Abstract
The CCN family of proteins consists of 6 members (CCN1-CCN6) that share conserved functional domains. These matricellular proteins interact with growth factors, extracellular matrix (ECM) proteins, cell surface integrins and other receptors to promote ECM-intracellular signaling. This signaling leads to propagation of a variety of cellular actions, including adhesion, invasion, migration and proliferation within several cell types, including epithelial, endothelial and smooth muscle cells. Though CCNs share significant homology, the function of each is unique due to distinct and cell specific expression patterns. Thus, their correct spatial and temporal expressions are critical during embryonic development, wound healing, angiogenesis and fibrosis. Disruption of these patterns leads to severe development disorders and contributes to the pathological progression of cancers, vascular diseases and chronic inflammatory diseases such as colitis, rheumatoid arthritis and atherosclerosis. While the effects of CCNs are diverse, this review will focus on the role of CCNs within the vasculature during development and in vascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
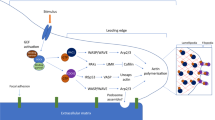
CCN proteins are complex components of the extracellular matrix (ECM) that affect many diverse biological processes. The acronym originates from the names of the first three proteins of the group that were first identified, (cysteine-rich 61 (CYR61) – CCN1 / connective tissue growth factor (CTGF) – CCN2 / nephroblastoma overexpressed (NOV) – CCN3)) and to date there are six proteins within this family. The other three, WISP1 (CCN4), WISP2 (CCN5) and WISP3 (CCN6), are Wnt-inducible secreted proteins that were later classified as CCNs based on homology to the seminal members. This group of proteins are characterized by a modular domain structure with strictly conserved cysteine residues which form disulfide bonds throughout each molecule (Holbourn et al. 2009). Each CCN protein contains an N-terminal secretion signal-peptide followed by domains with homology to insulin growth factor binding protein (IGFBP), von Willebrand factor type C (vWC), and thrombospondin type 1 repeat (TSP-1) (see Fig. 1). Additionally, all members except for CCN5 share a cysteine knot motif at their carboxyl end, most commonly found in proteins involved in dimerization and/or heparin binding (Krupska et al. 2015; Vilmos et al. 2001). This makes CCN5 an intriguing member of this group that can potentially act as a natural dominant negative to one or more of the other CCNs in vivo. While the literature citing the importance of CCN proteins in both development and in pathological conditions is extensive, this review will focus on the roles of CCNs within the vasculature.
General CCN domain structure with known binding partners. sp. – signal peptide, IGFBP – insulin-like growth factor binding domain, vWC – von Willebrand factor type C repeat, TSP-1 – thrombospondin type 1 repeat, CT – carboxy-terminal knot domain. Note: all members of the CCN family have identical structural properties except for CCN5 which lacks the CT domain. Inset box – known integrins and ECM proteins that bind CCNs and proteins not currently assigned to a binding domain. TrkA – Tropomyosin kinase receptor, M6P – mannose-6-phosphate receptor, RANK – receptor activator of NFκB
The major published function of CCN proteins deposited within the extracellular matrix is to promote ECM-cell signaling rather than to maintain the fidelity of the matrix, thus classifying them as matricellular proteins (Bornstein 2009; Bornstein and Sage 2002). Within the vessel, CCN-initiated signals contribute to the maintenance of vessel integrity during homeostasis which makes the tightly regulated of expression of CCNs important. Inciting of injury or disease disrupts this expression pattern and dysregulation occurs.
Role of CCNs in vascular development and homeostasis
CCN1 (Cyr61) is classified as a growth factor-inducible protein produced by an immediate-early gene that is widely expressed in the cardiovascular system during development. Both endothelial cells (EC) and vascular smooth muscle cells (VSMC) express CCN1 during development with diminished expression found within the patent vessel. Experiments performed using the CCN1 promoter show that cardiac expression of CCN1 occurs as early as E8.5 in a mouse embryo and persists until E11.5 (Kireeva et al. 1997; Mo and Lau 2006; Mo et al. 2002), with subsequent expression in all major arteries within the circulatory system. CCN1 null mice die by E14.5 due to improper chorioallantoic fusion or vascular structural defects (Mo et al. 2002), further emphasizing the importance of CCN1 in proper fetal vascular development.
Similar to CCN1, CCN2 (CTGF) is also a growth factor-inducible protein produced by an immediate-early gene, whose expression is rapidly upregulated in response to growth factors and other ECM stimuli (Hall-Glenn and Lyons 2011; Ponticos 2013). CCN1 and CCN2 also share similar spatial and temporal expression patterns, which suggests these two proteins have some amount of functional redundancy. CCN2 is expressed in EC, VSMC and pericytes within the blood vessels and heart during development and is an important regulator of EC-pericyte interactions (Chuva de Sousa Lopes et al. 2004; Hall-Glenn et al. 2012; Ivkovic et al. 2003). Not surprisingly, CCN2 null mice are lethal, dying shortly after birth, with severe pulmonary, skeletal and vascular defects observed. These mice show dysregulation of the microvascular with insufficient pericyte localization and vessel maturation along with disruption of basement membrane organization, which suggests CCN2 is important for both ECM organization and embryonic angiogenesis (Ivkovic et al. 2003).
While sharing many of the structural aspects of CCN1 and CCN2, within the vasculature, CCN3 (Nov) seems to be functionally divergent, acting as a counterbalance to protect from excess or aberrant vessel growth, especially in VSMC. CCN3 was first isolated from nephroblastoma tissue in newborn chicks infected with the MAV-1(N) avian retrovirus (Joliot et al. 1992). CCN3 is expressed in a wide variety of tissues including fibroblasts, EC and VSMC during development (Kocialkowski et al. 2001; Perbal 2001; Su et al. 2001) with continued expression in aortas of fully developed mice as well as in healthy human aortic tissue (Zhang et al. 2016). Recent studies have shown that CCN3 can affect vascular function by regulation of endothelial cell inflammation and neointimal hyperplasia (Lin et al. 2010; Shimoyama et al. 2010). CCN3 knockout mice are viable and develop to adulthood with a normal vasculature and ECM composition (Shimoyama et al. 2010).
CCN4 (WISP1) was initially identified as a gene in a mouse mammary epithelial cell line (Pennica et al. 1998) with later studies restricting CCN4 protein expression to osteoblast and osteoblastic progenitor cells during development and bone fracture repair (French et al. 2004). Thus, its main regulatory roles are in skeletal development and as a modulator of degenerative joint disease. More recent studies, however, have expanded its potential modulatory activities to include cardiac fibroblast proliferation and VSMC migration and proliferation (Liu et al. 2013).
CCN5 (WISP2) is a growth arrest-specific gene that was discovered in multiple laboratories (Pennica et al. 1998; Zhang et al. 1998) and first isolated using a subtractive hybridization approach in VSMC induced with heparin (Lake et al. 2003). It has been identified and studied in mice, rat and humans. It is expressed as early as E4.5 with continued expression observed throughout embryonic development (Myers et al. 2012). Significant levels of CCN5 persist in EC, VSMC, and heart myocardium which, similar to CCN3, suggests a regulatory role within the adult vasculature.
CCN6 (WISP3), to date, has not been shown to be involved with vascular development or any type of vascular disease. It was first identified in a search for homologous proteins (to CCN4 and CCN5) in an expressed sequence tag (EST) database. Its main function appears to be the maintenance of human arterial cartilage (Baker et al. 2012) but has also been postulated as a therapeutic target for certain types of breast and metastatic cancers. It is downregulated in aggressive breast cancers which allows activation of TAK1 and p38 kinases as well as AKT phosphorylation (Pal et al. 2012) and insulin-like growth factor 1 signaling (Kleer et al. 2004). The CCN6 knockout mouse has no observable gross phenotype (Kutz et al. 2005).
CCN proteins in cardiac and vascular disease
The modular domain structure of CCN proteins in conjunction with their ECM deposition allows them to bind a variety of molecules such as growth factors (GF), heparin sulfate proteoglycans (HSPG), and cell surface receptors (most notably integrins and low density lipoprotein receptors (LRPs)) within the heart and systemic vasculature. One of the important functions of CCNs appears to be as an adaptor molecule, binding growth factors, such as VEFG and TGFβ, and shuttling them near the cell surface via a second binding partner, namely integrins or HSPGs. CCNs binding to the cell surface itself also appears to have a direct role in the initiation of intracellular signaling. These ECM – cell surface interactions are critical for cellular growth and mobility during vascular development and aid in the propagation of vascular diseases such as atherosclerosis and restenosis.
The migratory and adhesive properties of CCN1 upon microvascular endothelial cells was originally reported by Babic in 1998 (Babic et al. 1998). Since then CCN1 has been shown to promote EC adhesion, migration and proliferation in various EC types and systems (Kireeva et al. 1998) as well as adhesion, proliferation and chemotaxis in VSMC (Grzeszkiewicz et al. 2002). While CCNs do not appear to be master regulators of vascular development, they seem to be important to sustain vessel integrity. The CCN1 knockout mouse embryo during development has large disorganized vessels, with EC and VSMC entering the media layer. The end result is cellular dysregulation, vessel dilation and hemorrhage (Chintala et al. 2015). In the mature vascular system CCN1 signaling is attenuated. However, during times of active vascular growth or repair, such as wound healing, or under pathological conditions, such as atherosclerosis or cancer, the expression of CCN1 increases at the sites of active angiogenesis. CCN1 has been shown to be upregulated in both a rat carotid artery balloon injury model as well as in mouse and human atherosclerotic plaques (Hilfiker et al. 2002; Mo et al. 2002; Sigala et al. 2006). Looking at CCN1 levels after injury in a rat carotid artery balloon injury model, Matsumae et al. found that CCN1 expression was upregulated after 14 and 28 days post-injury in VSMC within the media and neointima of the carotid artery. Knockdown of CCN1 using a CCN1 siRNA lentiviral-mediated vector led to significantly diminished intimal thickening when compared to control arteries and this reduction could be reversed with reintroduction of CCN1. These results suggest that CCN1 plays a critical role in neointimal hyperplasia after vascular injury and that inhibition of CCN1 may be a viable treatment to prevent restenosis after vascular interventions (Matsumae et al. 2008). CCN1 can also attenuate immune cell infiltration in a model of induced myocarditis (Rother et al. 2010) and may play an important role in the adaptation of the heart to cardiovascular stress (Hilfiker-Kleiner et al. 2004) which is consistent with the importance of CCN1 in vessel stabilization.
CCN2, similar to CCN1, is believed to function as a matricellular protein, converting growth factor and other forms of ECM signaling into active cellular responses (Abreu et al. 2002; Ponticos 2013) CCN2 has been shown to support cell adhesion, migration and proliferation during embryonic growth and at other times of active angiogenesis (Babic et al. 1999). Vascular defects in the CCN1 null mouse appear toward the later stages of embryonic development (Hall-Glenn et al. 2012) with VSMC dysregulation in size and placement as well as defects within the composition of the ECM. In adulthood, expression of CCN2 is low or absent in most tissues, yet can be rapidly upregulated after vascular injury or in sustained disease states, such as atherosclerosis. CCN2 can stimulate angiogenesis in times of disease or injury (Yan and Chaqour 2013), however this effect does not appear to be universal, for tissues used from CCN2 knockout mice show no impaired angiogenic response within the eye after ischemic or laser induced neovascularization (Kuiper et al. 2007). These disparate observations underscore the importance of localized CCN2 concentrations as well as pro-angiogenic growth factors in the surrounding milieu. CCN2 affects pro-angiogenic cell signaling through interactions with integrins and growth factors and downregulates Wnt signaling (which can affect CCN4, CCN5 and CCN6 transcript levels) through binding to LRP1 (Gao and Brigstock 2003; Segarini et al. 2001).
Within the scope of cardiac and vascular disease, increased CCN2 protein levels have been found in cardiac myocytes after acute myocardial infarction (MI) (Recchia et al. 2009), chronic heart failure (Ahmed et al. 2007; Ahmed et al. 2004) and in aortic aneurysms (Kanazawa et al. 2005). Similarly, CCN2 is expressed at very high levels within atherosclerotic plaques of mice (Geary et al. 2002) and atherosclerotic, but not normal human blood vessels (Oemar et al. 1997). While CCN2 has been shown to be important for VSMC growth and migration and neointimal thickening in a rat carotid artery angioplasty model (Rodriguez-Vita et al. 2005), the exact signaling pathways used by CCN2 by VSMC in active diseases, such as MI or atherosclerosis, have not been completely elucidated. From several elegant studies, however, mechanistic insight into the role of CCN2 in cardiac and vascular pathologies has been gleaned. CCN2 interacts with various integrins (αvβ3, α6β1, αMβ2) (Chen and Lau 2009; Schober et al. 2002), growth factors (VEGF (Inoki et al. 2002), TGF-β (Abreu et al. 2002)) and other ECM components (HSPG, fibronectin (Hoshijima et al. 2006)) to induce pro-angiogenic signaling. Interestingly, full length CCN2 can be cleaved into bioactive N- and C-terminal fragments (Hashimoto et al. 2002), with subsequent release of sequestered VEGF. Thus the angiogenic response regulated by CCN2 appears to be modulated by both full length CCN2 protein levels as well as the bioactivity of CCN2 fragments produced from the parent molecule by local metalloproteinases.
CCN3 is the first member of the CCN family that seems to have expression patterns and molecular functions that differ from the pro-angiogenic, pro-atherosclerotic natures of CCN1 and CCN2. Building on the original finding that CCN3 was anti-proliferative (Joliot et al. 1992), Ellis et al. demonstrated that CCN3 expression in VSMC in culture was downregulated upon exposure to growth factors (Ellis et al. 2000). Immunohistochemical staining of adult rat aorta showed that CCN3 was highly expressed within the VSMC where vascular homeostasis occurs. CCN3 protein levels substantially diminished, however, at day 7 after balloon injury to the carotid artery when vascular repair is prevalent, indicating CCN3, in part, inhibits VSMC-directed tissue and ECM remodeling. Interestingly, CCN3 protein levels increased at day 14 post injury where the vessels are again intact. These results, coupled with the finding that CCN3 affects VSMC adhesion, suggests that CCN3 may be necessary to release VSMC from the ECM to allow migration and proliferation at the site of injury (Ellis et al. 2000). Work from Shimoyama et al. built on these findings and showed that CCN3 was able to inhibit VSMC proliferation independent of growth factor TGF-β signaling. The anti-proliferative effects were caused, in part, by increased expression of cyclin-dependent kinase inhibitors p15 and p21 through a CCN3/Notch signaling pathway (Shimoyama et al. 2010). In vivo, CCN3 was found to be expressed and localized to the VSMC of mouse aortas and was significantly reduced under diabetic conditions. Knockout mice for CCN3 showed enhanced neointimal hyperplasia after injury when compared to wild-type mice, adding further evidence that CCN3 is an important regulator of VSMC function and its expression is critical to prevent unwanted neovascularization after tissue repair. Thus, CCN3 protein levels within the vasculature may be important in the regulation of atherosclerotic disease.
To examine this further, Liu et al. looked at the role of CCN3 in atherosclerosis using ApoE deficient mice. Mice deficient for Apolipoprotein E, when fed a high fat diet, develop severe atherosclerotic lesions. CCN3 protein levels in the carotid artery were reduced by over 86 % in ApoE−/− mice when compared to those of wild-type mice (Liu et al. 2014), which suggests that CCN3 expression is inhibited in the progression of atherosclerosis. Reintroduction of CCN3 through adenoviral mediated delivery attenuated atherosclerotic progression in part by suppressing gene expression of adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1). Increased CCN3 protein levels also caused the decreased expression of matrix metalloproteinases (MMP-2, MMP-9) and pro-angiogenic factors CCN1 and CCN2. Taken together, CCN3 is a potent inhibitor of inflammation and atherosclerosis in ApoE deficient mice (Liu et al. 2014).
Recent work from our lab has further expanded the protective role of CCN3 within the vasculature using models of induced abdominal aortic aneurysms (AAA) (Zhang et al. 2016). Aortic aneurysms are characterized by dysregulation of VSMC function, ECM composition and increased vascular inflammation, the end result being a weakening of the vessel wall with increased rupture potential (Boddy et al. 2008; El-Hamamsy and Yacoub 2009; Krishna et al. 2010; LeMaire and Russell 2011; Nordon et al. 2009). Little has been reported on the role of CCN3 within this specific vascular pathology. Compared to CCN protein levels within normal control tissues, CCN3 protein levels are significantly reduced in both induced AAA tissue in mice as well as human infrarenal aortic biopsies taken from AAA patients (Zhang et al. 2016), confirming previously published results (Lenk et al. 2007). These findings suggest a protective role for CCN3 against AAA formation. To confirm this, AAA were induced in both wild-type and CCN3-deficient mice. The absence of CCN3 caused increases in both the incidence and severity of AAA. The maximal abdominal aortic arch diameter was significantly larger in CCN3 null mice concomitant with enhanced reactive oxygen species (ROS) generation and VSMC apoptosis, leading to a diseased vascular state. This was augmented by severe ECM disruption marked by elastin degradation and increased matrix metalloproteinase activity. Loss of CCN3 also caused an increase in inflammatory infiltrates such as T-cells and macrophages as well as key inflammatory markers monocyte chemoattractant protein-1 (MCP-1) and VCAM-1. Interestingly, viral mediated overexpression of CCN3 attenuated much of the AAA pathology described above.
Since ROS have been shown to play a key role in VSMC apoptosis and the regulation of MMP activity (McCormick et al. 2007) we assessed the effects of oxidative stress upon AAA formation. CCN3-null mice showed reduced incidence and severity of AAA when treated with ROS inhibitors, with increased vessel wall integrity and diminished inflammatory cell numbers and MMP activity. Taken together, this suggests that CCN3, expressed within the VSMC of the media, protects against AAA formation, in part, by attenuating ROS formation, ECM degradation and inflammatory cell infiltration within the aorta.
Mechanistically, CCN3-deficient mice were shown to use different signaling pathways than the canonical ones normally associated with AAA formation (TGFβ, JNK and NFκB). Of note, pharmacological and genetic inactivation of ERK1/2 signaling dramatically reduced AAA incidence and severity in CCN3-deficient mice. These studies add important mechanistic insight into AAA progression within the context of CCN3 depletion and may provide a potential treatment strategy for patients with AAA.
While many studies have explored CCN4, CCN5 and CCN6 within tumors of various cancers, to date, little is known collectively about the roles of these proteins within the vasculature. CCN4 has been reported to promote VSMC migration and proliferation through interaction with α5β1 integrin (Liu et al. 2013) and it has been suggested that inhibition of CCN4 may provide a promising strategy for the prevention of restenosis after vascular interventions. CCN4 has also been shown to promote angiogenesis by inducing VEGF expression and endothelial progenitor cell recruitment in oral squamous cell carcinoma (Chuang et al. 2015).
While CCN5 has been reported to regulate VSMC proliferation and motility (Lake et al. 2003; Lake and Castellot 2003), it is also detected in the nucleus (Wiesman et al. 2010). Adding to previous reports suggesting a role for nuclear CCN3 and CCN2 proteins in the regulation of transcription (Perbal 1999; Wahab et al. 2001), CCN5 has been shown to be a transcriptional repressor of TGFβ in human breast tissue (Sabbah et al. 2011). As the only CCN family member without the C-terminal cysteine knot domain, it may have the potential to act as a negative regulator to other CCN proteins in vivo disrupting their functions (Perbal 2001; Perbal et al. 2016). More work is needed to explore this possibility as well as in vivo studies of CCN4 and CCN5 to further define their contributions to vascular development and disease. While CCN6 is involved in regulation of ECM degrading proteinases (Baker et al. 2012), no role of CCN6 within VSMC or the ECM surrounding the vasculature has been reported.
Conclusion
The family of CCN proteins have complicated functions within the vasculature. This is due to their modular domain structures which bind a variety of integrins, growth factors, cell surface receptors and many ECM-associated molecules. Little structural detail is known about these interactions. Do CCN proteins bind multiple partners simultaneously, forming tripartite or tetrapartite combinations as suggested by Perbal (Perbal 2001; Perbal et al. 2016)? If so, which combinations occur in the vascular system and what are their binding affinities? It appears that CCNs can have disparate roles based on their tissue-specific expression levels coupled with the local concentrations of their interacting partners. Within the vasculature, each microenvironment dictates CCN, growth factor, HSPG and integrin bioavailability and determines which ligands, or even other CCN family members, can interact with a specific CCN. Thus, the effect of a particular CCN may drastically differ from one vessel to the next, such as during development versus homeostasis. Much more work needs to be done at the functional level of these proteins to determine their therapeutic potential.
To date, there have only been a few genetic mutations and polymorphisms loosely correlated to human vascular disease (Bouchard et al. 2007; Cozzolino et al. 2010; Jun and Lau 2011; Yamada et al. 2009). Levels of CCN proteins in serum may serve as a prognostic tool for specific chronic diseases such as atherosclerosis and cancer, but there is little functional insight into how they may contribute to the onset and progression of disease. Since CCNs share some functional redundancy (especially CCN1 and CCN2), a combinatorial approach targeting multiple CCNs and/or other signaling pathways (TGFβ, TNFα, Wnt) is most likely needed to alleviate any CCN-specific disease pathology.
Future studies clarifying the role of CCNs within the vasculature and in other diseases where angiogenesis plays a central role (such as cancer and diabetic retinopathy) will be of great interest.
References
Abreu JG, Ketpura NI, Reversade B, De Robertis EM (2002) Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol 4:599–604
Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H (2004) Connective tissue growth factor–a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol 36:393–404
Ahmed MS, Oie E, Vinge LE, von Lueder TG, Attramadal T, Attramadal H (2007) Induction of pulmonary connective tissue growth factor in heart failure is associated with pulmonary parenchymal and vascular remodeling. Cardiovasc Res 74:323–333
Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998) CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A 95:6355–6360
Babic AM, Chen CC, Lau LF (1999) Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19:2958–2966
Baker N, Sharpe P, Culley K, Otero M, Bevan D, Newham P, Barker W, Clements KM, Langham CJ, Goldring MB, Gavrilovic J (2012) Dual regulation of metalloproteinase expression in chondrocytes by wnt-1-inducible signaling pathway protein 3/CCN6. Arthritis Rheum 64:2289–2299
Boddy AM, Lenk GM, Lillvis JH, Nischan J, Kyo Y, Kuivaniemi H (2008) Basic research studies to understand aneurysm disease. Drug News Perspect 21:142–148
Bornstein P (2009) Matricellular proteins: an overview. J Cell Commun Signal 3:163–165
Bornstein P, Sage EH (2002) Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14:608–616
Bouchard L, Tchernof A, Deshaies Y, Lebel S, Hould FS, Marceau P, Vohl MC (2007) CYR61 polymorphisms are associated with plasma HDL-cholesterol levels in obese individuals. Clin Genet 72:224–229
Chen CC, Lau LF (2009) Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol 41:771–783
Chintala H, Krupska I, Yan L, Lau L, Grant M, Chaqour B (2015) The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and notch signaling. Development 142:2364–2374
Chuang JY, Chen PC, Tsao CW, Chang AC, Lein MY, Lin CC, Wang SW, Lin CW, Tang CH (2015) WISP-1 a novel angiogenic regulator of the CCN family promotes oral squamous cell carcinoma angiogenesis through VEGF-A expression. Oncotarget 6:4239–4252
Chuva de Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL (2004) Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn 231:542–550
Cozzolino M, Biondi ML, Banfi E, Riser BL, Mehmeti F, Cusi D, Gallieni M (2010) CCN2 (CTGF) gene polymorphism is a novel prognostic risk factor for cardiovascular outcomes in hemodialysis patients. Blood Purif 30:272–276
El-Hamamsy I, Yacoub MH (2009) Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol 6:771–786
Ellis PD, Chen Q, Barker PJ, Metcalfe JC, Kemp PR (2000) Nov gene encodes adhesion factor for vascular smooth muscle cells and is dynamically regulated in response to vascular injury. Arterioscler Thromb Vasc Biol 20:1912–1919
French DM, Kaul RJ, D'Souza AL, Crowley CW, Bao M, Frantz GD, Filvaroff EH, Desnoyers L (2004) WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol 165:855–867
Gao R, Brigstock DR (2003) Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res 27:214–220
Geary RL, Wong JM, Rossini A, Schwartz SM, Adams LD (2002) Expression profiling identifies 147 genes contributing to a unique primate neointimal smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 22:2010–2016
Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF (2002) The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology 143:1441–1450
Hall-Glenn F, Lyons KM (2011) Roles for CCN2 in normal physiological processes. Cell Mol Life Sci 68:3209–3217
Hall-Glenn F, De Young RA, Huang BL, van Handel B, Hofmann JJ, Chen TT, Choi A, Ong JR, Benya PD, Mikkola H, Iruela-Arispe ML, Lyons KM (2012) CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS One 7:e30562
Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y (2002) Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 277:36288–36295
Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkotter HJ, Schieffer B, Drexler H (2002) Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation 106:254–260
Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, Drexler H (2004) Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation 109:2227–2233
Holbourn KP, Perbal B, Ravi Acharya K (2009) Proteins on the catwalk: modelling the structural domains of the CCN family of proteins. J Cell Commun Signal 3:25–41
Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M (2006) CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett 580:1376–1382
Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y (2002) Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J 16:219–221
Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791
Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B (1992) Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol 12:10–21
Jun JI, Lau LF (2011) Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10:945–963
Kanazawa S, Miyake T, Kakinuma T, Tanemoto K, Tsunoda T, Kikuchi K (2005) The expression of platelet-derived growth factor and connective tissue growth factor in different types of abdominal aortic aneurysms. J Cardiovasc Surg 46:271–278
Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF (1997) Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res 233:63–77
Kireeva ML, Lam SC, Lau LF (1998) Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem 273:3090–3096
Kleer CG, Zhang Y, Pan Q, Merajver SD (2004) WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia 6:179–185
Kocialkowski S, Yeger H, Kingdom J, Perbal B, Schofield PN (2001) Expression of the human NOV gene in first trimester fetal tissues. Anat Embryol (Berl) 203:417–427
Krishna SM, Dear AE, Norman PE, Golledge J (2010) Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis 212:16–29
Krupska I, Bruford EA, Chaqour B (2015) Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Hum Genet 9:24
Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM, Agostini HJ, Rakic JM, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO (2007) Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem 55:1139–1147
Kutz WE, Gong Y, Warman ML (2005) WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol 25:414–421
Lake AC, Castellot JJ Jr (2003) CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun Signal 1:5
Lake AC, Bialik A, Walsh K, Castellot JJ Jr (2003) CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol 162:219–231
LeMaire SA, Russell L (2011) Epidemiology of thoracic aortic dissection. Nat Rev Cardiol 8:103–113
Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H (2007) Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics 8:237
Lin Z, Natesan V, Shi H, Hamik A, Kawanami D, Hao C, Mahabaleshwar GH, Wang W, Jin ZG, Atkins GB, Firth SM, Rittie L, Perbal B, Jain MK (2010) A novel role of CCN3 in regulating endothelial inflammation. J Cell Commun Signal 4:141–153
Liu H, Dong W, Lin Z, Lu J, Wan H, Zhou Z, Liu Z (2013) CCN4 regulates vascular smooth muscle cell migration and proliferation. Mol Cell 36:112–118
Liu J, Ren Y, Kang L, Zhang L (2014) Overexpression of CCN3 inhibits inflammation and progression of atherosclerosis in apolipoprotein E-deficient mice. PLoS One 9:e94912
Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M, Kita T, Tanaka M (2008) CCN1 knockdown suppresses neointimal hyperplasia in a rat artery balloon injury model. Arterioscler Thromb Vasc Biol 28:1077–1083
McCormick ML, Gavrila D, Weintraub NL (2007) Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 27:461–469
Mo FE, Lau LF (2006) The matricellular protein CCN1 is essential for cardiac development. Circ Res 99:961–969
Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF (2002) CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 22:8709–8720
Myers RB, Rwayitare K, Richey L, Lem J, Castellot JJ Jr (2012) CCN5 expression in mammals. III Early embryonic mouse development. J Cell Commun Signal 6:217–223
Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM (2009) Review of current theories for abdominal aortic aneurysm pathogenesis. Vascular 17:253–263
Oemar BS, Werner A, Garnier JM, Do DD, Godoy N, Nauck M, Marz W, Rupp J, Pech M, Luscher TF (1997) Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation 95:831–839
Pal A, Huang W, Li X, Toy KA, Nikolovska-Coleska Z, Kleer CG (2012) CCN6 modulates BMP signaling via the Smad-independent TAK1/p38 pathway, acting to suppress metastasis of breast cancer. Cancer Res 72:4818–4828
Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ (1998) WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A 95:14717–14722
Perbal B (1999) Nuclear localisation of NOVH protein: a potential role for NOV in the regulation of gene expression. Mol Pathol 52:84–91
Perbal B (2001) NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol 54:57–79
Perbal B, Lau L, Lyons K, Kubota S, Yeger H, Fisher G (2016) Report on the 8th international workshop on the CCN family of genes - nice November 3-8, 2015. J Cell Commun Signal 10:77–86
Ponticos M (2013) Connective tissue growth factor (CCN2) in blood vessels. Vasc Pharmacol 58:189–193
Recchia AG, Filice E, Pellegrino D, Dobrina A, Cerra MC, Maggiolini M (2009) Endothelin-1 induces connective tissue growth factor expression in cardiomyocytes. J Mol Cell Cardiol 46:352–359
Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, Egido J (2005) Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res 97:125–134
Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, Westermann D, Savvatis K, Dannemann N, Skurk C, Hilfiker-Kleiner D, Cathomen T, Fechner H, Rauch U, Schultheiss HP, Heymans S, Eriksson U, Scheibenbogen C, Poller W (2010) Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation 122:2688–2698
Sabbah M, Prunier C, Ferrand N, Megalophonos V, Lambein K, De Wever O, Nazaret N, Lachuer J, Dumont S, Redeuilh G (2011) CCN5, a novel transcriptional repressor of the transforming growth factor beta signaling pathway. Mol Cell Biol 31:1459–1469
Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC (2002) Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood 99:4457–4465
Segarini PR, Nesbitt JE, Li D, Hays LG, Yates JR 3rd, Carmichael DF (2001) The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J Biol Chem 276:40659–40667
Shimoyama T, Hiraoka S, Takemoto M, Koshizaka M, Tokuyama H, Tokuyama T, Watanabe A, Fujimoto M, Kawamura H, Sato S, Tsurutani Y, Saito Y, Perbal B, Koseki H, Yokote K (2010) CCN3 inhibits neointimal hyperplasia through modulation of smooth muscle cell growth and migration. Arterioscler Thromb Vasc Biol 30:675–682
Sigala F, Georgopoulos S, Papalambros E, Chasiotis D, Vourliotakis G, Niforou A, Kotsinas A, Kavantzas N, Patsouris E, Gorgoulis VG, Bastounis E (2006) Heregulin, cysteine rich-61 and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with clinical data. Eur J Vasc Endovasc Surg 32:238–245
Su BY, Cai WQ, Zhang CG, Martinez V, Lombet A, Perbal B (2001) The expression of ccn3 (nov) RNA and protein in the rat central nervous system is developmentally regulated. Mol Pathol 54:184–191
Vilmos P, Gaudenz K, Hegedus Z, Marsh JL (2001) The twisted gastrulation family of proteins, together with the IGFBP and CCN families, comprise the TIC superfamily of cysteine rich secreted factors. Mol Pathol 54:317–323
Wahab NA, Brinkman H, Mason RM (2001) Uptake and intracellular transport of the connective tissue growth factor: a potential mode of action. Biochem J 359:89–97
Wiesman KC, Wei L, Baughman C, Russo J, Gray MR, Castellot JJ (2010) CCN5, a secreted protein, localizes to the nucleus. J Cell Commun Signal 4:91–98
Yamada Y, Ando F, Shimokata H (2009) Association of polymorphisms of SORBS1, GCK and WISP1 with hypertension in community-dwelling Japanese individuals. Hypertens Res 32:325–331
Yan L, Chaqour B (2013) Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of ocular neovascular and fibrovascular disease therapy. J Cell Commun Signal 7:253–263
Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P (1998) Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol 18:6131–6141
Zhang C, van der Voort D, Shi H, Zhang R, Qing Y, Hiraoka S, Takemoto M, Yokote K, Moxon JV, Norman P, Rittie L, Kuivaniemi H, Atkins GB, Gerson SL, Shi GP, Golledge J, Dong N, Perbal B, Prosdocimo DA, Lin Z (2016) Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J Clin Invest 126:1282–1299
Acknowledgments
This work was supported by NIH grants HL117759 and AA021390 (to Z.L.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klenotic, P.A., Zhang, C. & Lin, Z. Emerging roles of CCN proteins in vascular development and pathology. J. Cell Commun. Signal. 10, 251–257 (2016). https://doi.org/10.1007/s12079-016-0332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-016-0332-z