mostly by moths, reduce acorn production, stem growth and, in the case of cork oaks, also cork growth (Magnoler and Cambini 1973; Ferreira and Ferreira 1991). Trees weakened by intense defoliation are exceptionally vulnerable to xylophagous pests, whose increasing population levels may not affect exclusively trees that are stressed, weakened or decaying, but healthy and young trees as well (Sousa et al. 1995; Sousa and Debouzie 1999, 2002; Sousa and Inácio 2005). Moreover, pre-dispersive acorn predation by carpophagous insects, comprising weevils and moths with seed-boring larvae, severely constrains cork and holm oak regeneration by affecting the emergence and survival of seedlings (Nogueira 1967; Aizpúrua 1993; Soria et al. 1996, 1999a, b; Siscart et al. 1999; Branco et al. 2002a; Leiva and Fernández-Alés 2005; Jiménez et al. 2006; Bonal and Muñoz 2007; Jiménez et al. 2011).
Numerous entomopathogens and arthropods (parasitoids, predators and competitors) can contribute to restrict cork and holm oak pest populations by controlling their abundance and distribution at different stages of the life-cycle (Ferreira and Ferreira 1991; Romanyk and Cadahia 1992; Villemant and Ramzi 1995; Villemant and Andreï-Ruiz 1999). Concerning insectivorous vertebrates, birds play an important role as pest predators in several agroforestry ecosystems (e.g., Solomon et al. 1976; Holmes et al. 1979; Kroll and Fleet 1979; Campbell et al. 1983; Loyn et al. 1983; Joern 1986; Fowler et al. 1991; Bock et al. 1992; Mols and Visser 2002; Hooks et al. 2003; Fayt et al. 2005; Ji et al. 2008; Koh 2008; Van Bael et al. 2008; Whelan et al. 2008; Johnson et al. 2010; Bereczki et al. 2014). Insectivorous birds are a dominant guild of bird communities in Mediterranean oak woodlands (Herrera 1978a; Rabaça 1990; Almeida 1992a, 1997; Peris and Masa 1992; Pulido and Díaz 1992; Finlayson et al. 2002; Santos et al. 2002; Camprodon and Brotons 2006; Godinho and Rabaça 2011; Leal et al. 2011a) but there is no comprehensive study about their role in controlling cork and holm oak pests. Actually, there are very few studies on birds’ diet and foraging ecology in montados and dehesas, and these are often published in the grey literature and not in English.
The present review aims to document: (i) which bird species can regularly feed on cork and holm oak pests in Mediterranean oak woodlands of the Iberian Peninsula; (ii) the relationship between the foraging niches used by bird predators and the distinct life-cycle stages of pests; and (iii) potential correlations between birds’ phenology and the biology of consumed pests. We additionally examined the effect of typical management strategies on bird assemblages and provide recommendations to enhance pest control by birds in montados and dehesas. Overall, this review provides a comprehensive framework on the role of birds as potential predators in controlling cork and holm oak insect pests (hereafter named pests), which will be particularly important to stimulate further studies on this issue.
Cork and holm oak pests
We listed twenty species of insects whose regular damages in montados and dehesas cause considerable economic losses (Table 1).
Lepidopterous larvae, particularly of Catocala spp., Euproctis chrysorrhoea, Lymantria dispar, Malacosoma neustria and Tortrix viridana, are the most important defoliators of cork and holm oak trees (Toimil 1987, 1989; Ferreira and Ferreira 1991; Villemant and Fraval 1991, 1999; Romanyk and Cadahia 1992). In some areas, extreme defoliations are also attributed to larval sawflies (Periclista spp.) and weevils (Orchestes spp.) (Silva 1960; Nogueira 1967; Baeta-Neves et al. 1972; Toimil 1987, 1989; Ferreira and Ferreira 1991), and larvae of Coeliodes ruber, another weevil species, dig galleries inside little branches stopping trees to burgeon (Ferreira and Ferreira 1991). Despite other phytophagous insects, such as gall inducing (e.g., gall midges and gall wasps) and sapsucker insects (e.g., aphids), often occur on trees, their damage is usually negligible (Aldrey 1981; Skuhravá et al. 1996; Villemant and Fraval 1991; Inácio et al. 2002).
Three main groups of wood-boring insects attack cork and holm oak trees: ambrosia beetles (particularly Platypus cylindrus), longhorn beetles (Cerambyx cerdo and Phymatodes testaceus) and buprestids of the genus Coraebus (Ferreira and Ferreira 1991; Villemant and Fraval 1991; Romanyk and Cadahia 1992). Ambrosia beetles are xylomycetophagous, coping with many genera of endosymbiotic fungi which will feed their larvae inside galleries in the wood, therefore acting as a vector for fungal diseases (Sousa et al. 1997; Sousa and Debouzie 2002; Henriques et al. 2009; Inácio et al. 2011). Longhorn beetles are considered secondary pests, however they open outsized holes in trees which can act as entryways for fungal infection (Soria et al. 1994a; Martín et al. 2005). Buprestids’ activity can take place either on the branches or trunk of the trees; Coraebus florentinus makes longitudinal and annular larval galleries under the bark of the branches, interrupting sap flow and thus causing branch death, whilst C. undatus larvae feed under the trunk bark of cork oak trees, digging galleries in the cambium, where the new cork tissue is formed (Natividade 1945; Benitez Morera 1961; Merle and Attié 1992; Soria et al. 1992, 1994a; Suñer and Abós 1994). The last species accounts for the most significant economic losses in cork production (Merle and Attié 1992), although cork spoilage as a consequence of the nest construction activity of an ant species, Crematogaster scutellaris, has also been frequently reported (Natividade 1945; Montoya Oliver 1988; Villagran and Ocete 1990; Villemant and Fraval 1991; Soria et al. 1994b).
The viability of acorns can be restricted by weevils (Curculio elephas) and moths (Cydia spp.). These oviposite inside developing acorns of cork and holm oak within which the feeding larva completes growth; after seed dropping, the larva buries itself into the soil where pupation takes place (Nogueira 1967; Aizpúrua 1993; Soria et al. 1996, 1999a, b; Siscart et al. 1999; Branco et al. 2002a; Leiva and Fernández-Alés 2005; Jiménez et al. 2006; Bonal and Muñoz 2007; Jiménez et al. 2011). Even if larval activity does not directly affect the embryo in some cases, attacked acorns are more vulnerable to rotting fungi which are responsible for higher postgermination mortality (Branco et al. 2002b).
Foraging niches of bird predators
The foraging niches of bird predators were used to explore the relationship with the different life-cycle stages of pests (Fig. 2; Table 2). We identified resident, breeding and wintering bird species as potential regular predators of cork and holm oak pests in SW Iberian Peninsula whenever their diet in Mediterranean oak woodlands or elsewhere notably includes the above mentioned pests or their taxonomic counterparts.
Relationship between the seasonal foraging niches used by bird predators and the different life-cycle stages of cork oak and holm oak insect pests in SW Iberian Peninsula. Potential prey are identified by numbers which refer to species listed in Table 1. For the scientific name of birds see text or Table 2
Ground-foragers: predation on overwintering pupae and larvae of seed-borers
Insectivorous birds foraging on or in the ground may be of special relevance as predators of pests whose larvae overwinter or pupate in the soil, such as weevils (C. ruber and C. elephas), sawflies (Periclista spp.) and moths (Cydia spp.).
The Eurasian Hoopoe Upupa epops probes in soil with its long curved bill (5–6 cm) to forage on buried larvae and pupae (Cramp and Perrins 1998). This large insectivorous specialist species is resident in SW Iberian Peninsula though less abundant during winter (BirdLife International 2004; SEO/BirdLife 2012). At least during breeding, in spring and early summer, it is an important predator of lepidopterous larvae and pupae in various woodlands of southern Europe (Gonzalez-Cano 1981; Battisti et al. 2000; Fournier and Arlettaz 2001) where it contributes to the regulation of forest pest populations under non-outbreak conditions (Battisti et al. 2000).
The Common Starling Sturnus vulgaris and the Spotless Starling Sturnus unicolor are opportunistic feeders which forage largely on the ground (Cramp and Perrins 1998). Much of their food is taken below soil surface making use of a special open-bill probing technique: individuals push the closed bill into the soil, open it to create a hole, and during bill-opening, eyes can rotate forward avoiding the necessity of turning head to one side to see into the hole (Beecher 1978). Pupal and larval lepidopterous, sawflies, and weevils are described as common prey of both species during the breeding season, when starlings are mainly insectivorous (Cramp and Perrins 1998). Yet, while the worldwide distribution of the Spotless Starling is greatly restricted to the Iberian Peninsula (BirdLife International 2004), the Common Starling is essentially a winter visitor (Motis et al. 1983; Tellería et al. 1988; SEO/BirdLife 2012). In montados of SE Portugal, hymenopterous larvae and lepidopterous larvae and pupae comprised 18 and 15 %, respectively, of the items given by the Spotless Starling to nestlings (Almeida 1996a). In dehesas of W Spain, adult birds consumed many imago and larval coleopterous, hymenopterous and larval lepidopterous (Peris 1980a) while nestlings’ diet comprised mostly larval and pupal lepidopterous and imago coleopterous (Peris 1980b). In NE Spain, although in a farmland ecosystem, larval weevils predominated in the diet of Spotless Starling adults while first year birds ate mostly imago weevils (Escartín Porta et al. 1996).
Besides being eaten by ground foraging birds while pupating in the soil, larvae of seed-borers (C. elephas and Cydia spp.) are also unintentionally preyed by granivorous birds feeding on acorns (intraguild predation). Infested acorns are prematurely abscised and the larvae complete their development inside the acorns after these drop on the ground (Bonal and Muñoz 2007), making them vulnerable to predation before the insect pupates in the ground. Between November and March, fallen acorns are a main food for six to seven million wood pigeons Columba palumbus (Purroy et al. 1984; Purroy 1988; Díaz and Martín 1998; Bea and Fernández-García 2001; Bea et al. 2003) and 155,000 common cranes Grus grus (Soriguer and Herrera 1977; Almeida and Pinto 1992; Díaz et al. 1996; Cruz 1998) wintering in Iberian Peninsula, although cranes’ distribution is fairly localised (Fernández-Cruz et al. 1981; Alonso and Alonso 1986; Almeida 1992b, 1996b; Prieta and Del Moral 2008; SEO/BirdLife 2012). Many corvids occurring in Mediterranean oak woodlands occasionally include acorns in their diets (Soler and Soler 1991) but only the Eurasian Jay Garrulus glandarius strongly depends on acorns for food, foraging on ground except when collecting acorns in autumn for hoarding (Bossema 1979; Gómez 2003; Pons and Pausas 2007a, b). Bird predation on infested acorns may decrease insect numbers up to the point of reducing acorn infestation rates (Drew 1987; Herrera 1989), but granivorous birds are acorn predators too. Furthermore, they have a direct negative effect on oaks’ regeneration as predators of uninfested acorns, which can be selected over infested acorns by some birds (Dixon et al. 1997). Therefore, cork and holm oaks may not necessarily receive a net benefit from birds’ intraguild predation on seed-borer larvae.
On the other hand, small abundant passerines may notably consume seed-borer larvae when feeding on the endosperm of cracked acorns without affecting viable ones. Fallen acorns can be extensively used by tits (Herrera 1980), particularly by the Great Tit Parus major which may spend 18 % of its foraging time on the ground during winter (Almeida and Granadeiro 2000). Also, wintering European robins Erithacus rubecula, which reach very high densities in the Mediterranean oak woodlands of SW Iberian Peninsula (Herrera 1978a, 1980; Tellería et al. 1988; Peris and Masa 1992; SEO/BirdLife 2012), greatly rely on acorn endosperm to increase weight (Herrera 1977), and it may represent approximately half of the diet during the mid-winter period (Debussche and Isenmann 1985).
Studies on the autumn–winter diet of ground-foraging birds in Mediterranean habitats (Herrera 1977, 1978b, 1984a; Cabello et al. 1991a) pointed out the importance of ants for several species, in particular for the Eurasian Hoopoe, the European Robin and the Stonechat Saxicola torquata which present substantial winter populations in montados and dehesas (Tellería et al. 1988; Peris and Masa 1992; SEO/BirdLife 2012). Between October and February, the proportion of ants in the invertebrate fraction of the diet was 76 % for the European Robin (Herrera 1977), 54 and 63 % for the Stonechat (Herrera 1984a; Cabello et al. 1991a, respectively), and 66 % for the Eurasian Hoopoe (Herrera 1984a). Although ants of the genera Messor and Lasius were the most common in their diets (Herrera 1984a; Cabello et al. 1991a), worker ants of the cork-boring species C. scutellaris may be preyed as well.
Tree-foragers: predation on defoliators and wood-borers
Three guilds of insectivores can be considered among tree-foraging birds: foliage-gleaners, bark-gleaners, and excavators. Foliage-gleaning is broadly used by birds during the spring-summer period, coinciding with the sprouting of young leaves on oak trees and the larval and pupal development of defoliator moths (Catocala spp., E. chrysorrhoea, L. dispar, M. neustria and T. viridana), sawflies (Periclista spp.) and weevils (Orchestes spp.). Since many oak pests lay eggs on the trunk, branches and twigs of trees, bark-gleaners may be relevant egg predators of defoliator moths (as well as their pupae), buprestids (Coraebus spp.) and longhorn beetles (C. cerdo and P. testaceus). Moreover, colonies of C. scutellaris may also be preyed by bark-gleaners, eating the eggs, larvae, pupae and imagines of this cork-boring ant species. The excavators’ guild is represented by forest specialists, namely woodpeckers, that feed on wood-boring insects (C. florentinus, C. undatus, C. cerdo, P. testaceus and P. cylindrus) when their larvae, pupae and imagines are enclosed in galleries inside wood.
Tits are the most representative group of tree-foraging insectivores, accounting with four resident species in montados and dehesas: Great Tit, Blue Tit Cyanistes caeruleus, Crested Tit Lophophanes cristatus, and Long-tailed Tit Aegithalos caudatus (Herrera 1978a, c, 1979; Rabaça 1990; Almeida 1992a; Peris and Masa 1992; Almeida and Granadeiro 2000; Finlayson et al. 2002; Leal et al. 2011a). In particular, the former two species are the most important foliage-gleaners both due to their high densities in Mediterranean oak woodlands (Herrera 1978a; Rabaça 1990; Peris and Masa 1992; Pulido and Díaz 1992; Díaz and Pulido 1993; Almeida 1997; Santos et al. 2002; Camprodon and Brotons 2006; Leal et al. 2011a) and their constant foraging activity on cork and holm oak trees (Herrera 1978b; Díaz and Pulido 1993; Pulido and Díaz 1994; Leal et al. 2011b, 2013), spending annually more than 65 % of their foraging time on foliage branches (Leal et al. 2013). Tits are generalist species, although during the breeding season their diet comprises around 90 % of phytophagous insects, mainly lepidopterous larvae (Cramp and Perrins 1998). Worldwide, tits have proved to be effective predators of oak defoliators, including T. viridana, Catocala spp. and Coeliodes spp. (Betts 1955; Romanyk and Cadahia 1992), in particular during the larval stage (Murakami and Nakano 2000; Sanz 2001). Foraging tits typically aggregate in areas where prey density is higher (Díaz et al. 1998) and positive numerical responses to outbreaks of defoliator moths’ larvae have been described in the Iberian Peninsula (Pimentel and Nilsson 2007, 2009). With the decrease of lepidopterous larvae along the breeding season, the proportion of pupae and eggs taken by tits from branches and twigs increases in both nestlings’ and adults’ diet (Cramp and Perrins 1998). Summer diet of Blue Tit in dehesas showed that both young and adult birds ingested a large proportion of Coleoptera, although soft-bodied prey may be underestimated by faecal analyses (Pulido and Díaz 1994).
Four species forage exclusively on trees, the Great Spotted Woodpecker Dendrocopos major, the Lesser Spotted Woodpecker D. minor, the Nuthatch Sitta europaea, and the Short-toed treecreeper Certhia brachydactyla, gleaning most of their year-round prey from trunks and large branches (Almeida and Granadeiro 2000; Leal et al. 2011b, 2013). These species consume eggs, larvae and pupae of Lepidoptera, including T. viridana, coleopterous imagines and ants caught in bark surface, cracks and crevices (Cramp and Perrins 1998). Nuthatch’s diet in dehesas was exclusively composed of invertebrates between March and August: a monthly percentage of 76–100 % of the stomachs analysed contained Coleoptera and, between March and June, 10–36 % contained Lepidoptera (Ceballos 1969). Nuthatches often hammer with bill when foraging, but apparently they are not able to chisel into wood to get wood-boring insects, unless it is rotten (Cramp and Perrins 1998). On the other hand, woodpeckers proficiently excavate wood to expose wood-borers not only in dead and decaying wood but also on trunks and branches of living hardwood trees (Solomon 1969). Due to the morphological adaptations, provided by the head and neck muscles and bones, the Great Spotted Woodpecker can drill holes up to 10 cm deep, by hammering bark and wood with lateral and vertical blows of bill. Moreover, it can probe fissures with its tongue almost twice larger than bill (~4 cm), making use of a sharp tip to impale soft-bodied prey while harder insects adhere to tongue bristles coated with sticky saliva (Cramp and Perrins 1998). Larvae, pupae and imagines of many buprestids, bark beetles, longhorn beetles, and weevils are an essential part of woodpeckers’ diet in addition to surface-dwelling insects (Cramp and Perrins 1998). For that reason, woodpeckers have been reported to play a significant role in the regulation of wood-boring pests in some forestry ecosystems in the Iberian Peninsula (Valente and Branco 2008).
During spring and summer, the Chaffinch Fringilla coelebs forages considerably on trees, although the ground is the main foraging substrate for the remainder of the year in Mediterranean oak woodlands (Herrera 1980; Almeida and Granadeiro 2000). Invertebrates represent the bulk of Chaffinch’s diet during this period and nestlings are fed mainly with leaf-dwelling insects, including defoliator lepidopterous larvae (Cramp and Perrins 1998). The Eurasian Jay also feeds nestlings with a large number of lepidopterous larvae from leaves of trees, including T. viridana in oak woodlands (Bossema 1979). Accordingly, in montados of SE Portugal, lepidopterous larvae and pupae comprised 42 % of the Azure-winged Magpie Cyanopica cyanus nestlings’ diet in terms of biomass (Canário et al. 2002). Two breeding migrants, the Great Spotted Cuckoo Clamator glandarius and the Common Cuckoo Cuculus canorus, also feed on late-instar lepidopterous larvae, including numerous colonial, hairy, and aposematic species (Valverde 1971; Gonzalez-Cano 1981; Cramp and Perrins 1998; Hoyas and López 1998). Cuckoos are highly adapted to deal with urticating caterpillars (e.g., E. chrysorrhoea, L. dispar and M. neustria) owing to their soft gizzard wall structure, and pellets of noxious hairs can be regurgitated together with chitin (Cramp and Perrins 1998). In a 36-year study of Common Cuckoo’s stomach content from central Europe (Link 1889, cited in Cramp and Perrins 1998), L. dispar and M. neustria were important prey and stomachs were often full with larvae of these two moth species (e.g., 173 larvae of M. neustria were found in a single stomach).
During the autumn–winter period, insectivorous passerines wintering or transient in Mediterranean habitats rely heavily on plant material, mainly fleshy fruits taken from shrubs and endosperm of dropped acorns, and include insects only as a minor part of their diets (Herrera 1977, 1981; Jordano 1981; Jordano and Herrera 1981; Herrera 1983, 1984b; Jordano 1987a, b, 1989; Cabello et al. 1991b; Herrera 1998). Nevertheless, the Chiffchaff Phylloscopus collybita and the Firecrest Regulus ignicapillus are tree-foraging migrants whose diet can be exclusively insectivorous while wintering in the Iberian Peninsula (Guitián 1985; Jordano 1987a). In montados and dehesas, these species forage together with resident tits at the outermost branches and twigs of cork and holm oak trees (Herrera 1979, 1980; Almeida and Granadeiro 2000; Leal et al. 2011b, 2013) where they may well glean for overwintering eggs and larvae of some lepidopterous pest species.
Aerial-sweepers: predation on airborne imagines
Aerial-sweepers are typically associated to open agro-forest habitats and reach higher densities in semi-open than in dense Mediterranean oak woodlands (Herrera 1978a; Finlayson et al. 2002; Santos et al. 2002; Camprodon and Brotons 2006; Godinho and Rabaça 2011). This guild comprises a few migratory breeding species which abundantly catch insects in flight during spring and summer (Herrera 1978a), coinciding with the airborne imago stage of cork and holm oak pests.
Most European species of aerial-sweepers correspond to hirundines and swifts which are known to prey mostly on Diptera but also on flying imagines of the orders Coleoptera, Hymenoptera and Lepidoptera (Cramp and Perrins 1998). In particular, the breeding densities of the Barn Swallow Hirundo rustica are much higher in areas with more livestock farming and rural architecture, which contribute to provide food resources and nesting sites, respectively (Ambrosini et al. 2002), and therefore they are likely to be more abundant in montados and dehesas with these characteristics. Airborne imagines of hymenopterous and weevils may represent profitable prey given that barn swallows feed preferentially on large insects (~6 mm), despite their relatively lesser abundance (Turner 1982).
Nightjars are fairly specialized in the crepuscular and nocturnal predation of lepidopterous imagines (Cramp and Perrins 1998). Moths can represent >80 % biomass in the diet of adults and up to 93 % in the diet of nestlings of the European Nightjar Caprimulgus europaeus in central Europe (Sierro et al. 2001). In SW Iberian Peninsula, this species occurs together with the Red-necked Nightjar Caprimulgus ruficolis, which is more common as a breeder in this region (Cuadrado and Dominguez 1996; Santos et al. 2002; Martí and Del Moral 2003; Equipa Atlas 2008). Since the imagines of lepidopterous species damaging cork and holm oak are predominantly active at dusk and at night, these may be an important prey for nightjars in Mediterranean oak woodlands with a sparse tree cover.
The European Bee-eater Merops apiaster is a common breeding visitor to SW Iberian Peninsula (Martí and Del Moral 2003; Equipa Atlas 2008). Hymenoptera are the most important prey in its diet, particularly honey bees Apis mellifera; pellet analysis from Spanish dehesas showed a considerable percentage (6–28 %) of coleopterous imagines, including longhorn beetles and weevils (Herrera and Ramirez 1974; Martínez 1984; Arenas and Torres 1987). Similar results were obtained in Portuguese montados, where the percentage of Coleoptera in the pellets varied between 11 and 42 % and included imagines of large longhorn beetles and weevils as well (Costa 1991).
Discussion
In this review we recognize the potential of twenty-six bird species as predators of the most relevant cork and holm oak pests in the Iberian Peninsula, which correspond to more than a third of the bird assemblage of montados and dehesas (Herrera 1978a; Rabaça 1990; Almeida 1992a, 1997; Peris and Masa 1992; Pulido and Díaz 1992; Finlayson et al. 2002; Santos et al. 2002; Camprodon and Brotons 2006; Godinho and Rabaça 2011; Leal et al. 2011a). Tree-foraging birds represent the most important predatory guild, either considering the number of species involved or their abundance, because several small ubiquitous passerine species present at high densities in this ecosystem forage on trees. Throughout Europe, including Mediterranean oak woodlands, canopy defoliation occurs mainly in spring, because the larval development of most phytophagous insects coincides with the sprouting of young leaves on oak trees (Herrera 1980). During this period, foliage-gleaning birds can reduce by 22–100 % the populations of forest lepidopterous pests which they feed on (Crawford and Jennings 1989; Parry et al. 1997; Tanhuanpää et al. 2001). In autumn and winter, the predation of bark-gleaning birds on overwintering egg masses can be an important factor controlling lepidopterous pest populations in montados and dehesas given that predation rates on L. dispar egg masses can go up to 53–71 % (Higashiura 1989; Cooper and Smith 1995). Eggs of Coleoptera and Hymenoptera are also potentially taken by bark-gleaners, although they are currently not referred in their diet, possibly because of their diminutive size and reduced importance in terms of biomass. On the other hand, great and lesser spotted woodpeckers may control wood-boring Coleoptera in Mediterranean oak woodlands, as it is suggested by excavator species regulating wood-borers populations in North American temperate forests (Kroll and Fleet 1979; Fayt et al. 2005; Norris and Martin 2008; Edworthy et al. 2011).
A predator community dominated by generalist species such as tits, nuthatches, treecreepers and chaffinches, may be enough to stabilize prey populations at low abundance levels, in agreement with the predictions of the generalist predation hypothesis (Murdoch and Oaten 1975), although their effect is likely to be noticeable only in non-outbreak circumstances (Crawford and Jennings 1989; Holmes 1990; Parry et al. 1997). On the other hand, specialist predation is characterized by a numerical response to the abundance of prey (Murdoch and Oaten 1975), therefore large insectivorous specialists such as cuckoos and woodpeckers, generally having broad territories and occurring at low densities, may increase their local abundances during outbreaks of prey (Fayt et al. 2005; Barber et al. 2008; Koenig et al. 2011; Edworthy et al. 2011). In most cases, an effective regulation of prey populations is achieved through a combined effect of specialist and generalist predators (Symondson et al. 2002), as we suggest for the birds in the present review. In Mediterranean oak woodlands, different species of specialist and generalist birds may be complementary in space and time, and this review suggests that distinct foraging niches and periods allow a temporal succession of predation covering the complete life-cycle of most pests.
Conclusions and management implications
Seasonal differences in bird density and species richness in Mediterranean evergreen oak forests are less marked than in more northern European forests, with a larger number of resident and migrating species owing to the mild climate, the evergreen conditions and the geographical location along the migratory routes to Africa (López-Iborra and Gil-Delgado 1999). Therefore, an appropriate management of Mediterranean oak woodlands in SW Iberian Peninsula to sustain healthy bird communities should be advantageous to keep insect populations at low levels and prevent pest outbreaks. Management is a key factor promoting bird diversity in Mediterranean oak woodlands by creating distinct habitat types (Díaz et al. 1997; Tellería 2001; Bugalho et al. 2011). However, considerable changes in taxonomic and functional diversity of bird communities take place at a local scale according to management regime (Rabaça 1990; Almeida 1992a; Pulido and Díaz 1992, 1997; Camprodon and Brotons 2006; Godinho and Rabaça 2011; Leal et al. 2011b, 2013; Pereira et al. 2014a). A decrease in natural regulation of pests by birds may outcome from common management practices, such as undergrowth clearing, tree thinning, canopy pruning and cork extraction, as these significantly reduce foraging and nesting resources for tree-foraging birds which are the most relevant guild of pest predators.
Undergrowth clearing decreases both species richness and abundance in Mediterranean oak woodlands (Rabaça 1990; Almeida 1992a; Pulido and Díaz 1992; Camprodon and Brotons 2006; Pereira et al. 2014a). This practice largely affects bird species depending directly on shrubs (e.g., Sylvia warblers) but there is also a considerable decline in the density and diversity of small tree-foraging passerine species which regularly seek food or refuge in the understory (Rabaça 1990; Almeida 1992a; Pulido and Díaz 1992; Camprodon and Brotons 2006; Godinho and Rabaça 2011). As most managed Mediterranean oak woodlands have understory shrubs removed, the presence of other habitat fragments, such as olive groves and riparian galleries, may help to sustain higher densities of tree-foraging species in the surrounding woodland matrix, as it was observed for Great Tit, Blue Tit and Chaffinch (Leal et al. 2011a; Pereira et al. 2014b).
Tree density is generally correlated with an increase of forest bird species and a decrease of ground-foragers (Tellería 2001; Santos et al. 2002; Díaz et al. 2003; Camprodon and Brotons 2006; Pereira et al. 2014a). Therefore, non-thinned woodlands are expected to favour the abundance and richness of tree-foraging species as a result of improved foraging and nesting opportunities. In fact, Blue Tit abundance in Spanish dehesas was strongly correlated with tree density and with the availability of tree holes for nesting (Pulido and Díaz 1997).
Cork extraction from cork oak trees is usually carried out every 9 years reducing food availability for bark-gleaners, but in the meantime a new cork layer suitable for arthropod prey is developing. Densities of both bark- (Nuthatch, Short-toed Treecreeper) and bark-foliage-gleaners (Great Tit, Blue Tit) were lower in areas with younger cork (Almeida 1992a; Godinho and Rabaça 2011; Leal et al. 2011b) and even though species richness is apparently not influenced by cork age (Leal et al. 2011b), woodpeckers and other species with broad territories or occurring in low densities can leave from recently debarked areas (Almeida 1992a). Cork exploitation regimes comprising trees with different cork ages in the same area may support high densities of bark-gleaning species, although lower than those in areas with only old cork (Leal et al. 2011b).
Maintenance pruning is often conducted on cork and holm oak trees to remove outermost branches and foliage from the canopy. This practice predominantly affects foliage-gleaning species by reducing foraging substrate and consequently the amount of available prey, although the elimination of cavities may have a negative effect on hole-nesting species (Leal et al. 2013). Leal et al. (2013) showed that densities of Great Tit, Blue Tit and wintering Chiffchaff were lower in pruned than in unpruned areas, and suggested a similar pattern for other foliage-gleaning species (Crested Tit, Long-tailed Tit and Firecrest).
Finally, artificial nest-boxes have been used in various ecosystems to control pests by increasing breeding populations of hole-nesting predators (East and Perrins 1988; Wang and Liao 1990; Sanz 2001; Mols and Visser 2002; Bouvier et al. 2005). Small abundant tree-foraging passerines (Great Tit, Blue Tit, Nuthatch and Short-toed Treecreeper) are the most common hole-nesting species occurring in montados and dehesas. If local breeding populations of these species are limited by shortage of cavities in trees, the provision of artificial nest-boxes may enhance bird predation on most cork and holm oak pests.
References
Aizpúrua CG (1993) Cydia fagiglandana (Zeller, 1841). Lep. Tortricidae, en España. Bol San Veg Plagas 19:389–400
Aldrey N (1981) Contribución al conocimiento de los cinípidios gallícolas (Hym., Cynipidae) en la encina y el alcornoque, en la provincia de Salamanca. Bol Asoc Esp Entomol 5:59–74
Almeida V (1898) Àcerca dos montados de sobro. Agric Contemp 8:375–381
Almeida J (1992a) Alguns aspectos dos efeitos do maneio dos montados de sobro Quercus suber na avifauna nidificante. Airo 3:69–74
Almeida J (1992b) Censos de Grous Grus grus invernantes em Portugal. Airo 3:55–58
Almeida JF (1996a) Análise da dieta de pintos de Estorninho-preto Sturnus unicolor por aplicação do ‘método do colar’. Airo 7:63–68
Almeida J (1996b) Situation and conservation perspectives of the Common Crane in Portugal. Alytes 8:99–104
Almeida J (1997) Caracterização da avifauna nidificante num montado de azinho Quercus rotundifolia por aplicação do método dos mapas: dois anos de estudo. Airo 8:1–6
Almeida J, Granadeiro JP (2000) Seasonal variation of foraging niches in a guild of passerine birds in a cork-oak woodland. Ardea 88:243–252
Almeida J, Pinto M (1992) Selecção dos biótopos de alimentação pelo Grou-comum Grus grus: o caso de Moura (Alentejo). Airo 3:1–8
Alonso JA, Alonso JC (1986) Demographic parameters of the Common Crane (Grus g. grus) population wintering in Iberia. Aquila 93–94:137–143
Ambrosini R, Bolzern AM, Canova L, Arieni S, Moller AP, Saino N (2002) The distribution and colony size of barn swallows in relation to agriculture land use. J Appl Ecol 39:524–534
Arenas R, Torres JA (1987) Apuntes sobre la dieta alimenticia del Abejaruco (Merops apiaster) en la provincia de Córdoba. Oxyura 4:171–176
Azevedo NFS (1958) O carvão do entrecasco do sobreiro. Publ Dir Geral Serv Florest Aquícolas 25:159–179
Baeta-Neves CML, Cabral MTC, Nogueira CDS, Ferreira LJC (1972) Bio-ecologia da Tortrix viridana L. e combate da Lymantria dispar L. pela luta biológica. Instituto Superior de Agronomia, Lisbon
Barber NA, Marquis RJ, Tori WP (2008) Invasive prey impacts regional distribution of native predators. Ecology 89:2678–2683
Barbosa MA (1958) O carvão do entrecasco Hypoxylon mediterraneum (de Not.) Ces. et de Not.: Contribuição para o seu estudo. Publ Dir Geral Serv Florest Aquícolas 25:93–132
Battisti A, Bernardi M, Ghiraldo C (2000) Predation by the hoopoe on pupae of Thaumetopoea pityocampa and the likely influence on other natural enemies. Biocontrol 45:311–323
Bea A, Fernández-García JM (2001) Censo y distribución de los efectivos de paloma torcaz Columba palumbus invernantes en la península Ibérica. Naturzale Cuad Cienc Nat 16:103–115
Bea A, Beitia R, Fernández JM (2003) The census and distribution of wintering woodpigeons Columba palumbus in the Iberian peninsula. Ornis Hung 12–13:157–167
Beecher WJ (1978) Feeding adaptations and evolution in the starlings. Bull Chic Acad Sci 11:269–298
Benitez Morera A (1961) Los coleópteros bupréstidos del género Coroebus Lap. en los alcornocales de Cadiz. Bol Serv Plagas For 7:55–61
Bereczki K, Ódor P, Csóka G, Mag Z, Báldi A (2014) Effects of forest heterogeneity on the efficiency of caterpillar control service provided by birds in temperate oak forests. For Ecol Manag 327:96–105
Betts MM (1955) The food of titmice in oak woodland. J Anim Ecol 24:282–323
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. BirdLife International, Cambridge
Bock CE, Bock JH, Grant MC (1992) Effects of bird predation on grasshopper densities in an Arizona grassland. Ecology 73:1706–1717
Bonal R, Muñoz A (2007) Multi-trophic effects of ungulate intraguild predation on acorn weevils. Oecologia 152:533–540
Bossema I (1979) Jays and oaks: an eco-ethological study of a symbiosis. Behaviour 70:1–117
Bouvier JC, Toubon JF, Boivin T, Sauphanor B (2005) Effects of apple orchard management strategies on the great tit (Parus major) in southeastern France. Environ Toxicol Chem 24:2846–2852
Branco M, Ramos AP (2009) Chapter 9: Coping with pests and diseases. In: Aronson J, Pereira JS, Pausas JG (eds) Cork oak woodlands on the edge: ecology, adaptative management, and restoration. Island Press, Washington, pp 103–111
Branco M, Branco C, Merouani H, Almeida MH (2002a) Effect of insect predation on the quality and storage conditions of acorns of cork oak (Quercus suber L.). IOBC/WPRS Bull 25:163–167
Branco M, Branco C, Merouani H, Almeida MH (2002b) Germination success, survival and seedling vigour of Quercus suber acorns in relation to insect damage. For Ecol Manag 166:159–164
Brasier CM (1993) Phytophthora cinnamomi as a contributory factor in European oak declines. In: Luisi N, Lerario P, Vannini A (eds) Recent advances in studies on oak decline. Tipolitografia Radio, Bari, pp 49–57
Bugalho MN, Caldeira MC, Pereira JS, Aronson J, Pausas JG (2011) Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front Ecol Environ 9:278–286
Cabello AM, Soler M, Soler JJ (1991a) Alimentación de la Tarabilla Común (Saxicola torquata) en el sureste de la Península Ibérica durante el período Otoño-Invierno. Ardeola 38:317–326
Cabello AM, Soler M, Soler JJ (1991b) Alimentación del Acentor Común (Prunella modularis) durante su invernada en el sureste de la Península Ibérica. Ardeola 38:305–315
Câmara-Pestana J (1898) Nova doença dos sobreiros. Arch Rural 36:297–298
Campbell RW, Torgersen TR, Srivastava N (1983) A suggested role for predaceous birds and ants in the population dynamics of the western spruce budworm. For Sci 29:779–790
Camprodon J, Brotons L (2006) Effects of undergrowth clearing on the bird communities of the Northwestern Mediterranean Coppice Holm oak forests. For Ecol Manag 221:72–82
Canário F, Boieiro M, Vicente L (2002) The nestling diet of the Iberian Azure-winged Magpie Cyanopica cyanus cooki in southestern Portugal. Ardeola 49:283–286
Carvalho Mendes AMS, Graça JAR (2009) Chapter 5: Cork bottle stoppers and other cork products. In: Aronson J, Pereira JS, Pausas JG (eds) Cork oak woodlands on the edge: ecology, adaptative management, and restoration. Island Press, Washington, pp 59–69
Ceballos P (1969) Estudio de alimentación del Trepador azul (Sitta europaea) en encinares durante los meses de marzo-agosto. Bol Serv Plagas For 24:89–95
Cobos JM, Montoya R, Tuset JJ (1993) New damages to the Quercus woodlands in Spain. Preliminary evaluation of the possible implication of Phytophthora cinnamomi. In: Luisi N, Lerario P, Vannini A (eds) Recent advances in studies on oak decline. Tipolitografia Radio, Bari, pp 163–169
Cooper RJ, Smith HR (1995) Predation on gypsy moth (Lepidoptera: Lymantriidae) egg masses by birds. Environ Entomol 24:571–575
Costa LT (1991) Apiculture and the diet of breeding European Bee-eater Merops apiaster. Airo 2:34–42
Costa M, Morla C, Sainz H (eds) (1998) Los bosques ibéricos, una interpretacción geobotánica. Editorial Planeta, Barcelona
Costa A, Pereira H, Madeira M (2009) Landscape dynamics in endangered cork oak woodlands in Southwestern Portugal (1958–2005). Agrofor Syst 77:83–96
Cramp S, Perrins CM (eds) (1998) The complete birds of the western Paleartic. BWP on CD-ROM. Oxford University Press, Oxford
Crawford HS, Jennings DT (1989) Predation by birds on spruce budworm Choristoneura fumiferana: functional, numerical, and total responses. Ecology 70:152–163
Cruz CM (1998) Contribuição para o estudo da dieta de Grou Grus grus em áreas ocidentais de invernia na Península Ibérica. Airo 9:16–18
Cuadrado M, Dominguez F (1996) Phenology and breeding success of Red-necked Nightjar Caprimulgus ruficollis in Southern Spain. J Ornithol 137:249–253
Debussche M, Isenmann P (1985) Frugivory of transient and wintering European robins Erithacus rubecula in a Mediterranean region and its relationship with ornithochory. Ecography 8:157–163
Díaz M, Martín P (1998) Habitat selectivity by wintering woodpigeons (Columba palumbus) in Holm-Oak (Quercus ilex) dehesa of Central Spain. Gibier Faune Sauvag 15:167–181
Díaz M, Pulido F (1993) Relaciones entre la abundancia de artrópodos y la densidad del Herrerillo Común (Parus caeruleus) en dehesas durante el período reproductor. Ardeola 40:33–38
Díaz M, González E, Muñoz-Pulido R, Naveso MA (1996) Habitat selection patterns of common cranes Grus grus wintering in holm oak Quercus ilex dehesas of central Spain: effects of human management. Biol Conserv 75:119–123
Díaz M, Campos P, Pulido FJ (1997) The Spanish dehesas: a diversity of land use and wildlife. In: Pain D, Pienkowski M (eds) Farming and birds in Europe. Academic Press, London, pp 178–209
Díaz M, Illera JC, Atienza JC (1998) Food resource matching by foraging tits Parus spp. during spring-summer in a Mediterranean mixed forest; evidence for an ideal free distribution. Ibis 140:654–660
Díaz M, Pulido FJ, Marañón T (2003) Diversidad biológica y sostenibilidad ecológica y económica de los sistemas adehesados. Ecosistemas 2003/3
Dixon MD, Johnson WC, Adkisson CS (1997) Effects of weevil larvae on acorn use by blue jays. Oecologia 111:201–208
Drew RAI (1987) Reduction in fruit fly (Tephritidae: Dacinae) populations in their endemic rainforest habitat by frugivorous vetebrates. Aust J Zool 35:283–288
East ML, Perrins CM (1988) The effect of nestboxes on breeding populations of birds in broadleaved temperate woodlands. Ibis 130:393–401
Edworthy AB, Drever MC, Martin K (2011) Woodpeckers increase in abundance but maintain fecundity in response to an outbreak of mountain pine bark beetles. For Ecol Manag 261:203–210
Equipa A (2008) Atlas das Aves Nidificantes em Portugal (1999–2005). ICNB, SPEA, PNM, SRAM. Assírio & Alvim, Lisbon
Escartín Porta E, Basarán Conde E, Pedrocchi Renault C (1996) Datos relativos a la alimentación del estornino negro (Sturnus unicolor) en la provincia de Huesca. Lucas Mallada 8:29–39
Fayt P, Machmer MM, Steeger C (2005) Regulation of spruce bark beetles by woodpeckers: a literature review. For Ecol Manag 206:1–14
Fernández-Cruz M, Alonso J, Garcia-Rúa A, Pereira P, Pérez-Chiscano JL, Veiga JP (1981) La migración e invernada de la grulla común (Grus grus) en España. Resultados del Proyecto Grus (Crane Project). Ardeola 26–27:1–164
Ferreira MC, Ferreira GWS (1991) Pragas das Folhosas. Ministério da Agricultura, Pescas e Alimentação, Lisbon
Finlayson C, Finlayson G, Mosquera M, Fa D (2002) The breeding bird community in the Cork Oak woodlands of Cádiz and its significance in the context of the south and west of the Iberian Peninsula. Almoraima 27:27–31
Fournier J, Arlettaz R (2001) Food provision to nestlings in the Hoopoe Upupa epops: implications for the conservation of a small endangered population in the Swiss Alps. Ibis 143:2–10
Fowler AC, Knight RL, George TL, McEwen LC (1991) Effects of avian predation on grasshopper populations in North Dakota grasslands. Ecology 72:1775–1781
Gallego FJ, de Algaba AP, Fernandez-Escobar R (1999) Etiology of oak decline in Spain. Eur J For Pathol 29:17–27
Godinho C, Rabaça JE (2011) Birds like it Corky: the influence of habitat features and management of ‘montados’ in breeding bird communities. Agrofor Syst 82:183–195
Gómez JM (2003) Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 26:573–584
Gómez Guttierez JM, Pérez Fernández M (1996) The dehesas, silvopastoral systems in semiarid Mediterranean regions with poor soils, seasonal climate and extensive utilisation. In: Etienne M (ed) Western european silvopastoral systems. INRA Editions, Paris, pp 55–70
Gonzalez-Cano JM (1981) Predación de ‘Procesionaria del pino’ por vertebrados en la zona de Mora de Rubielos (Teruel). Bol Estac Cent Ecol 10:53–77
Guitián J (1985) Datos sobre el regimen alimenticio de los paseriformes de un bosque montano de la cordillera cantábrica occidental. Ardeola 32:155–172
Henriques J, Inácio ML, Sousa E (2009) Fungi associated to Platypus cylindrus Fab. (Coleoptera: Platypodidae) in Cork Oak. Revista Ciênc Agrár 32:56–66
Henriques J, Inácio ML, Lima A, Sousa E (2012) New outbreaks of charcoal canker on young cork oak trees in Portugal. IOBC/WPRS Bull 76:85–88
Herrera CM (1977) Ecología alimenticia del Petirrojo (Erithacus rubecula) durante su invernada en encinares del Sur de España. Doñana Acta Vertebr 4:35–59
Herrera CM (1978a) Ecological correlates of residence and non–residence in a Mediterranean passerine bird community. J Anim Ecol 47:871–890
Herrera CM (1978b) Datos sobre la dieta invernal del colirrojo tizón (Phoenicurus ochruros) en encinares de Andalucía occidental. Doñana Acta Vertebr 5:61–71
Herrera CM (1978c) Niche-shift in the genus Parus in Southern Spain. Ibis 120:236–240
Herrera CM (1979) Ecological aspects of heterospecific flocks formation in a Mediterranean passerine bird community. Oikos 33:85–96
Herrera CM (1980) Composición y estructura de dos comunidades mediterráneas de passeriformes. Doñana Acta Vertebr 7:1–340
Herrera CM (1981) Fruit food of Robins wintering in southern Spanish Mediterranean shrubland. Bird Study 28:115–122
Herrera CM (1983) Coevolución de plantas y frugívoros: la invernada mediterránea de algunos passeriformes. Alytes 1:177–190
Herrera CM (1984a) Significance of ants in the diet of insectivorous birds in southern Spanish Mediterranean habitats. Ardeola 30:77–81
Herrera CM (1984b) A study of avian frugivores, bird-dispersed plants and their interaction in Mediterranean shrubland. Ecol Monogr 54:1–23
Herrera CM (1989) Vertebrate frugivores and their interaction with invertebrate fruit predators: supporting evidence from a Costa Rican dry forest. Oikos 54:23–32
Herrera CM (1998) Long–term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12–year study. Ecol Monogr 68:511–538
Herrera CM, Ramirez A (1974) Food of Bee-eaters in southern Spain. Br Birds 67:158–164
Higashiura Y (1989) Survival of eggs in the gypsy moth Lymantria dispar. I. Predation by birds. J Anim Ecol 58:403–412
Holmes RT (1990) Ecological and evolutionary impacts of bird predation on forest insects: an overview. Stud Avian Biol 13:6–13
Holmes RT, Schultz JC, Nothnagle P (1979) Bird predation on forest insects: an exclosure experiment. Sciences 206:462–463
Hooks CRR, Pandey RR, Johnson MW (2003) Impact of avian and arthropod predation on lepidopteran caterpillar densities and plant productivity in an ephemeral agroecosystem. Ecol Entomol 28:522–532
Hoyas J, López F (1998) Distribución del críalo según la abundancia de procesionaria del pino. Quercus 149:20–22
Inácio ML, Naves P, Moreira M, Sousa EM (2002) Gall inducing insects associated with oak trees (Quercus spp.) in Portugal. IOBC/WPRS Bull 25:159–162
Inácio ML, Henriques J, Sousa E (2011) Contribution of symbiotic fungi to cork oak colonization by Platypus cylindrus (Coleoptera: Platypodidae). Silva Lusit 19:89–99
Ji R, Simpson SJ, Yua F, He QX, Yun CJ (2008) Diets of migratory rosy starlings (Passeriformes: Sturnidae) and their effects on grasshoppers: implications for a biological agent for insect pests. Biol Control 46:547–551
Jiménez A, Soria FJ, Villagrán M, Ocete ME (2006) Seguimiento del ciclo biológico de Cydia fagigalndana (Zeller) (Lepidoptera: Tortricidae) en un encinar del sur de España. Bol San Veg Plagas 32:159–168
Jiménez A, Maistrello L, Lopez MA, Ocete ME, Soria FJ (2011) Spatial distribution of Cydia fagiglandana (Zeller) in an exploited holm oak (Quercus ilex L.) forest. Span J Agric Res 9:570–579
Joern A (1986) Experimental study of avian predation on coexisting grasshopper populations (Orthoptera: Acrididae) in a sandhills grassland. Oikos 46:243–249
Joffre R, Vacher J, De Los Llanos C, Long G (1988) The dehesa: an agrosilvopastoral system of the Mediterranean region with special reference to the Sierra Morena area of Spain. Agrofor Syst 6:71–96
Joffre R, Rambal S, Ratte JP (1999) The dehesa system of southern Spain and Portugal as a natural ecosystem mimic. Agrofor Syst 45:57–79
Johnson MD, Kellermann JL, Stercho AM (2010) Pest reduction services by birds in shade and sun coffee in Jamaica. Anim Conserv 13:140–147
Jordano P (1981) Alimentación y relaciones tróficas entre los Passeriformes en paso otoñal por una localidad de Andalucía central. Doñana Acta Vertebr 8:103–124
Jordano P (1987a) Notas sobre la dieta no-insectívora de algunos Muscicapidae. Ardeola 34:89–98
Jordano P (1987b) Frugivory, external morphology and digestive system in Mediterranean sylviid warblers Sylvia spp. Ibis 129:175–189
Jordano P (1989) Variación de la dieta frugívora otoño-invernal del Petirrojo (Erithacus rubecula): efectos sobre la condición corporal. Ardeola 36:161–183
Jordano P, Herrera CM (1981) The frugivorous diet of Blackcap populations (Sylvia attricapilla) wintering in southern Spain. Ibis 123:502–507
Koenig WD, Walters EL, Liebhold AM (2011) Effects of gypsy Moth outbreaks on North American woodpeckers. Condor 113:352–361
Koh LP (2008) Birds defend oil palms from herbivorous insects. Ecol Appl 18:821–825
Kroll JC, Fleet RR (1979) Impact of woodpecker predation on over-wintering within-tree populations of the southern pine beetle (Dendroctonus frontalis). In: Dickson JG (ed) The role of insectivorous birds in forest ecosystems. Academic Press, New York, pp 269–281
Leal AI, Martins RC, Palmeirim JM, Granadeiro JP (2011a) Influence of habitat fragments on bird assemblages in Cork Oak woodlands. Bird Study 58:309–320
Leal AI, Correia RA, Granadeiro JP, Palmeirim JM (2011b) Impact of cork extraction on birds: relevance for conservation of Mediterranean biodiversity. Biol Conserv 144:1655–1662
Leal AI, Correia RA, Palmeirim JM, Granadeiro JP (2013) Does canopy pruning affect foliage-gleaning birds in managed cork oak woodlands? Agrofor Syst 87:355–363
Leiva MJ, Fernández-Alés R (2005) Holm-oak (Quercus ilex subsp. ballota) acorns infestation by insects in Mediterranean dehesas and shrublands—its effect on acorn germination and seedling emergence. For Ecol Manag 212:221–229
Link JA (1889) Monatsschr. Dtsch. Ver. Schutze Vogelwelt 14:439–453 (Cited in: Cramp and Perrins 1998)
López-Iborra G, Gil-Delgado JA (1999) Composition and dynamics of bird communities. In: Rodà F, Retana J, Gracia CA, Bellot J (eds) Ecology of Mediterranean evergreen oak forests. Springer, Berlin, pp 355–368
Loyn RH, Runnalls RG, Forward GY (1983) Territorial bell miners and other birds affecting populations of insect prey. Sciences 221:1411–1413
Luque J, Cohen M, Savé R, Biel C, Álvarez IF (1999) Effects of three fungal pathogens on water relations, chlorophyll fluorescence and growth of Quercus suber L. Ann For Sci 56:19–26
Luque J, Parlade J, Pera J (2000) Pathogenicity of fungi isolated from Quercus suber in Catalonia (NE Spain). For Pathol 30:247–263
Magnoler A, Cambini A (1973) Radial growth of cork oak and the effects of defoliation caused by larvae of Lymantria dispar and Malacosoma neustria. Bol Inst Prod Florest Cortiça 35:53–59
Marañón T (1988) Agro-sylvo-pastoral systems in the Iberian Peninsula: Dehesas and Montados. Rangel 10:255–258
Martí R, Del Moral JC (eds) (2003) Atlas de las aves reproductoras de España. SEO/BirdLife, Dirección General de Conservación de la Naturaleza, Madrid
Martín J, Cabezas J, Buyolo T, Patón D (2005) The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterraneum infection on Quercus suber forests. For Ecol Manag 216:166–174
Martínez C (1984) Notes sur l’alimentation du Guêpier d’Europe (Merops apiaster L.) dans une colonie du centre de l’Espagne. Alauda 52:45–50
Merle P, Attié M (1992) Coroebus undatus (Coleoptera: Buprestidae) sur chêne-liège dans le Sud-Est de la France: estimation des dégâts, relations entre ceux-ci et certains facteurs du milieu. Ann Sci For 49:571–588
Mols CMM, Visser ME (2002) Great tits can reduce caterpillar damage in apple orchards. J Appl Ecol 39:888–899
Montoya Oliver JM (1988) Los Alcornocales (Quercus suber L.). Ministerio de Agricultura, Pesca y Alimentación, Madrid
Motis A, Mestre P, Martínez A (1983) La colonización y expansión del estornino pinto (Sturnus vulgaris L.) y del estornino negro (Sturnus unicolor Temm.) en Cataluña (NE de la Península Ibérica). Misc Zool 7:131–137
Murakami M, Nakano S (2000) Species-specific bird functions in a forest-canopy food web. Proc R Soc Lond B 267:1597–1601
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Natividade JV (1945) Subericultura. Ministério da Economia, Direcção Geral dos Serviços Florestais e Agrícolas, Lisbon
Neves CM (1950) Introdução à entomologia florestal Portuguesa. Livraria Sá da Costa, Lisbon
Nogueira CDS (1967) Pragas dos montados de sobro e azinho. Rev Agron 5:66–74
Norris AR, Martin K (2008) Mountain pine beetle presence affects nest patch choice of red-breasted nuthatches. J Wildl Manag 72:733–737
Olea L, López-Bellido RJ, Poblaciones MJ (2005) European types of silvopastoral systems in the Mediterranean area: dehesa. In: Mosquera MR, Rigueiro A, McAdam J (eds) Silvopastoralism and sustainable land management. CABI Publishing, Wallingford, pp 30–35
Parry D, Spence JR, Volney WJA (1997) Responses of natural enemies to experimentally increased populations of the forest tent caterpillar, Malacosoma disstria. Ecol Entomol 22:97–108
Pereira P, Godinho C, Roque I, Marques A, Branco M, Rabaça JE (2014a) Time to rethink the management intensity in a Mediterranean oak woodland: the response of insectivorous birds and leaf-chewing defoliators as key groups in the forest ecosystem. Ann For Sci 71:25–32
Pereira P, Godinho C, Gomes M, Rabaça JE (2014b) The importance of the surroundings: are bird communities of riparian galleries influenced by agroforestry matrices in SW Iberian Peninsula? Ann For Sci 71:33–41
Peris SJ (1980a) Biología del estornino negro (Sturnus unicolor Temm.). 1. Alimentación y variación de la dieta. Ardeola 25:207–240
Peris SJ (1980b) Biología del estornino negro (Sturnus unicolor Temm.). 2. Dieta del pollo. Doñana Acta Vertebr 7:249–260
Peris SJ, Masa AI (1992) Comunidades nidificantes y invernantes de aves del encinar adehesado (Quercus rotundifolia) del Centro-oeste de la Peninsula Iberica. Airo 3:75–82
Pimentel C, Nilsson JA (2007) Response of great tits Parus major to an irruption of a pine processionary moth Thaumetopoea pityocampa population with a shifted phenology. Ardea 95:191–199
Pimentel C, Nilsson JA (2009) Response of passerine birds to an irruption of a pine processionary moth Thaumetopoea pityocampa population with a shifted phenology. Ardeola 56:189–203
Pinto-Correia T (1993) Threatened landscape in Alentejo, Portugal: the montado and other agro-sylvo-pastoral systems. Landsc Urban Plan 24:43–48
Pinto-Correia T, Mascarenhas J (1999) Contribution to the extensification/intensification debate: new trends in the Portuguese montado. Landsc Urban Plan 46:125–131
Plieninger T, Wilbrand C (2001) Land use, biodiversity conservation, and rural development in the dehesas of Cuatro Lugares, Spain. Agrofor Syst 51:23–34
Pons J, Pausas JG (2007a) Acorn dispersal estimated by radiotracking. Oecologia 153:903–911
Pons J, Pausas JG (2007b) Not only size matters: acorn selection by the European jay (Garrulus glandarius). Acta Oecol 31:353–360
Prieta J, Del Moral JC (2008) La grulla común invernante en España. Población en 2007, y método de censo. SEO/BirdLife, Madrid
Pulido F, Díaz M (1992) Relaciones entre la estructura de la vegetación y comunidades nidificantes de aves en las dehesas: influencia del manejo humano. Ardeola 39:63–72
Pulido FJ, Díaz M (1994) Diet and prey selection by adult and young blue tits (Parus caeruleus): the effect of correcting for prey digestibility. Ardeola 41:153–161
Pulido F, Díaz M (1997) Linking individual foraging behavior and population spatial distribution in patchy environments: a field example with Mediterranean blue tits. Oecologia 111:434–442
Purroy FJ (1988) Sobre la invernada de la Paloma Torcaz (Columba palumbus) en Iberia. In: Tellería JL (ed) Invernada de aves en la Península Ibérica. Monografías SEO, Madrid, pp 137–151
Purroy FJ, Rodero M, Tomialojc L (1984) The ecology of woodpigeons Columba palumbus wintering on the Iberian Peninsula. Acta Ornithol 20:111–146
Rabaça JE (1990) The influence of shrubby understory in breeding bird communities of Cork Oak (Quercus suber L.) woodlands in Portugal. Port Zool 1:1–6
Ribeiro NA, Surový P, Pinheiro A (2010) Adaptive management on sustainability of cork oak woodlands. In: Manos B, Paparrizos K, Matsatsinis N, Papathanasiou J (eds) Decision support systems in agriculture, food and the environment: trends, applications and advances. IGI Global, Hershey, pp 437–449
Romanyk N, Cadahia D (eds) (1992) Plagas de insectos en las masas forestales españolas, 2nd edn. Ministerio de Medio Ambiente, Madrid
Santos MNS (2003) Contribuição para o conhecimento das relações Quercus suber—Biscogniauxia mediterranea (syn. Hypoxilon mediterraneum). Silva Lusit 11:21–29
Santos T, Tellería JL, Carbonell R (2002) Bird conservation in fragmented Mediterranean forests of Spain: effects of geographical location, habitat and landscape degradation. Biol Conserv 105:113–125
Sanz JJ (2001) Experimentally increased insectivorous bird density results in a reduction of caterpillar density and leaf damage to Pyrenean oak. Ecol Res 16:387–394
SEO/BirdLife (2012) Atlas de las aves en invierno en España 2007–2010. Ministerio de Agricultura, Alimentación y Medio Ambiente, SEO/BirdLife, Madrid
Serrano MD, De Vita P, Fernández-Rebollo P, Coelho AC, Belbahri L, Sánchez ME (2012) Phytophthora cinnamomi and Pythium spiculum as main agents of Quercus decline in southern Spain and Portugal. IOBC/WPRS Bull 76:97–100
Sierro A, Arlettaz R, Naef-Daenzer B, Strebel S, Zbinden N (2001) Habitat use and foraging ecology of the nightjar (Caprimulgus europaeus) in the Swiss Alps: towards a conservation scheme. Biol Conserv 98:325–331
Silva FA (1960) Nota sobre a Periclista andrei Konow, Hymenoptera, Tenthredinidae, uma praga do sobreiro, Quercus suber L., em Portugal. Direcção Geral dos Serviços Florestais e Aquícolas, Lisbon
Siscart D, Diego V, Lloret F (1999) Acorn ecology. In: Rodà F, Retana J, Gracia CA, Bellot J (eds) Ecology of Mediterranean evergreen oak forests. Springer, Berlin, pp 89–103
Skuhravá M, Skuhravá V, Blasco-Zumeta J, Pujade J (1996) Gall midges (Diptera: Cecidomyiidae) of the Iberian Peninsula. Bol Asoc Esp Entomol 20:41–61
Soler JJ, Soler M (1991) Análisis comparado del régimen alimenticio durante el período otoño-invierno de tres especies de córvidos en un área de simpatría. Ardeola 38:69–89
Solomon JD (1969) Woodpecker predation on insect borers in living hardwoods. Ann Entomol Soc Am 62:1214–1215
Solomon DM, Glen DM, Kendall DA, Milsom NF (1976) Predation on overwintering larvae of codling moth (Cydia pomonella (L.)) by birds. J Appl Ecol 13:341–353
Soria FJ, Villagran M, Ocete ME (1992) Estudios poblacionales sobre Coroebus undatus en alcornocales de Andalucía Occidental. I: Relación infestación-bosque. Bol San Veg Plagas 18:377–383
Soria FJ, Villagran M, Del Tio R, Ocete ME (1994a) Estudios prospectivos de los principales perforadores del alcornoque en la Sierra Norte de Sevilla. Bol San Veg Plagas 20:643–651
Soria FJ, Villagran M, Ocete ME (1994b) Crematogaster scutellaris Oliv. (Hym. Formicidae) en tres alcornocales del SW español. Bol San Veg Plagas 20:637–642
Soria FJ, Cano EY, Ocete ME (1996) Efectos del ataque de fitófagos perforadores en el fruto de la encina (Quercus rotundifolia Lam.). Bol San Veg Plagas 22:427–432
Soria FJ, Villagrán M, Martín P, Ocete ME (1999a) Curculio elephas (Gyllenhal) (Col.: Curculionidae) y Cydia fagiglandana (Zeller) (Lep.: Tortricidae) en encina (Quercus rotundifolia Lam.): infestación y relaciones interespecíficas. Bol San Veg Plagas 25:125–130
Soria FJ, Cano E, Ocete ME (1999b) Valoración del ataque de Curculio elephas (Gyllenhal) (Coleoptera, Curculionidae) y Cydia spp. (Lepidoptera, Tortricidae) en el fruto de alcornoque (Quercus suber L.). Bol San Veg Plagas 25:69–74
Soriguer RC, Herrera C (1977) Análisis de dos contenidos estomacales de Grulla Común Grus grus. Ardeola 24:217–219
Sousa EMR, Debouzie D (1999) Distribution spatio-temporelle des attaques de Platypus cylindrus F. (Coleoptera: Platypodidae) dans des peuplements dus chênes-lièges au Portugal. IOBC/WPRS Bull 22:47–58
Sousa E, Debouzie D (2002) Contribution à la bioecologie de Platypus cylindrus F. au Portugal. IOBC/WPRS Bull 25:75–83
Sousa E, Inácio ML (2005) New aspects of Platypus cylindrus Fab. (Coleoptera: Platypodidae): life history on cork oak stands in Portugal. In: Lieutier F, Ghaioule D (eds) Entomological research in Mediterranean forest ecosystems. INRA Editions, Paris, pp 147–168
Sousa EMR, Debouzie D, Pereira H (1995) Le role de l’insect Platypus cylindrus F. (Coleoptera: Platypodidae) dans le processus de dépérissement des peuplements du chêne liège au Portugal. IOBC/WPRS Bull 18:43–49
Sousa E, Tomaz IL, Moniz FA, Basto S (1997) La répartition spatiale dês champignons associés à Platypus cylindrus Fab. (Coleoptera: Platypodidae). Phytopathol Mediterr 36:145–153
Suñer D, Abós L (1994) Estudio de la infestación de Coroebus undatus en los alcornocales catalanes. Sci Gerund 20:45–53
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Tanhuanpää M, Ruohomaki K, Uusipaikka E (2001) High larval predation rate in non-outbreaking populations of a geometrid moth. Ecology 82:281–289
Tellería JL (2001) Passerine bird communities of Iberian dehesas: a review. Anim Biodivers Conserv 24:67–78
Tellería JL, Santos T, Carrascal LM (1988) La invernada de los paseriformes (O. Passeriformes) en la Peninsula Ibérica. In: Tellería JL (ed) Invernada de Aves en la Península Ibérica. Monografías SEO, Madrid, pp 153–166
Toimil FJ (1987) Algunos insectos defoliadores de la encina (Quercus ilex L.) en la provincia de Huelva. Bol San Veg Plagas 13:173–188
Toimil FJ (1989) Comparación del período larval de las especies defoliadoras más importantes del encinar encontradas en la provincia de Huelva entre 1985 y 1988. Bol San Veg Plagas 4:365–374
Torres J (1985) El Hypoxylon mediterraneum (de Not.) Mill. y su comportamiento en los encinares y alcornocales andaluces. Bol Serv Def Plagas Insp Fitopatol 11:185–191
Turner AK (1982) Optimal foraging by the swallow (Hirundo rustica L.): prey size selection. Anim Behav 30:862–872
Tuset JJ, Hinarejos C, Mira JL, Cobos JM (1996) Implicación de Phytophthora cinnamomi Rands en la enfernedad de la seca de encinas y alcornoques. Bol San Veg Plagas 22:491–499
Valente C, Branco M (2008) A importância dos pica-paus como inimigos naturais das brocas do eucalipto. In: Branco M, Valente C, Paiva MR (eds) Pragas e doenças em Pinhal e Eucaliptal—desafios para a sua gestão integrada. ISA Press, Lisbon, pp 147–158
Valverde JA (1971) Notas sobre la biologia reproductora del Críalo Clamator glandarius (L.). Ardeola special number:591–647
Van Bael SA, Philpott SM, Greenberg R, Bichier P, Barber NA, Mooney KA, Gruner DS (2008) Birds as predators in tropical agroforestry systems. Ecol 89:928–934
Vannini A, Valentini R, Luisi N (1996) Impact of drought and Hypoxylon mediterraneum on oak decline in the Mediterranean region. Ann For Sci 53:753–760
Villagran M, Ocete ME (1990) Datos preliminares sobre la distribución de nidos de Crematogaster scutellaris Oliv., 1789 (Hym: Formicidae) en alcornocales de Andalucía Occidental. Relación con el perímetro del tronco. Bol San Veg Plagas 16:151–157
Villemant C, Andreï-Ruiz M-C (1999) Life-cycles and biological features of eggs predators of Lymantria dispar (Lepidoptera: Lymantriidae) in the Mamora cork oak forest, Morocco. Eur J Entomol 96:29–36
Villemant C, Fraval A (eds) (1991) La Faune du Chêne-Liège. Actes Editions, Rabat
Villemant C, Fraval A (1999) Les gradations de Lymantria dispar en Europe et en Afrique du Nord. IOBC/WPRS Bull 23:71–79
Villemant C, Ramzi H (1995) Predators of Lymantria dispar (Lep. Lymantriidae) egg masses: spatio-temporal variation of their impact during the 1988–89 pest generation in the Mamora cork oak forest (Morocco). Entomophaga 40:441–456
Wang YH, Liao YJ (1990) Attracting beneficial birds for the biological control of Dendrolimus punctatus (Lep. Lasiocampidae). Chin J Biol Control 6:25–26
Whelan CJ, Wenny DG, Marquis RJ (2008) Ecosystem services provided by birds. Ann NY Acad Sci 1134:25–60
Acknowledgments
This work was funded by Fundação para a Ciência e a Tecnologia (FCT), through the PhD grant SFRH/BD/78813/2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceia, R.S., Ramos, J.A. Birds as predators of cork and holm oak pests. Agroforest Syst 90, 159–176 (2016). https://doi.org/10.1007/s10457-014-9749-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-014-9749-7