Abstract
The CCN family is a novel class of extracellular signal modulators that has been recently established. Typical members are composed of four conserved modules connected tandem, each of which is rich in cysteines and highly interactive with other molecules. The mammalian CCN family consists of six members, most of which have been described as multifunctional factors for the developmental process of mesenchymal tissue including blood vessel formation/induction. Particularly, the angiogenic properties of the three classical members, CCN1, 2 and 3 have so far been characterized, and their physiological and pathological significance has thus been indicated. Recent research has uncovered a unique mechanism regarding these proteins in promoting and/or modulating developmental, physiological and pathological angiogenic events. Namely, CCN proteins exert their ability to drive angiogenesis, not by stimulating a particular behavior of a particular type of cells, but by manipulating the cell communication networks that integrate most of the associated molecules/cells toward angiogenesis. In this article, the role of the CCN proteins in a variety of angiogenic events as an organizer of microenvironmental cell society is comprehensively described, together with a brief summary of the recent findings on each CCN family member relevant to angiogenesis including cardiovascular development and diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis is a critical procedure that is involved in a variety of biological events in vertebrates [1]. First and foremost, the formation of the placenta and cardiovascular system are definitely indispensable for mammalian embryonic development, which thus leads to fundamental organogenesis [2]. After development, growth of most of organs and tissues is supported by the expanding blood stream network. In adult animals, even though continuous angiogenesis may no longer occur, physiological angiogenesis is readily initiated as a part of the normal biological response, where additional oxygen, nutrition or cellular infiltration is demanded. Angiogenic events occur under the collaboration of vascular endothelial cells and other types of cells of various origins in the microenvironment. During this process, several molecules that are known as angiogenic factors, such as vascular endothelial growth factor (VEGF), play the role of the key commander [2, 3]. Under the signals emitted by such commanders, a vast number of different extracellular matrix (ECM) components, such as adhesion molecules, and tools for the reconstruction of ECM, including proteinases, are produced by those cells. Then blood vessels are finally manufactured at the requested site based on a properly organized ECM structure. Obviously, in order to establish such an integrated construction, certain molecules have to organize the information from the commander and then have to assist the tissue components to be placed correctly. We thus consider that the CCN proteins should be managing these difficult tasks [4–8].
The first member of the CCN family emerged in 1989 under the initial name of Cyr61, because of its cysteine-rich primary structure [9]. Two years later, the second member was identified as a secreted protein with mitogenic and chemotactic activities for fibroblasts from human vascular endothelial cells, and thus it was designated connective tissue growth factor (CTGF) [10]. Then, the next year, a nephroblastoma-overexpressed protein (NOV) with significant homology to Cyr61 and CTGF was identified as a truncated form in a chicken nephroblastoma [11]. Three genes encoding structurally similar proteins are necessary and sufficient to make up a gene family. As such, the initial word “CCN” was born and given to this family of genes in 1993 based on a proposal by Bork, by assembling the initials of the three classical members in order [4]. Thereafter, three additional members were newly reported by 1998 with the proper names totally irrelevant to the family name, CCN [12–15]. However, since most of the initial names given to each member were gradually revealed to be contradictory and misleading, a unified nomenclature was thus proposed in 2003 under the agreement of several major researchers in the CCN research field [16]. Today, all of the CCN family members are usually represented by a unified name, such as CCN1 or CCN2, rather than by classical names.
The CCN family proteins: multi-handed modulator of the extracellular information network
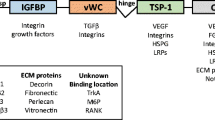
The primary structure of the CCN family proteins is quite unique. The basic organization of these proteins is, in a sense, a relatively simple one that consists of four conserved modules aligned in order. From the N-terminus, the insulin-like growth factor binding protein (IGFBP) module, von Willebrand factor type C repeat (VWC) module, thrombospondin type 1 repeat (TSP1) module and carboxyl-terminal (CT) module, which is absent only in CCN5, are connected in tandem, while no other functional domain exists [4–8]. Interestingly, all of these four modules are highly interactive with other proteins and proteoglycans [17–25]. Furthermore, these modules are encoded by single separate exons on the mRNAs, thus indicating that this gene family has evolved through exon shuffling, and thus each module is structurally independent from the others. This structure is as unusual as a tool composed of four hands or adaptors without any other devices. Such a tool may appear awkward, however, if duly used, it can be useful in a variety of situations. In fact, the CCN proteins exert multiple functions in different biological situations [26–33], which sometimes yields an apparently contradictory biological outcome induced by the same CCN protein under different conditions. For example, CCN2 was shown to induce apoptosis when it was overexpressed in breast cancer cells [34], while it is also known as a promoter of metastasis in the same type of cells in vivo [35, 36]. Therefore, even though the association with malignancies has been reported in all six members of the CCN family, the role of these proteins in carcinogenesis and malignant phenotypes still remains controversial [11, 12, 15, 37–45]. In terms of the development of mesenchymal tissue, most of the CCN family members have been shown to act as a promoter of cell growth and differentiation [26, 31–33, 46–49], with a few exceptions such as CCN3 with myoblasts [22]. In the case of the best-characterized CCN2, this factor is known to promote the proliferation and differentiation of fibroblasts, chondrocytes, osteoblasts and periodontal ligament cells as well as vascular endothelial cells [18, 26, 31–33, 48, 49]. How can a CCN protein perform such multiple jobs? The answer may be found in the novel modular structure of this family. Using its four (three for CCN5) conserved modules, a CCN protein is able to interact with multiple molecules in the microenvironment, thus resulting in the modulation of the extracellular molecular network therein. The molecular counterparts of the CCN proteins include adhesion molecules [5, 18, 21, 28, 50], cell surface signal transducing receptors [24, 25], proteoglycans [19, 28, 51] and even growth factors [19, 20]. CCN proteins may therefore also be called an integrator/modulator of extracellular information. The angiogenic effects of the CCN proteins are also the outcome of the negotiations with other molecules through the communication via four modules (Fig. 1).
(A) The primary structure of the CCN family proteins and their reported variants. The general structure is composed of four conserved modules is illustrated as a nascent translation product with a signal peptide for secretion (S) at the top. The module names abbreviated here are fully described in the text. The modules are then further abbreviated into a single letter when describing the structure of each member or its variant. Namely, “I”, “V”, “T” and “C” represent IGFBP, VWC, TSP1 and CT modules, respectively. In addition to the names under the unified nomenclature, a few classical and well-known names are also presented. As briefly noted in the figure, CCN2 variant proteins were reported by Brigstock et al. [52] Kubota et al. [53], Hinton et al. [54] and Boes et al. [55]. The N-terminal truncated form of CCN3 was identified in nephroblastomas by Joliot et al. [11]. In the cases of CCN4 and CCN6, variants were confirmed at mRNA level, which were generated through the alternative mRNA splicing [40–42]. (B) Multiple molecular interactions by the CCN proteins as exemplified by CCN2. In this panel also, each module is indicated by a single letter abbreviation, as explained above. The abbreviation HSPG stands for heparan sulfate proteoglycans. All of the other abbreviations are explained in the text
General mechanism of the angiogenic action of the CCN family proteins
According to the phenotypes of conventional KO mice of each CCN family member [56–58], although few members turn out to play a critical role in embryonic vascular development, no CCN family member may be defined as a master molecule of angiogenesis. In fact, even in the CCN1-KO with the most severe defect in angiogenesis, the formation of the blood vessels itself was distinctly observed until placental blood vessel fusion [58], thus representing its role rather as a developmental modulator.
The development of the embryonic vascular system is initiated under the signals mediated by bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs), as is generally observed in a number of morphogenetic events. In response to these signals, angioblasts and hematopoietic cells construct blood islands and vessels at proper temporal and spatial locations, which are regulated by a number of growth factors, such as VEGF, transforming growth factor (TGF)-β, platelet-derived growth factors (PDGFs), angiopoietin, sonic hedgehog (Shh), and ephrins, together with their receptors [2]. Of note, several CCN family members are known to interact with these growth factors [17, 20] or regulate the gene expression of some of them, and vice versa [39, 59]. As a result, these CCN family members are anticipated to modulate the effects of these growth factors toward the proper development of the cardiovascular system.
The other critical function of the CCN protein in angiogenesis is probably exerted under the interaction with the extracellular matrix (ECM) molecules and cell-surface adhesion molecule. This functional aspect of these CCN proteins may be metaphorized by a molecular scaffold for ECM construction. By interacting with integrins, fibronectins and other proteins and proteoglycans, several CCN proteins have been shown to promote adhesion and migration of various types of cells, including vascular endothelial cells (Table 1) [18, 28, 46, 47, 51]. In this context, the role of integrins in determining target cells for the CCN proteins is of particular interest, since several distinct subunits are differentially displayed onto the cells that are associated with angiogenic events. In addition to these two major mechanisms, several CCN proteins are capable of inducing the production of proteins that are critical for ECM remodeling which accompanies the physiological and pathological angiogenesis [27, 36] (Fig. 2).
(A) The general mechanism of angiogenic action of the CCN proteins. Through the interaction with integrins, angiogenic CCN proteins, such as CCN1, CCN2 and CCN3, promote the migration, adhesion and survival of vascular endothelial cells. CCN proteins are also known to bind to several ECM components including proteoglycans and adhesion proteins, which furnishes microenvironmental basement for the neovascularization by modulating the ECM architecture. The function of other growth factors is occasionally modulated by the direct interaction with these CCN proteins. The abbreviation “VEC” stands for “vascular endothelial cell”. (B) The hypothetical roles of CCN proteins in the regulation of the development of embryonic cardiovascular systems at early stages. Based on the findings obtained in vitro, the possible interactions of CCN proteins with the signaling molecules involved are illustrated. All of the full designations of the abbreviated names can be found in the text. Eph is the receptor of ephrin
The angiogenic property of each CCN family member
Until today, the angiogenic properties have been analyzed and reported for the three classical members. Concerning the other three members, CCN4, 5 and 6, no clear evidence has yet been presented so far. First, in this section, the current knowledge related to angiogenesis is summarized for CCN1, 2 and 3 individually (Fig. 3).
Assignment of the functioning stages for angiogenic CCN proteins in the angiogenic events throughout the life. According to the accumulating findings, CCN1 appears to play a critical role in the earlier stages of embryonic vascular development, while its function at later stages, such as tissue regeneration, may not be ruled out. In contrast, CCN2 is required for the angiogenic events at later stages of development and it is obviously involved in the regeneration of various tissues. Although no strict requirement of CCN3 in particular angiogenic events has yet been specified, it is plausible that CCN3 supports the CCN1 and CCN2 function in certain aspects of angiogenic events during the development and maintenance of the life. The angiogenic roles of CCN4, 5 and 6 still remain to be investigated further, since an association of these proteins with malignancies has been suggested
CCN1/Cyr61
CCN1 is not only the first identified member of the CCN family, but also the first member to have been described as an angiogenic factor. In 1998, Babic et al. reported the positive effect of CCN1 on angiogenesis and tumor growth [60]. Utilizing a baculovirus vector system to produce recombinant CCN1, they found that CCN1 was capable of enhancing migration and adhesion of microvascular endothelial cells in vitro, and inducing neovascularization on cornea in vivo. These effects were efficiently inhibited by the addition of CCN1-specific antibody, thus confirming the specificity of the observed effects. However, in order to obtain angiogenic effects comparable to basic fibroblast growth factor (bFGF), CCN1 of much higher concentration was required than bFGF. This dose-discrepancy might be due to either lower activity of the recombinant CCN1 than the intact one, or different mechanism of CCN1 in exerting angiogenic effects from that of bFGF.
The mechanism of the CCN1 action to enhance neovascularization by the promotion of migration and adhesion is mainly mediated by integrins (Fig. 1). Integrins are cell surface adhesion receptors that consist of two subunits, α and β. The α subunit is associated with divalent cations, whereas the β subunit contains a talin-binding domain, and thus is thought to transmit extracellular signals into the cells by activating focal adhesion kinase (FAK) [61]. In addition to the CCN family members, a number of different proteins have been identified as ligands for integrins. Interestingly, although most of the other ligands interact with integrins with a conserved peptide motif, arginine–glycine–aspartic acid (RGD), no such peptide sequence has been identified in the integrin binding sites in CCN1 [5, 7]. For CCN1-induced angiogenesis, integrin αvβ3 was initially shown to mediate the molecular interaction to realize the biological outcome [61]. A subsequent investigation with umbilical vein endothelial cells indicated that the angiogenic action mediated by integrin αvβ3 required its activation, whereas another integrin α6β1 constitutively supported the early proangiogenic events induced by CCN1 [62]. Furthermore, a recent report has described the collaboration of CCN1, integrin α6β1 and heparan sulfate proteoglycans to be able to promote the adhesion and chemotaxis of vascular smooth muscle cells [63]. Therefore, CCN1 is expected to play a significant role in organizing endothelial cells to construct almost all types of blood vessel structures. In addition to such integrin-mediated effects, CCN1 is known to promote the proliferation of vascular endothelial cells in an ECM-dependent manner in collaboration with other signaling molecules. Namely, owing to the strong affinity of CCN1 to heparan sulfate proteoglycans, it was found to displace another ECM-associated growth factor, such as bFGF, which eventually enhanced the proliferation of those cells [64].
Consistent with the findings above, conventional ccn1 KO mice that were created in 2002 displayed severe defects in angiogenesis during embryonic development [58]. The KO embryos suffered from defect in chorioallantoic fusion and placental vascular inefficiency. Such phenotypic changes were the most severe among all of the CCN KO mice analyzed, thus indicating angiogenesis to be a major mission of CCN1. In addition, since the observed vascular malformation was characterized by a specific defect in the vessel bifurcation related to the local impairment of VEGF gene expression, the collaboration of VEGF and CCN1 may thus be critical for the proper embryonic vascular development [58].
CCN2/CTGF
It is worthy of note that human CCN2 was originally discovered in, and purified from the conditioned medium of cultured vein endothelial cells [10]. Thereafter, following the characterization of CCN1 as a proangiogenic protein, the angiogenic potential of CCN2 was uncovered by two research groups independently. In 1998, Shimo et al. reported that knocking down the ccn2 expression resulted in the suppression of the proliferation and migration of normal vascular endothelial cells [48]. The next year, two articles directly describing the angiogenic activity of CCN2 in vitro and in vivo were published [18, 48]. Indeed, integrins were again revealed to be critical functional counterparts of CCN2 [18]. Furthermore, the CCN2-binding integrins known today are almost the same as those binding to CCN1 [7]. As expected, the enhanced migration and survival of endothelial cells by CCN2 were shown to be mediated by integrin αvβ3 [18]. The binding site in CCN2 to this particular integrin was located in the CT module, comprising a peptide sequence of NH2–IRTPKISKPIKFELSG–COOH, which does not contain any RGD motif of integrin binding. As such, CCN2 is believed to promote angiogenesis via the same mechanism as that of CCN1 [7]. However, their roles in embryonic angiogenesis are not the same, which is probably due to the four-dimensional difference in their gene expression patterns as realized by different gene regulatory systems. It should be also noted that CCN2 is processed into subfragments containing the CT module, which retains biological activities at least in part [65]. The functional significance of these subfragments still remains to be addressed, but they may represent a CCN2-specific biological role that is not assigned to CCN1.
The involvement of CCN2 in embryonic vascular development was also represented in the phenotypic changes in conventional CCN2 KO mice, albeit at a less striking level than in the CCN1 KO mice [56]. In CCN2 KO mice, no profound defect was observed in placental vascular development, and thus embryos usually survive until delivery. However, these CCN2 KO mice are also lethal upon delivery, probably due to the functional failure caused by a skeletal defect. A histological examination revealed the endochondral ossification process that determines the growth of most bones to be perturbed, which was characterized by the defect of vascular invasion into terminally differentiated layers of chondrocytes. Such phenotypes represent a critical role of CCN2 not only in cartilage growth, but also in angiogenesis during endochondral ossification. Endochondral ossification continues until the end of body growth. Therefore, CCN2 remains as a critical angiogenic factor for the skeletal growth of humans for longer than 10 years, but the task of CCN2 is not yet over, even after that.
Accumulating evidence has indicated that CCN2 is a major CCN family protein that promotes wound healing and tissue regeneration, which is also supported by the fact that CCN2 gene expression is temporally and spatially regulated during foliculogenesis and luteogenesis [66]. Moreover, although CCN2 is dispensable for embryonic vascular fusion to placenta, it is believed to facilitate the capillary development from maternal vessels in the placenta [30, 67], thus indicating that CCN2 also plays a role in mammalian reproduction.
In addition, in the case of CCN2, a novel role as an angiogenic modulator as well as a proanigiogenic molecule has been suspected, which is based on an investigation of the molecular interaction of CCN2 and VEGF [20]. According to the report by Inoki et al., when CCN2 co-existed, the VEGF-induced angiogenesis was rather repressed, although CCN2 is capable of inducing angiogenesis to some extent per se, which was based upon the direct interaction of CCN2 with VEGF, using the TSP1 and CT modules as interface. Of note, CCN2 and VEGF are capable of inducing gene expression in each other in vascular endothelial cells [7, 39, 59]. Considering the role of VEGF as a critical player in angiogenic events, the role of CCN2 as a modulator of the major information network is thus herein emphasized. Finally, one should not overlook the ability of CCN2 to promote the synthesis of ECM components, such as collagens, which are needed for the construction of a vascular bed that is prerequisite for neovascularization [65].
CCN3/NOV
As expected by the structural similarity with the other two classical CCN family members, the pro-angiogenic effects of CCN3 were investigated and reported in 2003, in which CCN3 was entitled “angiogenic regulator”. The mechanism of angiogenic action is also quite similar to CCN1 and 2, which is mediated by integrins αvβ3, α6β1 and α5β1, together with heparan sulfate proteoglycans [47]. Since the amino acid sequence of CCN3 does not contain any RGD motif, interaction of CCN3 and these integrins are carried out with proper peptide motif(s), as observed in CCN1 and CCN2. Unexpectedly, conventional KO mice of CCN3 appeared to display no distinct phenotypic abnormalities (Katsube et al., personal communication), thus representing the functional redundancy of the classical three members in embryonic vascular development and body growth. Nevertheless, it is still possible for CCN3 to act as a critical angiogenic modulator at particular biological situations, where both CCN1 and CCN2 are unavailable.
CCN4/Elm1/WISP1, CCN5/COP1/WISP2 and CCN6/WISP3
To date, although the involvement of CCN4 in mesenchymal tissue development has been described, no evidence has yet been reported concerning its angiogenic activity. In contrast, interesting findings in relation to vascular biology have been reported in the case of CCN5. Surprisingly, CCN5 was found to rather inhibit the proliferation of vascular smooth muscle cells (Table 1) [68]. Interestingly, CCN5 is the only member that lacks the CT module. Since the CT module generally acts as an interface for binding to proteoglycans, this inhibitory effect may thus be regarded as a trans-dominant effect on the ECM-associated action of CCN proteins. Nevertheless, the last CCN family member, CCN6, was shown to act as an antagonist of tumor angiogenesis, albeit the retention of the CT module [69]. These apparently contradictory findings may thus be due to the different experimental conditions and cellular background employed, or they may represent other mechanisms of action than the ones hypothesized above.
The ccn family members in the development of cardiovascular system
According to the molecular histochemical analysis of developing embryos, a few CCN family member genes are significantly expressed during cardiovascular development. Particularly, ccn1 and ccn2 gene expression appears to be prominent in the mesenchymes of developing cardiovascular tissues and/or the developed vasculature, thus indicating a certain role as a modulator of the ECM therein [58, 66, 67]. However, no CCN family member has yet been shown to be fundamentally essential for the initial development of the cardiovascular system itself. Even in the CCN1 KO mice, the primary vascular plexus was found to form normally. The vascular plexus next develops into a specified vein and artery leading to the establishment of circulatory flow, where Notch signaling plays a critical role. Interestingly, even though CCN1 KO mice showed dorsal aorta formation, the arterial wall was abnormally fragile and thus frequently caused hemorrhaging in the KO embryo. Together with the failure of chorioallantoic fusion and labyrinth formation in placenta observed in these mice, CCN1 is believed to be responsible for the vascular integrity, particularly in the arteries [58]. It is therefore anticipated that CCN1 should be required to organize the ECM network throughout the embryonic vascular development. Unexpectedly, CCN3 appeared to be dispensable for cardiovascular development, although it is known to interact with Notch molecules during embryonic development [22]. After the establishment of the arterial and venous fate, the vascular plexus has to be rearranged, following the morphological program, which is mediated by signaling molecules including Shh and BMP (Fig. 2). CCN2 is known to interact with several of such signaling molecules, but it appeared to be unnecessary for this process, as it is represented by no obvious malfunction in the cardiovascular system of CCN2 KO mice, at least before delivery. Collectively, the differential roles of the three functionally similar proangiogenic CCN family members in the development of cardiovascular system can be summarized: CCN1 is the primary modulator of the specialized ECM structure in cardiovascular development, whereas CCN2 is produced as a supplemental molecule that can partially substitute the role of CCN1. In spite of its angiogenic activities, CCN3 may not be critically involved in this particular biological process, as indicated by no distinct evidence for gene expression therein and no abnormal phenotypes associated with the KO mice.
Role of the CCN proteins in tumor angiogenesis, progression and metastasis
It is a critical issue for tumors to induce neovascularization in establishing the supply of nutrition and oxygen toward further growth, and also in preparing the entrance leading to metastasis. Therefore, the angiogenic potential of a neoplasm is one of the determinants of its malignant phenotypes, and it is represented by the quality and quantity of the angiogenesis-associated factors released from the tumor cells [1]. The CCN family proteins have been attracting the interest of a number of oncologists from two different viewpoints. The first one is based on the above-mentioned angiogenic effects of the three classical members, whereas the second arose from the association of certain malignancies with the overexpression of normal or truncated forms of particular members. The possible involvement of the truncated forms caused by alternative splicing or retroviral gene modification in a few animal and human malignancies has been indicated in CCN3, 4 and 6 [11, 41–43]. In the case of the full-length intact CCN proteins, a number of studies investigated the relationship of their gene expression and malignant phenotypes, and most of those found that CCN proteins themselves rather repress the proliferation and invasiveness of the tumor cells [34, 38, 40, 70]. However, a few studies have also described a significant relationship between the metastatic phenotypes and the CCN family member gene expression in vivo [35, 45], which can be ascribed to their angiogenic effects acted in both paracrine and ECM-associated (matricrine) manners.
Among the angiogenic CCN family members, the role in tumor angiogenesis has been best characterized in CCN2. Along with the substantial growth of solid tumors, the tumor cells deep inside of the tumor suffer from hypoxia. The hypoxic induction of angiogenic factors is thus a critical property that allows the tumor to grow further. It is already known that VEGF is induced by hypoxia via the induction of hypoxia inducible factor 1 (HIF1) which transactivates the vegf gene transcription [3]. Interestingly, it has been recently uncovered that CCN2 is induced by hypoxia through different regulatory systems. The production of the CCN2 molecule is enhanced by hypoxia through multiple steps except for mRNA transcription. In terms of gene expression, hypoxic stress increases the level of ccn2 mRNA without changing the transcription activity of the ccn2 gene [36, 71]. The steady-state mRNA level is determined by the balance of the formation and degradation of the mRNA. The hypoxic signal controls the latter step to prolong the half-life or ccn2 mRNA, which results in the net increase of the ccn2 gene expression [71]. This post-transcriptional regulation is mediated by a cis-acting element of structure-anchored repression (CAESAR) in the 3′-untranslated region (UTR) of the ccn2 mRNA [71–73]. The other mechanism of the hypoxic overproduction of CCN2 is a post-translational one that is based on the ECM-associated behavior of CCN2. After hypoxia, the CCN2 trapped in the ECM is rapidly released, which confers an apparent increase in the secretory production of CCN2 [36, 71]. This event is thought to be mediated by the induction and activation of matrix remodeling enzymes, such as matrix metalloproteinases [36]. Consequently, CCN2 is now regarded as a central mediator of hypoxia-inducible angiogenic factor of certain malignancies. It should be also noted that CCN2 has recently been described as one of the critical mediators that determine the highly metastatic phenotype in breast cancers [35].
Cardiovascular disorder and the CCN proteins
Before the unification of the terminology, CCN2 was widely known as connective tissue growth factor (CTGF). In pathological point of view, the name, CTGF, deserves to represent the effect of CCN2, since CCN2 is associated with fibrotic disorders of a variety of organs and tissues [74–77]. The involvement of CCN2 in cardiac fibrosis has also been evaluated [78], and a significant relationship has been identified between the plasma CCN2 level and the cardiac function parameters of patients with heart failure, such as myocardial infarction (Koitabashi et al., in preparation). These findings suggest the utility of CCN2 as a diagnostic and prognostic marker for heart failure, which is one of the most frequent adult diseases in advanced countries. Arteriosclerosis is another major clinical complexity of adult humans. The prognosis of this disorder highly depends on the fragility of the arterial wall that is deranged by the formation of the atherosclerotic plaque. The initial finding that CCN2 is highly expressed in advanced atherosclerosis lesions was reported in 1997 [79]. An increased expression of CCN1 was also observed in the atherosclerotic vessels but it appeared to be angiotensin II dependent [80]. Notably, the accumulation of CCN2 in the plaque has also been observed as well [81]. The pathogenic role of this accumulation of CCN2 is unclear, but it was shown to stimulate monocyte/macrophage migration in vitro [23]. Since the atherosclerotic plaque is formed by the accumulation of cholesterol in oxidized low-density lipoprotein (LDL) taken up by macrophages, these findings should therefore not be overlooked. In addition, CCN2 is a possible mediator of aortic fibrosis by a distinct pathway. Further investigations with human aortic endothelial cells showed CCN2 to be produced by an increased blood flow [82] and by stimulation with oxidized LDL though TGF-β [83]. Probably, CCN2 is also engaged in the regeneration of damaged cardiovascular tissue, but it ends up insufficient; hence fibrous tissue replaces the normal functional tissue after all. Therefore, minor therapeutic modifications of such regenerative action of CCN2 toward a better direction may potentially improve the states of those diseases.
Conclusions and prospects
Embryonic angiogenesis is the most fundamental step leading to organogenesis. A classical CCN family member, CCN1, is a critical modulator of the extracellular components in constructing most types of integrated vascular architecture throughout the establishment of the cardiovascular system. A similar role is assigned to another classical member, CCN2, in a different developmental process. Based on the accumulated data in vitro and the phenotypic changes observed in the KO mice, CCN2 is now believed to be such a central modulator of extracellular signaling and molecular architecture in the endochondral ossification process. Endochondral ossification is the most fundamental step for skeletalogenesis in vertebrates, which remains active until the onset of adulthood. After body growth is over, CCN2 continues to play a central role in modulating regenerative events and angiogenesis upon request [84, 85], which sometimes is advantageous for the metastasis of tumors. CCN1 was also indicated to mediate the regeneration of particular tissues [86]. Therefore, both CCN1 and CCN2 are engaged in similar missions in different mesenchymes, following different genetic programs encoding the proper temporal and spatial gene expression (Fig. 3). Since CCN3 is also angiogenic and it is expressed in developing mesenchymes, it is therefore expected to be a central modulator of the extracellular microenvironment of certain tissues. However, specific tissues in which CCN3 plays a critical role still remain to be identified. The same hypothesis can be proposed for the other CCN family members. Research in the future may help to clarify the roles CCN4, CCN5 and CCN6 in a particular cell society as a molecular modulator of the extracellular network.
From a clinical point of view, tumor angiogenesis is one of the major targets of chemotherapy of malignancies [87]. Unlike vascular development, the major modulator of this process has been shown to be the CCN2 molecule, which is thus now regarded as a potential target of anti-angiogenic therapy [88, 89]. In fact, a recent study has shown that an anti-angiogenic agent was capable of inhibiting the ccn2 gene induction by VEGF in human vascular endothelial cells [90]. All of the CCN family members are kept silent in adult animals after development and body growth. Therefore, an anti-CCN strategy may provide a safer choice without adverse effects than those with other agents, such as metabolic antagonists. In particular, CCN2 is involved in tumor angiogenesis, atherosclerosis and cardiac fibrosis, which constitute the pathological background of all of three major diseases in human society of advanced countries. Millions of patients may thus be awaiting the advance of CCN research regarding the fields of cardiology, angiology and oncology.
References
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Coultas L, Chawengsaksophak K, Rossant J (2005) Endothelial cells and VEGF in vascular development. Nature 438:937–945
Ribatti D (2005) The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol 128:303–309
Bork P (1993) The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 327:125–130
Lau LF, Lam SC (1999) The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 248:44–57
Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363:62–64
Perbal B, Takigawa M (eds) (2005) CCN proteins—a new family of cell growth and differentiation regulators. Imperial College Press, London
Takigawa M, Nakanishi T, Kubota S, Nishida T (2003) Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol 194:256–266
O’Brien TP, Yang GP, Sanders L, Lau LF (1990) Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol 10:3569–3577
Bradham DM, Igarashi A, Potter RL, Grotendorst GR (1991) Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 114:1285–1294
Joliot V, Martinerie C, Dambrine G et al (1992) Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 12:10–21
Hashimoto Y, Shindo-Okada N, Tani M et al (1998) Expression of the Elm1 gene, a novel gene of the CCN (connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses in vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med 187:289–296
Zhang R, Averboukh L, Zhu W et al (1998) Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol 18:6131–6141
Kumar S, Hand AT, Connor JR et al (1999) Identification and cloning of a connective tissue growth factor-like cDNA from human osteoblasts encoding a novel regulator of osteoblast functions. J Biol Chem 274:17123–17131
Pennica D, Swanson TA, Welsh JW et al (1998) WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA 95:14717–14722
Brigstock DR, Goldschmeding R, Katsube KI et al (2003) Proposal for a unified CCN nomenclature. Mol Pathol 56:127–128
Abreu JG, Ketpura NI, Reversade B, De Robertis EM (2002) Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol 4:599–604
Babic AM, Chen CC, Lau LF (1999) Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19:2958–2966
Desnoyers L, Arnott D, Pennica D (2001) WISP-1 binds to decorin and biglycan. J Biol Chem 276:47599–47607
Inoki I, Shiomi T, Hashimoto G et al (2002) Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J 16:219–221
Jedsadayanmata A, Chen CC, Kireeva ML et al (1999) Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J Biol Chem 274:24321–24327
Sakamoto K, Yamaguchi S, Ando R et al (2002) The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem 277:29399–29405
Schober JM, Chen N, Grzeszkiewicz TM et al (2002) Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood 99:4457–4465
Segarini PR, Nesbitt JE, Li D et al (2001) The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem 276:40659–40667
Wahab NA, Weston BS, Mason RM (2005) Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol 16:340–351
Asano M, Kubota S, Nakanishi T et al (2005) Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells. Cell Commun Signal 5:11
Brigstock DR (2002) Regulation of angiogenesis and endothelial cell function by connective growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 5:153–165
Gao R, Brigstock DR (2004) Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem 279:8848–8855
Igarashi A, Okochi H, Bradham DM, Grotendorst GR (1993) Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4:637–645
Kireeva ML, Latinkic BV, Kolesnikova TV et al (1997) Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 233:63–77
Nakanishi T, Nishida T, Shimo T et al (2000) Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology 141:264–273
Nishida T, Nakanishi T, Asano M et al (2000) Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol 184:197–206
Safadi FF, Xu J, Smock SL et al (2003) Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol 196:51–62
Hishikawa K, Oemar BS, Tanner FC et al (1999) Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J Biol Chem 274:37461–37466
Kang Y, Siegel PM, Shu W et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3:537–549
Kondo S, Kubota S, Shimo T et al (2002) Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis 23:769–776
Kubo M, Kikuchi K, Nashiro K et al (1998) Expression of fibrogenic cytokines in desmoplastic malignant melanoma. Br J Dermatol 139:192–197
Moritani NH, Kubota S, Nishida T et al (2003) Suppressive effect of overexpressed connective tissue growth factor on tumor cell growth in a human oral squamous cell carcinoma-derived cell line. Cancer Lett 192:205–214
Shimo T, Nakanishi T, Nishida T et al (2001) Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology 61:315–322
Soon LL, Yie TA, Shvarts A et al (2003) Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem 278:11465–11470
Tanaka S, Sugimachi K, Saeki H et al (2001) A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene 20:5525–5532
Tanaka S, Sugimachi K, Shimada M et al (2002) Variant WISPs as targets for gastrointestinal carcinomas. Gastroenterology 123:392–393
Tanaka S, Sugimachi K, Kameyama T et al (2003) Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology 37:1122–1129
Tong X, Xie D, O’Kelly J et al (2001) Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer. J Biol Chem 276:47709–47714
Xie D, Nakachi K, Wang H et al (2001) Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res 61:8917–8923
Chen CC, Chen N, Lau LF (2001) The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem 276:10443–10452
Lin CG, Leu SJ, Chen N et al (2003) CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem 278:24200–24208
Shimo T, Nakanishi T, Kimura Y et al (1998) Inhibition of endogenous expression of connective tissue growth factor by its antisense oligonucleotide and antisense RNA suppresses proliferation and migration of vascular endothelial cells. J Biochem (Tokyo) 124:130–140
Shimo T, Nakanishi T, Nishida T et al (1999) Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem (Tokyo) 126:137–145
Hoshijima M, Hattori T, Inoue M et al (2006) CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett 20:580:1376–1382
Nishida T, Kubota S, Fukunaga T et al (2003) CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol 196:265–275
Brigstock DR, Steffen CL, Kim GY et al (1997) Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem 272:20275–20282
Kubota S, Eguchi T, Shimo T et al (2001) Novel mode of processing and secretion of connective tissue growth factor/ecogenin (CTGF/Hcs24) in chondrocytic HCS-2/8 cells. Bone 29:155–161
Hinton DR, Spee C, He S et al (2004) Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care 27:758–764
Boes M, Dake BL, Booth BA et al (1999) Connective tissue growth factor (IGFBP-rP2) expression and regulation in cultured bovine endothelial cells. Endocrinology 140:1575–1580
Ivkovic S, Yoon BS, Popoff SN et al (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791
Kutz WE, Gong Y, Warman ML (2005) WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol 25:414–421
Mo FE, Muntean AG, Chen CC et al (2002) CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 22:8709–8720
Suzuma K, Naruse K, Suzuma I et al (2000) Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem 275:40725–40731
Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 95:6355–6360
Ginsberg MH, Partridge A, Shattil SJ (2005) Integrin regulation. Curr Opin Cell Biol 17:509–516
Leu SJ, Lam SC, Lau LF (2002) Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 277:46248–46255
Grzeszkiewicz TM, Lindner V, Chen N et al (2002) The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology 143:1441–1450
Kolesnikova TV, Lau LF (1998) Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene 16:747–754
Grotendorst GR, Duncan MR (2005) Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J 19:729–738
Friedrichsen S, Heuer H, Christ S et al (2005) Gene expression of connective tissue growth factor in adult mouse. Growth Factors 23:43–53
Surveyor GA, Brigstock DR (1999) Immunohistochemical localization of connective tissue growth factor (CTGF) in the mouse embryo between days 7.5 and 14.5 of gestation. Growth Factors 17:115–124
Delmolino LM, Stearns NA, Castellot JJ Jr (2001) COP-1, a member of the CCN family, is a heparin-induced growth arrest specific gene in vascular smooth muscle cells. J Cell Physiol 188:45–55
Kleer CG, Zhang Y, Pan Q et al (2002) WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene 21:3172–3180
Gupta N, Wang H, McLeod TL et al (2001) Inhibition of glioma cell growth and tumorigenic potential by CCN3 (NOV). Mol Pathol 54:293–299
Kondo S, Kubota S, Mukudai Y et al (2006) Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-untranslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene 25:1099–1110
Kubota S, Kondo S, Eguchi T et al (2000) Identification of an RNA element that confers post-transcriptional repression of connective tissue growth factor/hypertrophic chondrocyte specific 24 (ctgf/hcs24) gene: similarities to retroviral RNA–protein interactions. Oncogene 19:4773–4786
Kubota S, Takigawa M (2002) Driving the driver: molecular regulation of CTGF/Hcs24/CCN2 that regulates chondrocyte growth and differentiation. In Recent Research Developments in Biophysics and Biochemistry. Research Signpost, Kerala, pp 995–1012
Hayashi N, Kakimuma T, Soma Y et al (2002) Connective tissue growth factor is directly related to liver fibrosis. Hepatogastroenterology 49:133–135
Ito Y, Aten J, Bende RJ et al (1998) Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 53:853–861
Sawai K, Mori K, Mukoyama M et al (2003) Angiogenic protein Cyr61 is expressed by podocytes in anti-Thy-1 glomerulonephritis. J Am Soc Nephrol 14:1154–1163
Igarashi A, Nashiro K, Kikuchi K et al (1996) Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol 106:729–733
Dean RG, Balding LC, Candido R et al (2005) Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem 53:1245–1256
Oemar BS, Werner A, Garnier JM et al (1997) Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation 95:831–839
Hilfiker A, Hilfiker-Kleiner D, Fuchs M et al (2002) Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation 106:254–260
Cicha I, Yilmaz A, Klein M et al (2005) Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol 25:1008–1013
Yoshisue H, Suzuki K, Kawabata A et al (2002) Large scale isolation of non-uniform shear stress-responsive genes from cultured human endothelial cells through the preparation of a subtracted cDNA library. Atherosclerosis 162:323–334
Sohn M, Tan Y, Wang B et al (2006) Mechanisms of low-density lipoprotein-induced expression of connective tissue growth factor in human aortic endothelial cells. Am J Physiol Heart Circ Physiol 290:H1624–1634
Igarashi A, Okochi H, Bradham DM, Grotendorst GR (1993) Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4:637–645
Nishida T, Kubota S, Kojima S et al (2004) Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor). J Bone Miner Res 19:1308–1319
Chen CC, Mo FE, Lau LF (2001) The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 276:47329–47337
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438:967–974
Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M (2006) Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther 5:1108–1116
Shimo T, Kubota S, Yoshioka N, Ibaragi S, Isowa S, Eguchi T, Sasaki A, Takigawa M (2006) Pathogenic role of connective tissue growth factor (CTGF/CCN2) in osteolytic metastasis of breast cancer. J Bone Miner Res 21:1045–1059
Kondo S, Tanaka N, Kubota S et al (2006) Novel angiogenic inhibitor DN-9693 that inhibits post-transcriptional induction of connective tissue growth factor (CTGF/CCN2) by vascular endothelial growth factor (VEGF) in human endothelial cells. Mol Cancer Therap 5:129–137
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (S)(M.T.) and (C)(S.K.), for Exploratory Research (M.T) from Ministry of Education, Culture, Sports, Science and Technology of Japan and Japan Society for the Promotion of Science; the Kurozumi Medical Foundation (S. K.); the Foundation for Growth Science in Japan (M. T.); the Sumitomo Foundation (M. T.); and the Foundation of Sanyo Broadcasting (S. K.). We also thank Ms. Yuki Nonami for her valuable secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kubota, S., Takigawa, M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis 10, 1–11 (2007). https://doi.org/10.1007/s10456-006-9058-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-006-9058-5