Abstract
A method for rapid in vitro propagation of Cassia siamea Lam. using cotyledonary node explants, excised from 14-day old aseptic seedlings, has been established. Murashige and Skoog (MS) medium supplemented with different concentrations of 6-benzyladenine (BA), kinetin (Kn) and thidiazuron (TDZ) singly or in combination with auxins was used for regeneration studies. Among the single treatment of three cytokinins BA at 1.0 µM was found to be optimum for direct shoot regeneration as it induced an average of 8.20 ± 0.66 shoots per explant. The regeneration frequency further enhanced with the application of auxin along with optimal BA concentration. The highest frequency for shoot regeneration (90%), the maximum number of shoots per explant (12.20 ± 0.73) and the maximum shoot length (6.40 ± 0.07) cm were obtained on the medium consisted of MS + 1.0 µM BA + 0.5 µM NAA. Successful in vitro rooting was induced from cut end of the microshoots when placed on half-strength MS + IBA (2.5 µM). The regenerated shoots with well developed root system were successfully acclimatized and established in pots containing sterilized garden soil and garden manure (1:1) and grown under greenhouse conditions with 85% survival rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cassia siamea Lam. (Siamese senna) is a fast growing, multipurpose nitrogen fixing tree species belonging to family Fabaceae (sub family Caesalpinaceae). The plant is native to South East Asia and is very popular in arid regions, particularly West Africa, it has been extensively planted as an avenue and shade tree in tea estates and found useful for afforestation of degraded and wastelands where organic manure is deficient. It decreases soil erosion, while improving soil fertility in the plantation site, well adapted to a variety of climatic conditions within the tropics, and highly resistant to drought. The plant also provides excellent fuel wood, premium quality timber, good fodder and green manure and compost (Anonymous 1992). Therefore, the tree species possess a great potential to be employed as a tool for traditional agroforestry systems and social and community forestry (Ouinsavi and Sokpon 2008) by conservation of germplasm through in vitro techniques.
Medicinally, it is reported to exhibit laxative and purgative properties and has been traditionally used for the treatment of liver problems, urticaria, loss of appetite from gastrointestinal trouble and rhinitis. Young leaves and flower extracts of the plant contain a type of chromone called barakol, two novel alkaloids cassiarins A(1) and B(2), anthraquinone glycosides and other alkaloids which are effective against insomnia, anxiety, periodic fever, malaria and showed a potent antiplasmodic activity (Morita et al. 2007).
C. siamea is commonly propagated through seeds. However, several fungi, sap suckers and wood borers are reported to attack the plant and a considerable damage is caused by Dendrophthoefalcata (a stem parasite). Thus, reducing the survival percentage of the seedlings and increasing its mortality rate. Besides, wild-stand seedlings are highly variable in terms of growth and biomass production. More uniform, healthy and vigorously growing planting stock of C. siamea that can benefit agroforestry systems can be obtained through micropropagated superior trees. Micropropagation techniques can be used as an alternative tool for rapid establishment and conservation of control pollinated seeds. Although, the regeneration of forest trees in general and legumes in particular have been a difficult task (Lakshmisita et al. 1992), the strategies to regenerate legumes by exploiting tissue culture techniques have been evolved steadily during the past few years (Rhagavaswamy et al. 1992; Gulati and Jaiswal 1996; Shahzad et al. 2006; Husain et al. 2008). Thus, to ensure an availability of large number of planting propagule for afforestation programmes, biomass production and germplasm conservation an alternative and feasible method (micropropagation) is of great value. As far as literature is available there has been no report of any attempt for micropropagation of C. siamea. In the present study the cotyledonary node explant (CN) was selected for the development of efficient regeneration protocol. CN explants have already been recommended, as an excellent tissue, in several earlier studies conducted in other forest tree species (Pradhan et al. 1998; Sinha et al. 2000; Anis et al. 2005).
Materials and methods
Explant collection and establishment of aseptic seedlings
The certified seeds of C. siamea obtained from Prem Nursery and Seed Store, Dehradun, India were used for raising aseptic seedlings. Seeds were first thoroughly washed under running tap water for 30 min to remove adherent particles. Seeds were kept in 1% (w/v) Bavastin (Carbendenzim Powder), a broad spectrum fungicide, for 25–30 min, followed by thorough washing with 5% (v/v) Teepol, a liquid detergent, by continuous shaking for 15 min. Seeds were washed with sterile double distilled water (DDW) for 3–4 times under the laminar flow hood followed by a short treatment with 70% (v/v) ethanol for 30–60 s, rapidly washed with sterile DDW and then surface sterilized using 0.1% (w/v) freshly prepared mercuric chloride (HgCl2) for 4 min. Finally, the seeds were washed (5–6 times) with sterile DDW to remove the traces of sterilant. The sterilized seeds were inoculated in the culture flask (100 ml, Borosil) containing 0.8% (w/v) agar solidified germination medium composed of MS (Murashige and Skoog 1962) basal medium or half-strength MS medium with 3% (w/v) sucrose and Gibberellic acid (GA3) or without GA3. Ten culture flasks were taken for each treatment and 10 seeds were inoculated in each flask.
CN measuring 1–1.5 cm excised from 14-day-old aseptic seedlings raised on half-strength MS supplemented with GA3 (1.0 µM) were used as explants.
Culture media and conditions
The MS medium supplemented with 3% (w/v) sucrose and 0.8% (w/v) agar (Hi-media, India) were used throughout the experiments. The pH of the medium was adjusted to 5.8 using 1 N NaOH or 1 N HCl prior to autoclaving at 121°C at 1.06 kg cm−2 pressure for 20 min. All the cultures were maintained at 24 ± 2°C under 16 h photoperiod with a photosynthetic photon flux density (PPFD) of 50 µmol m−2 s−1 provided by cool white fluorescent tubes (40W; Phillips, India) and with 50–60% relative humidity.
Shoot induction, multiplication and maintenance
The MS medium supplemented with various cytokinins 6-Benzyladenine (BA), Kinetin (Kn), Thidiazuron (TDZ) at different concentrations (0.1, 0.5, 1.0, 2.0 and 5.0 µM) individually or in combination with different auxins—Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA) or α-Naphthalene acetic acid (NAA) (0.1, 0.5 and 1.0 µM) were used for multiple shoot induction through CN, excised from aseptic seedlings. For shoot multiplication and long-term establishment the regenerating tissues were subcultured onto the fresh medium comprised of MS plus BA (1.0 µM) after every 4 weeks. The percentage of explants producing shoots, number of shoots per explant and shoot length were recorded after 8 weeks of culture.
In vitro rooting in microshoots
Regenerated shoots of about 3–4 cm length were excised and transferred to the rooting media composed of MS basal and half-strength MS supplemented with different auxins like IAA, IBA and NAA (1.0, 2.5, 5.0 and 10.0 µM). Data were recorded for rooting percentage, mean number of roots and root length after 4 weeks of transplantation onto the rooting medium.
Hardening and acclimatization
Plantlets with well developed root and shoot system were removed from the culture medium and washed gently under running tap water to remove any adherent gel from the roots and transferred to thrermocol cups containing sterile soilrite. These were kept under diffuse light conditions (16:8 h photoperiod) covered with transparent polythene bags to ensure high humidity. These were irrigated after every 3 days with ¼ strength MS salt solution (without vitamins) for 2 weeks. Polythene bags were removed gradually after 2 weeks in order to acclimatize the plantlets and after 4 weeks they were transferred onto earthen pots containing sterilized garden soil and garden manure (1:1) and maintained in green house under normal day length conditions.
Data collection and statistical analysis
The data for percentage regeneration, number of shoots per explant and shoot length were recorded after 8 weeks and for rooting experiment after 4 weeks. All the experiments were conducted with ten replicates per treatment and repeated thrice. The data were analyzed statistically using SPSS ver.12 (SPSS Inc., Chicago, USA). The significance of differences among means was carried out using Tukey’s multiple range test at P = 0.05 and the results are expressed as a mean ± SE of three repeated experiments.
Results and discussion
Establishment of aseptic seedlings and explant collection
To raise aseptic seedlings, the seeds were germinated under controlled conditions on MS basal and half-strength MS media with or without GA3. A maximum of 90% seed germination along with healthy growth was achieved on half strength MS + 1.0 µM GA3 (Table 1; Fig. 1a). The media lacking GA3 showed poor response (40–50%) for seed germination. On increasing the concentration of GA3 from 1.0 to 5.0 µM the germination percentage was reduced to 60%. GA3 is known to break dormancy of several types of seeds at a critical concentration. It stimulates seed germination via synthesis of α-amylase and other hydrolases (Shepley et al. 1972). Thus, the supplementation of GA3 has been found to enhance the rate of seed germination in C. siamea in consonance with earlier findings in Clitoria ternatea (Shahzad et al. 2007). CN explants excised from 14-day-old aseptic seedlings of C. siamea were used for regeneration studies throughout the experiment.
In vitro plant regeneration in C. siamea through CN explant. a Aseptic seedlings; b development of multiple shoots on MS + BA (1.0 µM) after 4 weeks; c multiplication and proliferation of shoots on MS + BA (1.0 µM) + NAA (0.5 µM) after 4 weeks; d fully developed, elongated microshoots after 8 weeks; e rooted plantlet on half-strength MS + IBA (2.5 µM); f an acclimatized plantlet
Induction and multiplication of shoots
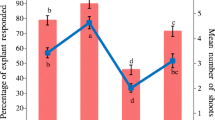
The response of various cytokinins (BA, Kn and TDZ) for shoot regeneration from CN explants is depicted in Table 2. The explants cultured on MS basal medium without growth regulators (control) did not show any regeneration response. However, the addition of plant growth regulators enhanced the multiplication rate and the number of shoots per explant. The percentage response varied with the type of growth regulator used and its concentration. All concentrations of BA, Kn and TDZ (0.1, 0.5, 1.0, 2.0 and 5.0 µM) alone resulted in direct shoot bud differentiation from the explant within 3 weeks of incubation. The initial response was noticed in the form of swelling of the explants within 8–10 days of incubation and then differentiation of green protuberances occurred which transferred into direct shoot buds in successive weeks. Among the three cytokinins tested, BA was found to be more efficient than others with respect to initiation and subsequent proliferation of shoots (Table 2). Of the various concentrations of BA tested, 1.0 µM proved to be most effective as in this medium an average of 8.20 ± 0.66 shoots per explant were developed in 80% of cultures (Fig. 1b). Our results are in consistence with earlier findings in woody tree species like Sterculia urens (Purohit and Dave 1996), Dalbergia sissoo (Pradhan et al. 1998) and Pterocarpus marsupium (Husain et al. 2008) where BA was found to be more effective than other cytokinins for mass multiplication.
Increasing the concentration of BA (5.0 µM) resulted in decrease in regeneration potential (64%) and the number of shoots per explant (5.20 ± 0.37). On this medium induction of callus from basal cut end was very much pronounced, which might be one of the reason for reduction of multiple shoot regeneration. Inhibitory effect of higher concentrations of BA on shoot multiplication has also been reported in other plant species such as Withania somnifera (Sen and Sharma 1991), Albizia chinensis (Sinha et al. 2000) and Simmondsia chinensis (Agrawal et al. 2002). There are several reports available in which the production of callus was considered inhibitory for direct shoot regeneration (Lakshmanan et al. 1997; Pattnaik and Chand 1997). Shoot induction was also observed in the explants cultured on MS medium fortified with Kn and TDZ. Kn was found to be less effective than BA as it induced an average of 4.80 ± 0.37 shoots per explant with 72% regeneration frequency at an optimal concentration of 1.0 µM. However, the regenerated shoots were healthy and showed good growth as compared to TDZ supplemented medium. Thus, among three cytokinins tested, TDZ was proved to be least effective (BA > Kn > TDZ). The MS medium supplemented with 0.5 µM TDZ produced only 4.40 ± 0.37 shoots per explant with 60% regeneration potential, furthermore the regenerated shoots were stunted with poor internodal elongation (Table 2). When the concentration of TDZ was increased beyond optimum level (0.5 µM) a gradual decrease was observed in regeneration frequency, number of shoots per explant and average shoot length. Similar inhibitory effects of TDZ on growth and elongation at higher concentration have also been observed in other woody trees Pyrus malus (Van Nieuwekerk et al. 1986), Rhododendron (Preece and Imel 1991), Albizia julibrissin (Sankhla et al. 1994), Albizia chinensis (Sinha et al. 2000) and Vitex negundo (Ahmad and Anis 2007). The formation of stunted shoots or inhibition of internodes elongation may be due to the high cytokinin activity of TDZ since cytokinins are known to inhibit stem elongation (Mok et al. 1982; Huettman and Preece 1993). Unlike adenine-type cytokinins (BA and Kn), TDZ was more effective at lower concentrations and therefore, a comparison of those cytokinins with TDZ at equimolar concentrations is not possible.
The synergistic effect of auxins such as IAA, IBA and NAA (0.1, 0.5 and1.0 µM) with optimal concentration of BA (1.0 µM) was also evaluated. Among various combinations used, BA (1.0 µM) + NAA (0.5 µM) was found to be most effective (Table 3) (Fig. 1c). MS medium supplemented with BA (1.0 µM) + NAA (0.5 µM) exhibited 90% shoot regeneration and induced maximum (12.20 ± 0.73) shoots per explant with an average shoot length of 6.40 ± 0.07 cm (Fig. 1d). Upon increasing the concentration of NAA to 1.0 µM, regeneration frequency (80%) as well as the number of shoots per explant was reduced (10.60 ± 0.75) moderately. Higher concentration of NAA reduced the regeneration frequency and resulted in increased basal callusing, which is not desirable for direct shoot regeneration. Our results substantiate with earlier findings in Psoralia corylifolia (Saxena et al. 1997), Acacia catechu (Kaur et al. 1998), Mucuna pruriens (Faisal et al. 2006) and Balanites aegyptiaca (Anis et al. 2010) where the addition of lower concentration of auxin with cytokinin promoted shoot regeneration. Among the different combinations of BA - IAA used, the highest shoot regeneration frequency (74%), maximum number of shoots per explant along with the maximum shoot length were recorded on MS medium augmented with BA (1.0 µM) + IAA (0.5 µM) (Table 3) followed by BA - IBA combinations, wherein the highest shoot regeneration frequency (72%), maximum number of shoots (8.40 ± 0.51) per explant and the maximum shoot length (6.06 ± 0.15 cm) were recorded on MS medium supplemented with BA (1.0 µM) + IBA (0.5 µM).
In vitro rooting in microshoots
In vitro raised microshoots (3–4 cm) were transferred to rooting media comprised of full strength MS and half-strength MS along with various concentrations of auxins; IAA, IBA and NAA (1.0, 2.5 and 5.0 µM). Full strength MS medium failed to induce rooting in all the treatments whereas half-strength MS with different concentrations of auxins induced rooting from basal cut end of microshoots within 2 weeks of transferring (Fig. 1e). The best rooting in terms of rooting percentage and root length was achieved in the medium fortified with IBA (2.5 µM) (Table 4), giving a maximum of 3.60 ± 0.24 roots per shoot with a root length of 7.88 ± 0.28 cm. The roots produced were thick, long and well developed with secondary branching. Comparatively lesser number of roots were produced when IBA was replaced with other auxins, NAA and IAA. However, among all the three auxins tested the lower concentrations of auxins were more satisfactory than the higher concentrations because the higher concentration favored basal callusing before root induction, which resulted into poor vascular connection between the shoot and induced roots, consequently affecting the survival percentage of plants (Data not shown). Our results are similar with earlier findings in other leguminous plants such as Pterocarpus marsupium (Chand and Singh 2004), Cassia angustifolia (Agrawal and Sardar 2006), Pterocarpus santalinus (Prakash et al. 2006) and Clitoria ternatea (Shahzad et al. 2007) where lower concentration of IBA was proved to be the best for rhizogenesis.
Hardening and acclimatization
The most critical and important step of micropropagation studies is the transfer of regenerants from artificial to natural environment to ensure the maximum application of the technique. Plantlets with well-developed root and shoot system were successfully hardened off inside the growth room in sterile planting substrate (soilrite) for 4 weeks and irrigated with ¼ strength of MS in the first two weeks (Fig. 1f). The pots were covered with polythene bags to ensure high relative humidity for the first two weeks. Thereafter exposed gradually to controlled environment followed by their transfer to earthen pots containing sterilized garden soil and garden manure (1:1) and maintained in green house under normal day light condition. About 85% of regenerated plants survived after transferring from soilrite to soil-garden manure mixture. All the plants were exhibiting normal morphology and growth when compared with naturally grown plants.
Conclusions
In conclusion, an efficient and effective protocol was developed for micropropagation of C. siamea, a leguminous tree of great importance. This protocol provides a successful and rapid technique that can be used for the propagation and ex situ conservation of this important species.
Abbreviations
- BA:
-
6-Benzyladenine
- Kn:
-
Kinetin
- TDZ:
-
Thidiazuron
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
α-Naphthalene acetic acid
- GA3 :
-
Gibberellic acid
- MS:
-
Murashige and Skoog medium
- PGR:
-
Plant growth regulator
References
Agrawal V, Sardar PR (2006) In vitro propagation of Cassia angustifolia through leaflet and cotyledon derived calli. Biol Plant 50:118–122
Anonymous (1992) Cassia. The Wealth of India. Raw Materials, vol 3. CSIR, New Delhi, India, pp 363–365
Agrawal V, Prakash S, Gupta SC (2002) Effective protocol for in vitro production through nodal explants of Simmondsia chinensis. Biol Plant 45:449–453
Ahmad N, Anis M (2007) Rapid clonal multiplication of a woody tree, Vitex negundo L through axillary shoot proliferation. Agrofor Syst 71:195–200
Anis M, Husain MK, Shahzad A (2005) In vitro plantlet regeneration of Pterocarpus marsupium Roxb., an endangered leguminous tree. Curr Sci 88:861–863
Anis M, Varshney A, Siddique I (2010) In vitro clonal propagation of Balanites aegyptiaca (L.) Del. Agrofor Syst 78:151–158
Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium Roxb. In Vitro Cell Dev Biol Plant 40:464–466
Faisal M, Siddiqui I, Anis M (2006) An efficient plant regeneration system for Mucuna pruriens L (DC) using cotyledonary node explants. In Vitro Cell Dev Biol Plant 42:59–64
Gulati A, Jaiswal PK (1996) Micropropagation of Dalbergia sissoo from nodal explants of mature trees. Biol Plant 38:169–175
Huettman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org Cult 33:105–119
Husain MK, Anis M, Shahzad A (2008) In vitro propagation of a multipurpose leguminous tree (Pterocarpus marsupium Roxb.) using nodal explants. Acta Physiol Plant 30:353–359
Kaur K, Verma B, Kant U (1998) Plants obtained from the Khair tree (Acacia catechu Wild) using mature nodal segment. Plant Cell Rep 17:427–429
Lakshmanan P, Lee CL, Goh CJ (1997) An efficient in vitro method for mass propagation of a woody ornamental plant Ixora coccinea L. Plant Cell Rep 16:572–577
Lakshmisita G, Sreenatha KS, Sujata SG (1992) Plantlet production from shoot tip cultures of red sandalwood (Pterocarpus santalinus). Curr Sci 62:532–535
Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T (1982) Cytokinin activity of N-phenyl-1, 2, 3- Thidiazil-5-ylurea (thidiazuron). Phytochem 21:1509–1511
Morita H, Oshimi S, Hirasawa Y, Koyama K, Honda T, Ekasari W, Indrayanto G, Zain NC (2007) Cassiarin A and B novel antiplasmodial alkaloids from Cassia siamea. Org Lett 9:3691–3693
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ouinsavi C, Sokpon N (2008) Traditional agroforestry systems as tools for conservation of genetic resources of Milicia excelsa Welw. C.C. Berg in Benin. Agrofor Syst 74:17–26
Pattnaik SK, Chand PK (1997) Rapid clonal propagation of tree mulberries, Morus cathayana Hemsl., Morus lhou Koiz., and M. serrata Roxb., through in vitro cultures of apical shoot bud and nodal explants from mature trees. Plant Cell Rep 16:503–508
Pradhan C, Kar S, Pattnaik S, Chand PK (1998) Propagation of Dalbergia sissoo Roxb. through in vitro shoot proliferation from cotyledonary nodes. Plant Cell Rep 18:122–126
Prakash E, Khan PSSV, Rao TJVS, Meru ES (2006) Micropropagation of red sanders (Pterocarpus santalinus L.) using mature nodal explants. J For Res 11:329–335
Preece JE, Imel MR (1991) Plant regeneration from leaf explants of Rhododendron P.J.M. hybrids. Sci Hortic 48:159–170
Purohit SD, Dave A (1996) Micropropagation of Sterculia urens Roxb. An endangered tree species. Plant Cell Rep 15:704–706
Rhagavaswamy BV, Himabendu K, Sita GL (1992) Efficient plant regeneration from cell suspension derived callus of East Indian rose wood (Dalbergia latifolia Roxb.). Plant Cell Rep 11:126–131
Sankhla D, Davis TD, Sankhla N (1994) Thidiazuron- induced in vitro shoot formation from roots of intact seedlings of Albizia julibrissin. Plant Growth Regul 14:267–272
Saxena C, Palai SK, Samataray S, Rout GR, Das P (1997) Plant regeneration from callus cultures of Psoralia corylifolia Linn. Plant Growth Regul 22:13–17
Sen J, Sharma AK (1991) Micropropagation of Withania somnifera from germinating seeds and shoot tips. Plant Cell Tiss Org Cult 26:71–73
Shahzad A, Ahmad N, Anis M (2006) An improved method of organogenesis from cotyledonary callus of Acacia sinuata (Lour.) Merr. using Thidiazuron. J Plant Biotechnol 8:15–19
Shahzad A, Faisal M, Anis M (2007) Micropropagation through excised root culture of Clitoria ternatea and comparison between in vitro regenerated plants and seedlings. Ann Appl Biol 150:341–349
Shepley S, Chen C, Chang JLL (1972) Does Gibberellic Acid stimulates seed germination via amylase synthesis. Plant Physiol 49:441–442
Sinha RK, Majumdar K, Sinha S (2000) In vitro differentiation and plant regeneration of Albizia chinensis (OBS.) MERR. In Vitro Cell Dev Biol Plant 36:370–373
Van Nieuwekerk JP, Zimmerman RH, Fordham I (1986) Thidiazuron stimulation of apple shoot proliferation in vitro. Hortic Sci 21:316–518
Acknowledgments
The author Anwar Shahzad is greatly acknowledged the financial support promoted by the Department of Science and Technology (DST), Government of India, New Delhi in the form of SERC Fast Track Scheme Vide no. SR/FT/L-23/2006. The authors are also thankful to the Department of Science and Technology (DST) Government of India, New Delhi for providing research assistance under DST-FIST Programme 2005 (Project No. SR/FST/LSI-085/2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parveen, S., Shahzad, A. & Saema, S. In vitro plant regeneration system for Cassia siamea Lam., a leguminous tree of economic importance. Agroforest Syst 80, 109–116 (2010). https://doi.org/10.1007/s10457-010-9301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-010-9301-3