Abstract
This study dealt with the effects of pruning on branch and leaf area (F a) production of ponderosa pines growing in silvopastoral systems in Patagonia. We hypothesized that pruning positively influences the number of branches per whorl and their basal area growth rate, changing F a production. In addition, we studied some water relations in order to explain potential differences in branch growth rates between treatments. Two mathematical models were developed to estimate branch and total F a. The averaged diameter at the third year of pruning was, for high-pruned trees 3.1 and 3.6 cm at the bottom and middle of the crown, against 4 and 4.4 cm for low-pruned trees. Pruning did not produce changes in the number of branches per whorl (approximately 7.6 branches per whorl). Water stress may be responsible of this lower branch growth in pruned trees. Water potential, stomatal conductance and transpiration were lower in high- than in low-pruned trees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of tree branches is one of several factors that affect the quality of final wood products. According to Mäkinen and Hein (2006), low stand densities may have negative effects on timber quality. These authors found that one the most pronounced effect of low stand density was the increase in branch diameter, particularly at lower crowns levels. In general, the diameter of the thicker branch in a crown increases with the distance from the apex. Mäkinen and Hein (2006) found that branch diameter of Picea abies (L.) Karst. did not differ in the uppermost crown among stand densities, but at the lower portion of the crown (the base of the crown), diameter of the branches was usually lower in trees growing at higher densities. On the other hand, Ishii and Wilson (2001) found that a model of crown structure developed for young trees could be fit to the upper crown of old-growth Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco). Based on these results, one may suppose that the pruning of the lower portion of the crown could result in branch diameter becoming independent of the stand density or age of the tree. However, depending on the ecophysiological capacities of the species, leaf area (F a) production can increase in response to pruning, increasing also branch diameter in order to maintain an adequate supply of water to the leaves. In this sense, a trend (Gyenge 2005) or a significant higher F a production (Montagu et al. 2003) were observed in pruned compared to unpruned trees. Based on the relationship between F a and sapwood area of the branches, the potential increase in F a growth after pruning could increase branch diameter or the number of branches, which can have important implications on wood quality.
From an ecophysiological point of view, in places without nutrient limitations, the amount of F a developed in each branch depends on the availability of water (in soil and for the leaves) and solar radiation. The maximum F a supported by each branch depends on its diameter, which is related to the specific water conductance of the wood (“pipe theory”, e.g., Callaway et al. 1994). On the other hand, F a depends on the amount of solar radiation reaching each branch, which decreases by the interception of the upper branches (Stenberg et al. 1998). For this reason, the effect of tree spacing in F a distribution along the canopy depends on the shade tolerance of leaves of each species, but also on tree size and age (see Garber and Maguire 2005a). Following a green pruning, some species can increase photosynthesis of the remnant F a by the increase of stomatal conductance (gs) (Pinkard et al. 1998, Pataki et al. 1998). This can occur if the remnant conducting tissue of the stem (which before the pruning, was conducting water to the pruned branches) can supply water to the remnant foliage. Other species tend to compensate for leaf loss by producing more foliage after pruning (e.g., Montagu et al. 2003). In both cases, an imbalance between green and respiratory organs can lead to significant reduction in growth if the extracted F a exceeds the compensatory mechanisms. This threshold depends on the interconnection between tracheids or vessels inside the hydroactive xylem (Schulte and Brooks 2003), and the plantation density and/or the degree of canopy closure (Pinkard 2002). For these reasons, the impact of green pruning on growth depends on the interaction between physiological characteristics of each species and resource availability. In addition, the duration of the impact of pruning depends on the capacity of the tree to reach carbon and water equilibrium between above- and below- ground components of the plant (functional homeostasis, Magnani et al. 2002).
Traditionally, the most important economic activity in semi-arid Patagonia (South Argentina) has been sheep livestock. Presently the sustainability of livestock production systems is threatened by desertification, with its negative effects on pasture quality and quantity. Recently, forest plantations based on fast growing species have been promoted through a subsidy policy in the country. In Patagonia, the exotic ponderosa pine (Pinus ponderosa Doug. ex Laws) is the species most commonly planted. In all cases, pine plantations have been designed to produce wood, and thus, have been planted at high densities. However, considering cultural, economic and environmental aspects, silvopastoral systems (SPS) may be an interesting alternative for small and medium land-owners in semiarid Patagonia. For this reason, since 1998 some studies have been carried out in this region to determine the feasibility of SPS using low density ponderosa pine plantations and native grasses (e.g., Fernández et al. 2006). Those studies reported results considering the forage component of the systems but no information is available about pine performance under these conditions.
The primary objective of this study was to test the hypothesis that pruning in pines growing in low density plantations positively influences the number of branches per whorl and their basal area growth rate, increasing branch diameter even in positions closed to the apex compared to unpruned trees. Also, pruned trees would have a higher increment of F a annual production than unpruned ones. If our hypothesis is correct, then the upper part of the crown of a pruned tree will be different than that of an unpruned tree, and different canopy models have to be developed for different pruning schedules. In addition, we aimed to understand the role of water relations in branch growth rate of low- and high-pruned trees. To test our hypothesis, we used ponderosa pines growing in SPS in N.·W. Patagonia as a study case.
Materials and methods
Study site and experimental treatments
The study site was located in Lemú Cuyén Ranch (40.3°S, 71.1°W, 900 m of altitude) in Neuquén Province, Patagonia, Argentina. The climate of the region is characterized by cold and humid winters, and hot and dry summers. During the period 1978–1999 average annual rainfall was 684 ± 283 mm (with approximately 579 mm in fall-winter and 105 mm in spring–summer). Maximum and minimum annual average temperatures are 17.1°C ± 0.5 and 4°C ± 2.1, respectively. The soil is a silty clay loam, with 27–40% silt and 20% clay (A. Marcolín, personal communication, 1998).
All measurements were carried out in a plantation of ponderosa pine established in 1985 (approximately 15 years old from plantation at the beginning of the experiment). In a stand of 2 ha with an original density of 500 trees ha−1 (500 SPS), ten study plots (40 × 40 m each) were randomly distributed in winter 1999. This trial was used to investigate several aspects of tree and forage performance in SPS (reported in Fernández et al. 2006), but for the purpose of this study, we only measured trees growing at 500 pines ha−1. Average tree height and diameter at breast height (dbh) at the beginning of this experiment (winter 1999) were 7.77 ± 0.31 m (±E.E, n = 163) and 21 ± 0.61 cm, respectively. At that time, all the trees growing at similar density in a contiguous stand, were pruned at 3 m height. After that, pines of three plots of the silvopastoral system were pruned again to 4.5 m height in winter 2001 and 2003 (Trees #7–14 and #17–20, Table 1). In winter 2004, trees growing in the contiguous stand were pruned to 6.5 m (trees # 1–6, Table 1). Trees pruned at winter 1999 are referred to “low-pruned”, whereas those trees pruned at winters 2001 and 2003 are called “high-pruned” trees. A detailed description of these trees is shown in Table 1. Measured trees were randomly selected in each plot.
Leaf area model
The relationship between F a and the diameter of each branch (DIAM) at 5 cm from the insertion of the branch was estimated for ponderosa pines growing under the situations described in the previous section. During three successive winters (2001–2003), we harvested one randomly selected branch from the top, middle and bottom of tree crowns from the silvopastoral system. One model for each year was developed and compared using F-tests with α = 0.05 (Neter and Waserman 1974). Two orthogonal diameters at 5 cm from the branch insertion were measured. All leaves of the branches were collected and oven dried to constant weight. Previously, 20 fascicles from each branch were randomly selected and measured to estimate F a. Total surface (A) of the fascicles was estimated using geometric formulae representing the fascicle as six rectangular surfaces with side r and length ℓ (area = r ℓ), and an external cylinder (area = 2πr ℓ). Combining the formula for six rectangular surfaces plus a cylinder, A of the fascicles was:
After that, fascicles were oven dried and weighted. Total F a of each branch was estimated from dry weight of all fascicles and the ratio F a: dry weight of the sub-sampled fascicles.
In order to develop a simple predictive model of F a of the entire tree, in winter 2004 we measured the DIAM of all branches, the diameter of the stem at the base of the living crown (DLC) and dbh of several trees growing at silvopastoral system and in a contiguous stand (Trees #1 to #16, Table 1). We also used data from the 8 pines (Fa and DLC) in which we measured annual F a production (trees #17 to #24, see below). These last trees were not used to build the equation for F a estimation from DIAM.
Branch diameter and leaf annual production
We measured DIAM of all branches of the same low-pruned (pruned at winter 1999) and high-pruned trees (pruned in winter 2001) during years 2002 (n = 6), 2003 and 2004 (n = 8). These trees were numbered #17–24 (Table 1). The information was used to determine mean and maximum diameter in each whorl, and the number of branches per whorl. Whorl 1 was the closest to the apex of the tree. Data of successive years were used to estimate basal increments (branch growth rates) and leaf annual production after deducing F a from branch DIAM with the previously determined leaf area model.
ANOVA tests were used to compare mean diameter and mean maximum diameter of each whorl between high-pruned and low-pruned trees. Also, we analyzed statistical differences using t-tests between high- and low-pruned trees in averaged diameters of branches grouped in three layers in each tree: top (first 3 whorls), bottom (last 4 whorls with green needles) and middle (remnant branches not included in the other two categories). The number of branches per whorl was analyzed using t-tests comparing the top, middle and bottom layers of high- and low-pruned trees at the end of the experiment (year 2004). When necessary, data were transformed to agree with the normality and homocedasticity assumptions of ANOVA.
Ecophysiological measurements
In order to detect if pruning treatment had an effect on tree water relations, some ecophysiological variables were studied at the leaf and tree levels during the driest period of the year 2002 (end of summer, February-March) in the same trees in which we measured branch and leaf annual production. This period was the first dry period after the pruning treatment.
At the leaf level, we measured during February-21-2002, leaf water potential (Ψ, in MPa; at predawn, 10:00, 13:00 and 16:00 h) and stomatal conductance (gs, mol m−2 s−1 , at 10:00 and 13:00 h) of one-year old needles (three fascicles) located in the north-faced part of the crown (4.5 m height) of four trees of each treatment. Stomatal conductance was measured with a LICOR 6200 system (Infra-red gas analyzer System, Li-Cor Co., USA). After that, Ψ of the same needle was measured with a pressure chamber (PMS Instrument Company, Corvallis, OR, Model 1003). Daily course of both variables were analyzed by repeated measures ANOVA. In addition, during 3 days of the same dry period (Feb-12, Feb-22, and March-12 of 2002) we measured Ψ at predawn. One way ANOVA was used, after transformation to the reciprocal of each value, to compare predawn Ψ of low- and high-pruned trees within each measurement date.
At the tree level, we measured xylem sapflow density (Js, ml cm−2 min−1) using 2 cm-long heated sapflow sensors (Granier 1985) from February to March 2002 and the same period in 2003. This variable was measured in the 8 low- and high-pruned trees in which measured leaf and branch annual production. Two pairs of sensors were installed on the southeast and southwest sides of the trunk of each tree, surrounded with a shield to eliminate bias caused by radiation heating. Measurements were automatically taken every 10 min and stored in a data-logger (IMKO GMBH, Ettlingen, Germany) for further processing. The characteristics of the measured trees are shown in Table 1. The sample unit was each tree (average of the two pairs of sensors). Daily transpiration of each tree was estimated multiplying Js by the sapwood area of each tree, taking into account the decrease of Js in the radius of the trunk according to the formula proposed by Irvine et al. (2002) and validated in Gyenge et al. (2003). This author estimated a decline of sap flux density with sapwood depth with a linear function of sapwood depth: % sap flux density = −0.691 * sapwood depth + 100.99, r 2 adj. = 0.99, P < 0.01. Therefore, this relationship was used to extrapolate sap flux density as determined over 0–20 mm sapwood depth to the remainder of the xylem.
Climate data were recorded with an automatic meteorological station located, for security reason, 3 km away from the experimental site, in the same valley. In order to check the assumption that meteorological conditions were similar in both places, we performed simultaneous measurements. The results showed similar meteorological conditions (Licata 2007). Data were collected every 5 min and the 30 min averages were stored in a data logger (Campbell CR10X, Campbell Scientific Inc., Utah, USA). Vapor pressure deficit (VPD, kPa) was estimated from temperature and relative humidity of the air assuming that the leaf has the same temperature as the air (Ewers and Oren 2000).
Results
Leaf area model
The harvested branches had 1–7.5 cm of DIAM. No statistical differences were found between the models developed for each year (P > 0.05). The relationship between F a (m2) and DIAM (mm) was (parameter ± S.E., n = 39, P < 0.05, R2 adj 0.87, Fig. 1):
Relationship between diameter at 5 cm from the insertion of the branch (DIAM, mm) and foliar area (F a, m2) of ponderosa pine trees growing at 500 trees ha−1 in Patagonia Argentina (mean values and 95% confidence interval (dotted thin line), F a = a + b DIAM2, n = 39, P < 0.05, R 2 adj 0.87) and of pines growing in different places in USA (data from Callaway et al. 1994)
Using equation 1, we estimated that a difference in pruning height of 1.5 m (3–4.5 m of the crown base height) represented, on average, a 40% in F a between treatments. On average, F a was 94 and 55 m2 in low- and high-pruned trees.
For comparison purposes, the models developed by Callaway et al. (1994) for two populations of ponderosa pine growing in a humid place (mountain, 950 mm annual precipitation) and in a drier site (desert, 450 mm) in native forests in USA are also shown in Fig. 1. Estimations of F a from the model (1) are similar than those obtained using a model developed for ponderosa pines growing in humid places in USA (Callaway et al. 1994, Fig. 1). In contrast, the model predicts higher F a in thicker branches (diameter higher than 47 mm, Fig. 1) than the model developed for ponderosa pines growing in desert places (Callaway et al. 1994).
When relating diameters of the bole with total F a of the crown, due to the shape of the bole, a closer relationship was found using DLC (cm) than dbh of the tree (Fig. 2). The model obtained was (parameter ± S.E., n = 24, P < 0.001, r 2 adj 0.75):
Relationship between leaf area (F a) of the whole crown and diameter at breast height (dbh, n = 24, P = 0.06, R 2 adj 0.11) and at the base of the living crown (DLC, n = 24, P < 0.001, R2 adj 0.75) in ponderosa pine growing at 500 trees ha−1 in Patagonia, Argentina. Adjusted equation: a + b (dbh or DLC)
Branch diameter and leaf annual production
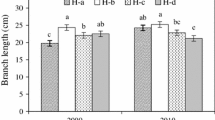
Maximum diameters were found from 7th to 12th whorl (Fig. 3). Mean maximum diameter of branches was approximately 5.5 cm (values found in the 6th whorl, Fig. 3). No differences were observed between mean diameter and mean maximum diameter of branches in each whorl comparing trees of the different plots at the beginning of the experiment (P > 0.05, Fig. 3 a, b). In following year, significant differences occurred in mean diameter and mean maximum diameter between low- and high-pruned pines in whorl 6 (P = 0.038 and 0.0322, respectively) and only in mean diameter at whorl 7 (P = 0.018). Surprisingly, in all cases, diameters were higher in low-pruned than in high-pruned trees (data not show). At the end of 2004, low-pruned trees had, comparing the same whorls, higher diameters of branches than high-pruned ones (P < 0.05, Fig. 3c, d) in 5th and 7th whorls (mean diameter) and in 2nd and 4th whorls (mean maximum diameter).
Branch mean diameter (cm ± SD, a and c) and branch mean maximum diameter (cm, b and d) of branches in each whorl (1 = top of the tree) at the beginning (a and b) and at the end of the study (c and d) in ponderosa pine trees growing at 500 trees ha−1 in Patagonia, Argentina. The values are the average of three trees in a and b, and four trees in c and d. The arrows indicate significant differences between treatments (α = 0.05). High- and low-pruned referred to pines with 4.5 and 3 m of pruned height (see Table 1)
In addition, when we grouped the whorls in three layers (top, middle and bottom), mean branch diameter of low-pruned trees at the middle and bottom of the canopy was higher than branches of the high-pruned trees at the third year after pruning (Table 2).
In contrast, no differences were found in the average number of branches per whorl between trees and at different heights in the crown (7.6 branches per whorl, Table 3). Maximum and minimum average number of branches per whorl was 8.8 and 6.8, respectively (Table 3). However, the lack of statistical differences could be due to the high variability in the number of branches of high-pruned trees, which presented higher values at the top and middle of their canopy (Table 3).
High-pruned trees showed lower annual basal area increment of branches than low-pruned ones. This explains the lower diameters of branches at the end of the experiment in the high-pruned pines. In this sense, branch basal area increment was 20 against 40 cm2 year−1, in high- and low-pruned pines, respectively (Fig. 4). The highest branch diameter growth rates were observed in the first eight whorls in both treatments.
Annual basal area increment (cm2 year−1) of branches during two consecutive growing seasons in ponderosa pine growing at 500 trees ha−1 in Patagonia, Argentina. High- and low-pruned referred to pines with 4.5 and 3 m of pruned height (see Table 1)
As in model (1), F a estimation depends on DIAM, leaf area growth followed the diameter growth patterns of branches. However, there were no significant differences between annual F a increment between treatments, probably due to the high variability within low-pruned trees (12.7 ± 3.3 and 21.2 ± 7.9 m2 year−1 for high- and low-pruned trees, respectively). When relative annual increment is considered, high-pruned trees produced more leaf area, compared to the initial area they have, than low-pruned trees. In this regard, a certain degree of compensatory leaf growth was observed.
Ecophysiological measurements
Pre-dawn water potential was measured in three days within one growing season. The first 2 days of measurements were within a period of high VPD values (average of 2.6 k Pa, with maximum values of 4.5 kPa), and low soil water content before the rain event of late February 2002 (Fig. 5). The last day of measurement (in March) was after small rains events typical of early autumn in the studied region. Stomatal conductance and leaf water potential along the day were also measured in one day within the first dry period. Comparing low- and high-pruned trees, the daily pattern of leaf water potential was marginally non-significantly different (Repeated measures ANOVA, P = 0.057). However, Tukey HSD test applied for each hour indicated that Ψ at predawn and at 16:00 h (maximum evaporative demand) were lower in high-pruned than in low-pruned pines (P = 0.006, Fig. 6). Stomatal conductance was also lower in high-pruned than in low-pruned trees (P = 0.0004), and decreased in all trees from 10:00 to 13:00 h (Fig. 6) in correlation with the decrease in Ψ (Fig. 7). Stomatal conductance of low-pruned pines at 13:00 h was similar than the gs of high-pruned pines at 10:00 h. Predawn Ψ values during the season were lower in high-pruned trees than in low-pruned trees (P < 0.05) during the dry period, and no significant differences were observed in March (P = 0.338) when 20 mm of precipitation fell some days before the measurement date.
Daily pattern of stomatal conductance (gs ± SD) and leaf water potential (±SD) at 4 h during one sunny day (Sep-21-2002) in ponderosa pine trees growing at 500 trees ha−1 in Patagonia, Argentina. Stars indicate statistical differences between low- and high-pruned trees (α = 0.05). High- and low-pruned referred to pines with 4.5 and 3 m of pruned height (see Table 1)
Relationship between stomatal conductance (gs ± SD) and leaf water potential of low- and high-pruned trees in ponderosa pine growing at 500 trees ha−1 in Patagonia, Argentina. High- and low-pruned referred to pines with 4.5 and 3 m of pruned height (see Table 1)
The amount of water used by each tree (liters day−1) in both years depended positively on F a (Fig. 8a). High-pruned trees were those with lower F a (empty symbols in Fig. 8a). However, when daily water use was normalized by F a (Fig. 8b, liters day−1 m−2), trees with lower F a (high-pruned trees) were those which used relatively more water. Within each year, the relationship between daily water used per m2 of leaf area and F a of the tree was described by the same general formula y = a + b F a 3, but with different parameter values (n = 6, P < 0.1, Fig. 8b).
Water used at individual (liters day−1, ±SD) and normalized by leaf area (liters day−1 m−2, F a) by low- (circles) and high- pruned trees (triangles) during the driest part of the summer of 2002 (empty symbols) and 2003 (filled symbols) in ponderosa pine trees growing at 500 trees ha−1 in Patagonia, Argentina. High- and low-pruned referred to pines with 4.5 and 3 m of pruned height (see Table 1)
Maximum water used per unit of F a, under the measured climatic conditions, was near 0.7 l day−1 m−2 (Fig. 8b), and this value decreased with total F a of the trees. The form of the equation indicated a fast saturation response as F a decreases, which does not allow for a compensation in water use (and maybe in carbon fixation) when some amount of foliar area is removed by pruning.
Discussion
Leaf area model
Estimations of F a from the model (1) are similar than those obtained using a model developed for ponderosa pines growing in humid places, but differed with the model developed from ponderosa pines growing in desert places in USA (Callaway et al. 1994). These results suggest that thin branches of ponderosa pine maintain a similar relationship between DIAM and F a among different environments, and probably, among different ages. However, as drought stress increases, this relationship changes for thick branches (higher than 47 mm) and relatively more sapwood is needed to provide water to the foliage. Considering that in general the upper branches of the pines are the thinnest ones, it is possible that the upper crown of ponderosa pines are similar in different environments and tree ages, as was also found for Douglas-fir (Ishii and Wilson 2001). On the contrary, differences in this relationship in the branches below certain threshold in the crown are expected in trees growing in sites with different water balances. In the particular case of the studied SPS (500 pines ha−1), average diameter of all branches was lower than the threshold diameter value at which the relationship F a:DIAM changes, whereas maximum diameter of the branches at the middle of the canopy was higher than that DIAM value (Table 2). These observations could give an opportunity to develop a general model for F a estimation in ponderosa pine, at least restricted to branches with a diameter lower than 4.7 cm.
Model (2) is based on the pipe theory. The pipe model theory emphasizes the importance of the sapwood as conductive tissue, but also the mechanical support function of stems. Kantola and Mäkelä (2004) found that at the tree-level the pipe model ratios were found to be very stable, and this could be independent of site quality, age or crown class in the case of Picea abies (L.) Karst. In contrast, Maherali and DeLucia (2001) found that ponderosa pines acclimate to sites with different water balances changing the relationship between F a and conducting tissue, as was observed in this study compared to data of thick branches of pines growing in desert places. Thus, it is expected that the relationship F a:DLC changes with site quality in this species. Zhang et al. (2004) found that cultural treatments (fertilization, complete vegetation control) have significant impacts on foliage biomass growth. However, DLC is closely related to current crown status regardless of cultural treatments, and for this reason, is a more robust predictor of the leaf area than dbh, as was found in this study. For management purposes, DLC is easier to measure than crown height or length. A model of F a based on DLC measurements could be useful in order to develop pruning schedules based on size crown and also, on an ecophysiological basis. For example, several management programs indicate that the pruning must be done at a height in which the stem has a particular diameter, independently of site quality (e.g., Kurtz and Ferruchi 2000). As plant performance (i.e., growth, transpiration, CO2 assimilation) is related to resource availability, the threshold diameter might be estimated and optimized.
Branch diameter and leaf annual production
The primary objective of this study was to test the hypothesis that pruning in pines growing in low density plantation positively influences the number of branches per whorl, the basal area growth rate of each branch, increasing the branch diameter even in positions closed to the apex. Firstly, green pruning had no effect over the number of branches per whorl (average of 7.7 branches per whorl). This lack of differences in the number of branches per whorl was also observed in a study in that were compared trees growing at different stand densities (Mäkinen and Hein 2006). However, the higher variability in the number of branches per whorl observed in the high-pruned trees at the top and middle of the canopy, suggests an effect of green pruning in our study. More information is needed in order to validate this hypothesis. In addition, we found trends that support the idea that pruning influences vertical distribution of branch basal area and F a, but only in the middle and bottom of the crown. Branches situated at the top of the canopy (thinner branches) were similar between trees independently of the treatment. As the size of the thickest branch of the tree determines the log grade, in the case of the studied pines the pruning of branches downward 8th whorl would not increase log quality.
Other studies have shown that the negative relationship between tree density and maximum branch diameter could be explained by the amount of radiation reaching each canopy layer (Stenberg et al. 1998). Below a certain threshold of radiation level, survival of leaves decreases and also, the increment of branch sapwood area does decrease. Accordingly, branch diameter profile may have a peak near the level of maximum lateral extension of the crown (Mäkinen and Hein 2006; Garber and Maguire 2005a). Garber and Maguire (2005a) found that foliage amount (and thus, branch diameter) in lower crown positions is largely influenced by the degree of light attenuation imposed by shoots at higher crown position. In a similar way, trees in a superior social position (tallest trees) have greater maximum branch diameters (Garber and Maguire 2005b). Also, in old growth Douglas-firs, the minimum relative level of photosynthetically active radiation was found in the same height of the crown where a marked decrease of live branch biomass was found (Ishii and Wilson 2001). Based on this knowledge, branches of trees growing in SPS could reach diameters that would decrease log quality. However, successive pruning may solve this problem even decreasing annual branch diameter growth (see below).
In general, the upper part of the crown is the most important regarding carbon assimilation, and, without pruning treatments, it seems to remain similar in shape and size between older or greater and smaller conifer tree. Several studies have shown that thinning or water availability do not produce changes in the upper part of the canopy. In this regard, Mäkinen and Hein (2006) and Kantola and Mäkelä (2004) found that for the first five whorls or meters down to the crown, branch size of P. abies did not differ between thinning intensities or ages. However, our results indicate that branches at the middle of the crown (whorl 5th and 7th) have a higher diameter growth in low-pruned than in high-pruned pines. Differences in branch diameter at the middle and bottom of the canopy between pruning treatments were also found when whorls were grouped in three main layers, being higher in low-pruned trees. This was an unexpected result. A possible explanation of the decrease in growth can be derived from an ecophysiological point of view. In this regard, hydraulic limitations could be responsible to the decrease in branch growth rate of the high-pruned trees: cavitation of sapwood by air ingression (Ueda and Shibata 2000), root death (Schroth 1999), and/or partial constriction in the base of the branches may limit hydraulic conductance of the tree (Schulte and Brooks 2003) (see below).
Ecophysiological measurements
The negative effect of high pruning on growth points out the low capacity of ponderosa pine to compensate for loss of leaf area. Our data suggest that high-pruned trees experiment more water stress than low-pruned ones, at least during periods of high evaporative demand and low soil water content (see predawn water potential in Fig. 5). Minimum Ψ values reached by leaves of the trees of both treatments agreed with the lower water potential values measured in other studies in N·W. Patagonia (Gyenge et al. 2003), indicating a stomatal regulation of water loss (Grulke and Retzlaff 2001; and see functional homeostasis, Magnani et al. 2002). An increase in stomatal resistance also means a decrease in carbon fixation capacity. In this regard, Grulke and Retzlaff (2001) demonstrated a stomatal limitation on photosynthesis of ponderosa pine when gs decreased below 0.08 mol m−2 s−1. The low gs values of high-pruned trees during the day (Fig. 6) suggest lower photosynthesis rates, which may explain the observed lower growth rates of branches. A similar decrease in growth rate was detected at the stem level (Gyenge 2005). However, in contrast to what was observed in branch growth, in the case of the stem, a decrease in growth rate was only measured the first growing season after pruning and not later. Future research is needed considering the effects of water stress in different periods after pruning and the causes why different tree organs are affected differentially.
Pruning of branches at the bottom of the canopy decreased whole tree transpiration (Fig. 7a) in spite of a certain degree of compensation (higher water used per unit of F a in high-pruned tress, Fig. 7b). This is a normal response. A linear relationship between water use and F a was also observed in Pinus radiata D. Don (Teskey and Sheriff 1996) and several Eucalypts species (Hatton et al. 1998). However, the degree of pruned F a and the decrease in water use were not linearly related, depending this relationship on the degree of compensation. In this regard, full compensation was observed in P. taeda after a 55% of extracted F a (Pataki et al. 1998), and therefore all trees used similar amounts of water even with such a degree of F a reduction. In this study, ponderosa pines showed a 10% difference in water use (lower in high-pruned pines) between high- and low-pruned pines differing in 25–40% of F a. These results suggest a lower compensatory capacity after pruning of ponderosa pine compared to other Pinus species. In this sense, ponderosa pine seems to be very susceptible to pruning, at least, in annual dbh growth (Mowat1947; Gyenge 2005).
Conclusions
In contrast to what was expected at the beginning of the study, pruning treatments applied to ponderosa pines growing in low density plantations did not result in higher branch diameters maybe due to a low hydraulic compensation capacity of the species. Higher water stress was detected in high-pruned pines compared to low-pruned trees, which may explain the observed differences in branch diameter growth between treatments, as well in stem growth. However, fortunately, differences in branch growth lasted for more years after pruning than differences in stem growth. Future research is needed to elucidate how ponderosa pines respond to pruning in sites with different water balances as well with different stand densities. In addition, further study is needed on the trade-off between losses of stem growth and gains in bole wood quality as a consequence of different amounts of extracted F a in these plantations characterized by relatively short rotation periods.
References
Callaway RM, Delucia EH, Schlesinger WH (1994) Biomass allocation of montane and desert ponderosa pine: an analog for response to climate change. Ecology 75:1474–1481. doi:10.2307/1937470
Ewers BE, Oren R (2000) Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol 20:579–589
Fernández ME, Gyenge JE, Schlichter TM (2006) Growth of Festuca pallescens in silvopastoral systems in Patagonia, part 1: positive balance between competition and facilitation. Agrofor Syst 66:259–269. doi:10.1007/s10457-005-0590-x
Garber SM, Maguire DA (2005a) The response of vertical foliage distribution to spacing and species composition in mixed conifer stands in Central Oregon. For Ecol Manag 211:341–355
Garber SM, Maguire DA (2005b) Vertical trends in maximum branch diameter in two mixed-species apacing trials in the central Oregon cascades. Can J Res 35:295–307. doi:10.1139/x04-164
Granier A (1985) Une nouvelle méthode pour la mesure du flux de séve brute dans le tronc des arbres. Ann Sci For 42:193–200. doi:10.1051/forest:19850204
Grulke NE, Retzlaff WA (2001) Changes in physiological attributes of ponderosa pine from seedling to mature tree. Tree Physiol 21:275–286
Gyenge JE (2005) Uso de agua y resistencia a la sequía de las principales especies forestales del noroeste patagónico, pino ponderosa y ciprés de la cordillera. PhD. thesis, Universidad Nacional del Comahue, 364 pp
Gyenge JE, Fernández ME, Schlichter TM (2003) Water relations of ponderosa pines in Patagonia Argentina: implications for local water resources and individual growth. Trees (Berl) 17:417–423. doi:10.1007/s00468-003-0254-2
Hatton T, Reece P, Taylor P et al (1998) Does leaf water efficiency vary among eucalypts in water-limited environments? Tree Physiol 18:529–536
Irvine J, Law BE, Anthoni PM et al (2002) Water limitations to carbon exchange in old-growth and young ponderosa pine stands. Tree Physiol 22:189–196
Ishii H, Wilson ME (2001) Crown structure of old-growth Douglas-fir in the western cascade range, Washington. Can J Res 31:1250–1261. doi:10.1139/cjfr-31-7-1250
Kantola A, Mäkelä A (2004) Crown development in Norway spruce (Picea abies (L.) Karst.). Trees (Berl) 18:408–421. doi:10.1007/s00468-004-0319-x
Kurtz VD, Ferruchi R (2000) La poda como parte de la estrategia para la obtención de madera de calidad. XV Jornadas Forestales de Entre Ríos, Concordia (E.R.), Argentina, pp B2-1–B2-23
Licata J (2007) Impact of exotic Ponderosa pine (Pinus ponderosa Doug. ex Laws.) Plantations on water resources in Northwestern Patagonia, Argentina. PhD thesis, Oregon State University, 151 pp
Magnani F, Grace J, Borghetti M (2002) Adjustment of tree structure in response to the environment under hydraulic constraints. Funct Ecol 16:385–393. doi:10.1046/j.1365-2435.2002.00630.x
Maherali H, DeLucia EH (2001) Influence of climate-driven shifts in biomass allocation on water transport and storage in ponderosa pine. Oecologia 129:481–491
Mäkinen H, Hein S (2006) Effect of wide spacing on increment and branch properties of young Noway spruce. Eur J For Res 125:239–248. doi:10.1007/s10342-006-0115-9
Montagu KD, Kearney DE, Smith RGB (2003) The biology and silviculture of pruning planted eucalypts for clear wood production–a review. For Ecol Manag 179:1–13
Mowat EL (1947) Effect of pruning on growth of Ponderosa pine. Forest research notes, PNW-38. USDA Forest Service, pp 1–3
Neter J, Waserman W (1974) Applied linear statistical models. Regression, analysis of variance and experimental design. R.D. Irwin Inc., Homewood, p 842
Pataki DE, Oren R, Phillips N (1998) Responses of sap flux and stomatal conductance of Pinus taeda L. trees to stepwise reductions in leaf area. J Exp Bot 49:871–878. doi:10.1093/jexbot/49.322.871
Pinkard EA (2002) Effects of pattern and severity of pruning on growth and branch development of pre-canopy closure Eucalyptus nitens. For Ecol Manag 157:217–230
Pinkard EA, Beadle CL, Davidson NJ, Battaglia M (1998) Photosynthetic responses of Eucalyptus nitens (Deane and Maiden) Maiden to green pruning. Trees (Berl) 12:119–129
Schroth G (1999) A review of belowground interactions in agroforestry, focusing on mechanisms and management options. Agrofor Syst 43:5–34. doi:10.1023/A:1026443018920
Schulte PJ, Brooks JR (2003) Branch junctions and the flow of water through xylem in Douglas-fir and ponderosa pine stems. J Exp Bot 54:1597–1605. doi:10.1093/jxb/erg169
Stenberg P, Smolander H, Sprugel D, Smolander S (1998) Shoot structure, light interception, and distribution of nitrogen in an Abies amabilis canopy. Tree Physiol 18:759–767
Teskey RO, Sheriff DW (1996) Water use by Pinus radiata trees in a plantation. Tree Physiol 16:276–279
Ueda M, Shibata E (2000) Change in trunk water transport area of heavily pruned Hinoki cypress, Chamaecyparis obtuse. Jpn J For Environ 42:57–60
Zhang Y, Borders BE, Will RE et al (2004) A model for foliage and branch biomass prediction for intensively managed fast growing loblolly pine. For Sci 50:65–80
Acknowledgements
We gratefully acknowledge personal of EEA INTA Bariloche for their support. This research was partially funded by INTA (Instituto Nacional de Tecnología Agropecuaria), FONCyT-MINCyT through the PICT 08-09415, and SAGPyA through the PIA 14/98.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyenge, J.E., Fernández, M.E. & Schlichter, T.M. Effect of pruning on branch production and water relations in widely spaced ponderosa pines. Agroforest Syst 77, 223–235 (2009). https://doi.org/10.1007/s10457-008-9183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-008-9183-9