Abstract
Microbial organisms can cause huge crises in decorated caves, as seen in emblematic sites such as Lascaux cave. The preservation of such sites involves understanding the healthy microbial behavior of caves before the damage occurs. Indeed, knowledge of normal cave behavior is a prerequisite to identifying potential imbalance. This study seeks to determine whether models of aerobiological behavior could be identified in several caves of different sizes, ranging from rock shelters to large caves that are open or closed to the public. Aerial rates of fungi and bacteria were monitored over 3 years in nine sites in Dordogne (France). This study revealed that in a context of caves where public visits were carefully managed, fungal and bacterial rates were more affected by the size of the caves than by the opening of sites to the visitors. The study confirmed that large caves can generally be described as “self-purifying caves” as they were strongly affected by the exterior environment at their entry but much less so at locations further inside the cave, while small caves can be described as “non-self-purifying caves” since they were strongly affected by the exterior environment throughout their whole length. The results also highlighted the difficulty to determine a limit value of microbial rates valid for all caves because of the specificities of each one.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Dordogne region of France is well known for the number and the quality of human occupation sites dating from the Upper Paleolithic (mainly Magdalenian, ~ 17,000–12,000 years ago; Leroi-Gourhan 1984). In particular, the Vézère valley boasts 147 prehistoric sites and 25 ornamented caves, 15 of which have been UNESCO World Heritage Sites since 1979 (UNESCO 1979). The most famous of these is Lascaux cave. This cave is interesting for several reasons. The cave was discovered in 1940 and is today considered one of the finest examples of rock art paintings. This attracted many visitors as soon as the cave was opened to the public. The presence of these visitors led to microbial damage and notably the strong growth of green algae on the walls, and the cave was closed indefinitely in 1963 (Dupont et al. 2007). Despite this closure, the Lascaux cave suffered from other microbial outbreaks, the most recent being a fungal development in 2006 (Bastian et al. 2010). Lascaux is not an isolated case; other major rock art sites such as Altamira cave in Spain have been affected by microbial outbreaks (Saiz-Jimenez et al. 2011). This has made the preservation of such heritage a major concern.
Several studies have sought to characterize microbial life on the rock surface (Schabereiter-Gurtner et al. 2004; Angelova and Groudeva 2014), in cave soil (Adetutu et al. 2011; Out et al. 2016) and in cave atmosphere (Docampo et al. 2011; Martin-Sanchez et al. 2014). The studies in cave atmosphere are particularly interesting, with some authors recently trying to use the quantity of airborne fungi as an indicator of disturbance in caves (Porca et al. 2011; Pusz et al. 2017). This approach required the initial identification of the non-disturbed signature of caves; the term “ornamented cave” covers a wide range of sites that vary in terms of size, volume and accessibility to the public. Identifying the non-disturbed aerobiological behavior of a cave requires long-term monitoring to understand normal variations that happen over time. However, very few studies have monitored airborne microbial rates at several sampling points and several sampling dates in a given cave (Docampo et al. 2011; Taylor et al. 2013; Martin-Sanchez et al. 2014).
A previous study focusing on five caves in the Gard region (France) demonstrated that each cave has its own specific aerobiological behavior signature (Leplat et al. 2019). It also showed that caves could be divided into two groups according to that behavior, namely “self-purifying caves” and “non-self-purifying caves.” The present study seeks to explore these results in depth by widening the diversity of study cases at very low volumetric sites and by introducing the human factor as a variable that could impact cave health, as some of the studied sites are open to visitors, whereas the others are not.
2 Materials and methods
2.1 Samplings
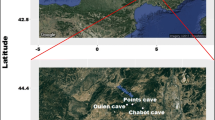
This study monitored airborne microbiological particles in nine ornamented sites of the Dordogne region (France), namely seven caves and two rock shelters (Fig. 1). Six of these nine sites are listed as World Heritage Sites by UNESCO. The two rock shelters can be considered as small caves as they are separated from the exterior by a wall. Some of the caves are open to visitors; the others are not (Table 1). In this study, a “closed cave” is defined as a cave that is inaccessible to the public, where the entrance is closed with walls and doors and equipped with openings for ventilation. An “open cave” is defined as a cave that is accessible to the public, but where the entrance is also closed with walls and doors and equipped with openings for ventilation. No major microbiological outbreak has occurred on the walls of these caves, which can therefore be considered representative of undisturbed caves. Five surveys were conducted over a period of 3 years to evaluate the rates of airborne microorganisms in the studied caves. Two surveys were carried out at the beginning of summer (June 2014 and June 2015), and three others were carried out during autumn (October 2013, October 2014 and October 2016). All the caves were tested on each of the five dates, with the exception of Reverdit shelter, which was not sampled in October 2016. At each sampling date, one to six points were sampled in each cave according to the size of the caves (Fig. 2a, b). An additional sampling point was added outside each cave in October 2016 to obtain an external air measurement.
Cave locations (graphics programs: R, package ggmap + Inkscape). The nine sites are located in the Dordogne area, France. Seven of the caves, i.e., Sorcier cave, La Mouthe cave, Font-de-Gaume caves, Combarelles I and II caves Cap Blanc shelter and Reverdit shelter, are located in the valley of the Vézère. The two others, i.e., Mammouth cave and Pigeonnier cave, are located in the Dordogne valley. The maps were generated from Google Earth [Google, Mountain View, USA; David and Hadley 2013) on the basis of geographical coordinates supplied by the French Ministry of Culture
a, b Sampling maps of the nine studied caves from Leroi-Gourhan (1984; graphics program: Inkscape). Each number represents one sampling point where fungal and bacterial rates were assessed. The sampling points were chosen in such a way as to cover the widest possible surface of the caves
A Duo SAS Super 360 air sampler (VWR-pbi, Milan, Italy) was used to detect cultivable microorganisms in cave air. Fifty liters of air was collected at each sampling point using a 219-hole impactor containing appropriate culture media in 55-mm Petri dishes. Bacteria were isolated on nutrient agar (Merck KGaA, Darmstadt, Germany), and fungi were isolated on malt extract agar (Merck KGaA, Darmstadt, Germany). The Petri dishes containing impacted media were then taken to the laboratory for analysis.
2.2 Counting of cultivable microorganisms
The plates were incubated in a BD 115 incubator (Binder GmbH, Tuttlingen, Germany) for 3 days at 30 °C for bacteria and for 7 days at 24 °C for fungi. These incubation temperatures are higher than those found in the caves and were chosen to allow rapid microbial growth. The number of microbial colonies grown in each Petri dish was counted after incubation. Each count was corrected using the table of the most probable count, as recommended in the manufacturer instructions. The results were expressed as colony-forming units per cubic meter (CFU m−3).
2.3 Identification of fungal strains
Bacterial strains were counted but not identified. Fungal strains were separated through subculturing on malt extract agar. The resulting isolated fungal strains were identified to the genus level by crosschecking their macroscopic and microscopic characteristics with those listed in reference books (Domsch et al. 1980; Seifert et al. 2011).
Genera that could not be distinguished by their morphology were identified through molecular identification. DNA was extracted as described by Edel et al. (2001). ITS1F/ITS4 primers were used to amplify the nuclear ribosomal internal transcribed spacer (ITS) region (Gardes and Bruns 1993; White et al. 1990). PCR was performed in 25 µl reactions, with 1 µl of template DNA, 1 U of Taq DNA polymerase (Invitrogen, Carlsbad, USA), 2.5 µl of 10X Taq DNA polymerase buffer, 1 µl of 2 mmol l−1 dNTPs (Thermo Fisher Scientific, Waltham, USA) and 1.5 µl of each 10 µmol l−1 primer (Eurogentec, Seraing, Belgium). Amplifications were performed on a PrimeG thermocycler (Bibby Scientific, Stone, UK) using the following parameters: a 4-min step at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 55 °C, and 40 s at 72 °C and a final 10-minute extension at 72 °C. PCR products were sequenced by Genoscreen (Lille, France) using the same primer set. Isolates were identified by BLAST in comparison with the NCBI GenBank database (Altschul et al. 1990).
2.4 Statistical analyses
2.4.1 Analyses of airborne microbiological particle counts
Statistical analyses were carried out using R 3.4.2 with α = 5% (R Core Team 2014). The results of microbiological counts were analyzed with a generalized linear mixed model (GLMM). GLMMs are now commonly used to analyze data from ecological surveys (Bolker et al. 2009). They are suitable for the analysis of non-normal data collected from complicated designs that involve random and fixed effects.
Five qualitative variables were used to design two different GLMMs: Sampled cave (nine modalities corresponding to the nine studied caves); Sampling season (two modalities: Early summer for June 2014 and June 2015, and Autumn for October 2013, October 2014 and October 2016); Sampling depth (three modalities: Entry for the first sampled point in each cave, Deep cave for all other points in each cave and Outside cave for the external air measurement); Cave size (two modalities, Small cave for caves that are around ten meters deep and Large cave for caves that are around one hundred meters deep or more; and public access (two modalities; Open cave or Closed cave). Two GLMMs were necessary because the cave size and public access variables are redundant when combined with the sampled cave variables, and cannot therefore be studied in the same model.
The first GLMM for this study was:
where θ is the general effect, αi is the sampled cave effect, βj is the sampling season effect, γk is the sampling depth effect, μik is the experimental error associated with each sampled point and εijkl is the residual error. α, β and γ were considered as fixed effects when μ was considered a random effect. The term ηijkl is the linear predictor. This term is obtained by submitting the variable pijkl to the link function of the model. The choice of the link function depends on the pijkl distribution law. The shapes of the distributions for bacterial and fungal counts were checked before applying the GLMM. The negative binomial link was then chosen to study these counts. This distribution is well adapted to counts with over-dispersed data (Booth et al. 2003). The parameters of GLMM were estimated through the penalized quasi-likelihood technique (package MASS; function glmmPQL; Wolfinger and O’connell 1993). A Wald Chi-square test was performed to determine the significance degree of each explanatory variable in the GLMM (package aod; function wald.test; Fox 2015). GLMM was completed with Tukey’s post hoc test in order to check the significance of differences between means (package lsmeans; function lsmeans; Bretz et al. 2016).
The second GLMM for this study was:
where θ is the general effect, βi is the sampling season effect, γj is the sampling depth effect, πk is the cave size effect, σl is the public access effect, μjkl is the experimental error associated with each sampled point and εijklm is the residual error. β, γ, π and σ were considered as fixed effects when μ was considered as a random effect.
2.4.2 Spatial distribution of airborne microbiological particles in the caves: distance index
A distance index was calculated to check whether the distance between the sampling spot and the cave entrance, as well as the distance between this spot and the end of the caves, had an effect on the counts of airborne microbiological particles. The end of the caves was defined as the point from which no further progress could be made by humans. It is therefore possible that although this point was not the real end of the cave, it was nevertheless a major physical obstacle to going any further. The index was normalized in order to enable comparison between caves of different sizes:
Id thus ranged from − 1 to 1, where − 1 is the value obtained at the cave entrance and 1 is the value obtained at the end of the caves.
The strength of the relationship between the microorganism counts and the Id value was assessed through linear and quadratic regression (package stats; function lm; Chambers and Hastie 1992). Pearson’s correlation coefficient and root mean squared error (RMSE, package nsRFA; function RMSE; Armstrong and Collopy 1992) were chosen to illustrate the strength of the regressions. RMSE shows the average distance between the regression curve and the observations. The significance of the difference between the linear and the quadratic regressions was assessed through the comparison of the analysis of variance tables associated with each regression model (package stats; function anova; Chambers and Hastie 1992). The significance of the difference between obtained RMSE values was estimated by bootstrapping the RMSE from original data (1000 replicates; package boot; function boot; Davison and Hinkley 1997). The intervals containing 95% of the obtained values were then compared and were considered to be significantly different when they did not overlap.
2.4.3 Structure of fungal communities
The structure of fungal communities was analyzed by principal component analysis (PCA; package FactoMinR; function PCA; Husson et al. 2017). The isolated fungal genera were used as quantitative dependent variables. Genera that represented less than 0.3% of the total fungal population were grouped together under the name “other genera” to facilitate the interpretation of the model. Sampled cave, sampling season, sampling depth, cave size and public access were used as supplementary qualitative variables.
3 Results
3.1 Airborne microbiological particle counts
3.1.1 Fungal particles
The rates of airborne fungal particles ranged from 0 to 8150 CFU m−3 over the five sampling campaigns (Fig. 3). La Mouthe, Combarelles I and Font-de-Gaume caves showed a fixed pattern of airborne fungal particles despite a small number of differences. The fungal rates were clearly high at the entrances of the La Mouthe and Combarelles I caves, before decreasing and possibly increasing again at the end of the caves. A similar pattern was observed in Font-de-Gaume cave, but the fungal rates were the highest at the second sampling point before decreasing and possibly increasing again at the end of the cave.
A second group of caves, consisting of Mammouth cave and possibly Combarelles II cave and Reverdit shelter, showed two different patterns according to the sampling season. The pattern in Mammouth cave was characterized by a decrease in fungal rates from the entrance to the back of the cave in October, while in June the rates assessed at the second sampling point were at the same level or higher than the rates assessed at the first sampling point and then decreased at the third sampling point. In case of Combarelles II cave and Reverdit shelter, the fungal rates assessed in June were systematically higher than the rates assessed in October.
Finally, a third group of caves, consisting of Pigeonnier cave, Sorcier cave and Cap Blanc shelter, showed no clearly fixed pattern of fungal particles.
The fungal rates assessed outside the caves in October 2016 were systematically higher than the rates assessed inside the corresponding caves.
The generalized linear mixed models showed that the sampled cave, sampling depth, cave size and public access variables all had an effect on fungal particles rates (Table 2). The fungal rates assessed in the Sorcier and Mammouth caves were significantly higher than the rates assessed in the Combarelles I, La Mouthe and Font-de-Gaume caves (Table 3). The rates assessed in the other caves had intermediate values. The fungal rates were significantly higher outside the caves than in the caves and were significantly higher at the entrance of the caves than at depth. The fungal rates were significantly higher in small caves than in large caves. Finally, the fungal rates were significantly higher in closed caves than in caves that were open to the public.
3.1.2 Bacterial particles
The rates of airborne bacterial particles ranged from 20 to 9780 CFU m−3 over the five sampling campaigns. As for the fungal particles, three groups of caves can be distinguished regarding the bacterial rates. The Combarelles I and La Mouthe caves showed a clearly fixed pattern despite a small number of differences (Fig. 4). These patterns were cave dependent, while the same general pattern characterized by high rates at the entrance and a decrease with depth was observed in the case of fungal particles. For example, the second and the fifth sampling points definitely provided high bacterial rates in Combarelles I cave, despite the abnormally low values recorded in October 2014 (second sampling point) and June 2015 (fifth sampling point).
A second group of caves consisting of Font-de-Gaume cave, Cap Blanc shelter and possibly Combarelles II cave showed two different patterns according to the sampling season despite some abnormal points.
Finally, a third group of caves, consisting of Mammouth, cave, Sorcier cave, Pigeonnier cave and Reverdit shelter, showed no clearly fixed pattern of bacterial particles.
Unlike the fungal rates, the bacterial rates assessed outside the caves in October 2016 were not systematically higher than the rates assessed inside the corresponding caves.
The GLMMs showed that the sampling depth and the cave size had an effect on bacterial counts (Table 2). The rates counted outside the caves were significantly higher than the rates counted at depth inside the caves (Table 3). Intermediate rates were recorded at the entrance of the caves. In addition, the rates in small caves were significantly higher than the rates in large caves.
3.2 Spatial distribution of the airborne microbiological particles in the caves: distance index
3.2.1 Fungal particles
The linear correlation coefficient between the distance index and the fungal rates was significant but low, showing a very poor linear relationship (Fig. 5a, Table 4). The quadratic correlation coefficient was significantly better than the linear coefficient, but also remained low. The RMSE associated with quadratic regression was also better than that associated with linear regression, but the difference was not significant.
Study of the relationship between airborne microbiological particles and the distance index: fungi (a), bacteria (b) (graphics program: R). The study first considered all data and then divided the data according to cave size. In each case, linear and quadratic regressions were applied to the data collected
The breakdown of results according to the size of the caves showed that the trend observed on all data was supported by the results of large caves, while no linear or quadratic relationship can be established between distance index and fungal rates in small caves. The correlations coefficient of the linear and quadratic regressions in large caves is better than the coefficients obtained from the entire data set. The quadratic model was significantly better adapted to the results than the linear model, even if the correlation remained weak. The RMSE associated with the quadratic regression was lower than the RMSE associated with the linear regression, but the difference was not significant.
3.2.2 Bacterial particles
No linear or quadratic relationship was established between the distance index and the bacterial rates for small or large caves (Fig. 5b, Table 4).
3.3 Structure of fungal communities
Over 40 fungal genera were isolated during the five sampling campaigns, of which Cladosporium (44.81% of the isolated strains) and Penicillium (38.43%) were the most abundant (Table 5).
The first dimension of the principal component analysis performed on all data explained 18.3% of the observation variance, while the second dimension explained 9.5%. The fungal genera Botrytis, Cladosporium, Epicoccum, Penicillium and Aspergillus provided the highest positive contribution to the construction of the first dimension (Table 6); the fungal genera Paecilomyces, Verticillium, the sterile strains and the strains belonging to the Basidiomycetes division provided the highest positive contribution to the construction of the second dimension, whereas “other genera” provided the highest contribution to the construction of the negative part of the second dimension. No difference was observed in the fungal communities according to the sampling season or to public access (Fig. 6). On the other hand, the fungal communities of the large caves were very different from the communities of small caves. The small caves shifted to positive values for both dimensions of the PCA. We can therefore consider new PCA that was produced by separating small caves and large caves.
Representation of the fungal communities according to selected supplementary qualitative variables of the PCA performed on all data (graphics program: R). All data are represented according to three supplementary qualitative variables used in PCA: sampling season (a), cave size (b) and public access (c). The colored circles represent the probable position of the observation barycenter associated with each modality
The first dimension of the PCA explained 23.5% of the observation variance and the second dimension explained 11.1% in large caves, while the first dimension explained 18.1% of the variance and the second dimension explained 12.4% in small caves. The fungal communities of all large caves were clearly different from the fungal communities identified outside the caves, while the Cap Blanc shelter was the only small cave for which fungal communities were different from outside caves communities (Fig. 7). The fungal communities of large caves were not different from each other, but the communities of Combarelles II and Mammouth caves strongly shifted to positive values on the PCA first dimension. The fungal communities at the entrance of and at depth in the large caves were different from the outside communities and differed among themselves. Conversely, the fungal communities at the entrance of the small caves were not different from outside communities. Moreover, the communities at depth of small caves were different from outside communities but could not have been distinguished from the communities at the entrance.
Representation of the fungal communities according to selected supplementary qualitative variables of the PCA performed according to cave size (graphics program: R). The data are shown for large caves (a) and small caves (b) according to two supplementary qualitative variables used in PCA: sampled cave and sampling depth. The colored circles represent the probable position of the observation barycenter associated with each modality
4 Discussion
4.1 Size of the caves as the main factor affecting their aerobiological behavior
The most valuable and important result in a framework of ornamented cave preservation was that the aerobiological behavior of caves was mainly driven by cave size and environmental factors rather than by public access. The effects of cave visitation on airborne biological particles are already known. The presence of visitors generally causes an increase in airborne fungal and bacterial particles (Wang et al. 2010a, b; Taylor et al. 2013; Fernandez-Cortes et al. 2011; Bercea et al. 2018; Mulec et al. 2017). The composition and the richness of microbial communities are also affected, notably through the introduction of non-indigenous species (Pusz et al. 2018; Griffin et al. 2014). In our study, opening caves to visitors had no effect on bacterial counts and the fungal counts in caves open to the public were significantly lower than those in closed caves; indeed, the size of caves had an effect on both bacterial and fungal counts, which were higher in small caves than in large caves. Moreover, the study of the composition of fungal populations through PCA showed that the populations of closed caves and caves open to the public were not significantly different.
4.2 Aerobiological behavior in large caves
4.2.1 General behavior: La Mouthe, Combarelles I and Font-de-Gaume caves
La Mouthe cave can be compared with Combarelles I and Font-de-Gaume caves. These three caves are of comparable sizes. Combarelles I and Font-de-Gaume are open to the public while La Mouthe is not, yet no significant differences were found in fungal and bacterial counts. Pusz et al. (2015) observed the same result when comparing data for the recently discovered cave of Jarkovicka, which was not open to visitors, to data published for other caves in the region. They also conclude that tourist traffic was perhaps not the main driver of particle increase in cave air. In our study, sampling was carried out outside visiting hours, either in the morning before the cave opened to visitors or after the last visit of the day. It is probable that microbial counts increased during visits, but the recovery capacity of the Combarelles I and Font-de-Gaume caves was enough to mask any difference with non-visited caves, namely due to the way these caves are managed. The management of these caves is meticulous, with a limited number of visitors, controlled group size and with the lights turned on only when necessary to decrease the risks of microbial outbreaks that have occurred in other caves in the past (Dupont et al. 2007).
These three caves can therefore be classified as “self-purifying caves” (Leplat et al. 2019). Their fungal aerobiological behavior showed the pattern defined for that type of cave: The highest rates were counted at the cave entrance, but were lower than rates counted outdoors. The rates then decreased with depth, before a final possible increase at the far end of the cave. This pattern has already been observed in many caves (Garcia-Anton et al. 2013; Docampo et al. 2011; Fernandez-Cortes et al. 2011; Griffin et al. 2014). All these studies underlined the importance of external pressure on the dispersal of fungal spores inside caves, which can consequently follow the air flow in the cave and be gradually deposited, mainly through gravitation (Kuzmina et al. 2012; Taylor et al. 2014).
The drivers of bacterial behavior in the air of the cave were different, despite the clearly fixed pattern of bacterial distribution for each cave. No link can be established between outdoor rates of bacteria and the rates assessed inside the cave. The rates counted inside the caves were in turn higher or lower than the outdoor rates, and no link could be made between bacterial rates and the distance from the cave entrances. This result has already been observed in several studies (Fernandez-Cortes et al. 2011; Bercea et al. 2018). Dredge et al. (2013) explained this difference by the size of the particles and therefore by the difference in their aerial transport, the transport of bacterial particles being consistent with that observed for aerosol between 0.1 and 10 µm. The pattern of bacterial distribution in each cave could also be the reflection of environmental variations of temperature and humidity inside the cave as well as local variations in available input matter or in aerosolization phenomenon (Wang et al. 2010b; Mulec et al. 2017).
The analysis of the fungal community compositions confirmed the results obtained through fungal counts. Cladosporium and Penicillium were the two most abundant genera identified inside and outside the caves, which was consistent with previous results (Ogórek 2018; Porca et al. 2011; Man et al. 2018; Pusz et al. 2018). Along with Epicoccum, Botrytis and Aspergillus, these two genera were strongly and positively involved in the building of the first dimension of the PCA associated with large caves. These five genera are all known to be markers of outside air (Ogórek et al. 2014). Therefore, the representation of outdoor fungal population strongly shifted to positive values on the first dimension of PCA representation. The representation of the fungal population at the cave entrance was significantly different from the outdoor population, despite being strongly affected by the external environment. Finally, the representation of the population at depth shifted to negative values on the PCA first dimension, as these populations were less affected by external environment and were certainly more representative of the fungal endemic population of caves (Leplat et al. 2019).
4.2.2 Unusual behaviors: Combarelles II and Mammouth caves
Combarelles II and Mammouth can be considered large caves in view of the definition given at the outset of this study, but they seemed to have a different behavior to those observed in La Mouthe, Combarelles I and Font-de-Gaume caves. The fungal counts were moderately higher in Combarelles II cave and significantly higher in Mammouth cave than in the three other large caves. Moreover, the representation of the fungal populations of these two caves on the PCA showed that they were more affected by the first dimension of the PCA than other large caves were.
In case of Combarelles II cave, this observation was the result of an experimental artifact. The only point sampled in Combarelles II was located at the entrance of the cave, which was not tested at depth. The results about this cave were therefore only comparable with the results obtained at the entrance of other large caves. We can reasonably assume that the results for this cave would have been closer to the results obtained for other large caves if the sampling had been more complete.
The results are much more interesting in case of Mammouth cave, which was sampled along its full length. Several factors could explain the high rates of fungal particles counted in this cave, which cannot be affected by visitors since the cave is closed to the public. First, the cave is divided into two parts separated by a wall. The first part of the cave could be therefore considered as a small cave. Second, the ceiling of the second part of the cave is covered by the roots of the vegetation growing on ground level above the cave. This factor has already been associated with high fungal rates (Leplat et al. 2019), probably by providing a favorable oligotrophic habitat and a stock of available organic matter (Saiz-Jimenez 2012; Jurado et al. 2009; Vanderwolf et al. 2013). Along with cave size, this result underlined the importance of environmental factors in the aerobiological behavior of caves and the difficulty to establish a general pattern for this behavior even for caves with comparable sizes.
4.3 Aerobiological behavior in small caves
While all the large caves except the Mammouth cave could be classified as “self-purifying caves,” the small caves can be classified as “non-self-purifying caves.” In other words, they were directly affected by outside air along their full length. These caves exhibited no clear distribution of fungal particles: For caves where several points were sampled, the highest fungal rates were counted either at the entrance or the far end of the cave, so no link could be established between the fungal rates and the distance from the entrance. The small size of these caves certainly did not allow the gradual discharge of fungal propagules that occurred in large caves where the fungal rates are high at the entry before collapsing (Griffin et al. 2014; Garcia-Anton et al. 2013). Therefore, the fungal rates in small caves were mostly representative of the variation of propagules amount in outside environment (Docampo et al. 2011), as well as of the air entrance when opening the door (Fernandez-Cortes et al. 2011), than the reflection of a behavior specific to the caves.
The study of the fungal population composition in small caves confirmed the strong influence of outside air along their full length: There was no significant difference between the population at the cave entrances and the population at depth. Nor was any significant difference observed between the populations inside the small caves and the population outside the caves, with the exception of the population of Cap Blanc shelter, which strongly shifted to positive values on the first dimension of the PCA unlike other populations.
Aspergillus, Verticillium, Paecilomyces and Engyodontium were the fungal genera involved the most in the building of this first PCA dimension for small caves and were isolated in the Cap Blanc Shelter. The genera Verticillium, Paecilomyces and Engyodontium could effectively be representative of the cave environment, since several species in these genera are known to be entomogenous (Shah and Pell 2003; Jurado et al. 2008). This kind of fungi has already been found to play a major role in cave environment (Pusz et al. 2018; Bastian et al. 2009, 2010). The rate of fungi counted in Cap Blanc shelter was relatively low in comparison with the rates observed in other small caves, even if the difference was not significant. Cap Blanc shelter therefore appeared to be less affected by the outside environment than other small caves. This is certainly due to the very particular layout of the shelter, which is open to visitors. A building has been built directly around the shelter. Visitors first enter the building and then pass a second door to enter the shelter. In this way, the shelter is never in direct contact with the outside environment, as the building plays the role of a large airlock. Airlocks have already been proved to be a suitable barrier to limit the impact of external environment in caves, in terms of both climatological stability and inputs of fungal and bacterial particles (Cigna 1993; Houillon et al. 2017; Porca et al. 2011).
4.4 Sorting of the caves according to risk index
Porca et al. (2011) proposed an index based on the concentration of fungal spores in cave air, sorting them into five risk categories ranging from caves with no fungal problem to irreversibly disturbed caves. Based on averages values, four out of the nine caves in our study would be classified in category 3, two in category 4 and three in category 5. Porca et al. (2011) explained that categories 4 and 5 seem to occur when the cave is suffering a fungal outbreak or massive visits. In our study, three of the five caves sorted in categories 4 and 5 are not open to the public, while three out of the four caves sorted in category 3 are open to the public. Indeed, the caves were almost sorted according to their total volume with the exceptions noted before: Counts were unusually high in Mammouth cave, which is a large cave, and counts were unusually low in Cap Blanc shelter, which is a small cave. In general, it may even be difficult to attain the safest category of the index, defined as fungal rates under 50 CFU m−3, in caves that are closed to visitors (Pusz et al. 2015; Leplat et al. 2019). As interesting as this index is, it probably does not sufficiently consider the total volumes of the caves and the high specificity that can occur in each cave, as already noted in the literature (Pusz et al. 2018). It nevertheless remains crucial to carry out meticulous monitoring, particularly in small caves that are open to the public.
5 Conclusions
This study shows that in a context of decorated caves that are carefully managed for public access, the size of the cave has more effect on the fungal and bacterial rates in the air of the caves than opening the sites to visitors. The results confirm that aerobiological behavior in large caves follows a cave-dependent fixed pattern. The large caves can generally be classified as “self-purifying caves” since they are strongly affected by the exterior environment at their entrance but much less so at depth. On the contrary, it was practically impossible to determine a fixed pattern of aerobiological behavior in small caves that are strongly affected by the outdoor environment. The small caves can therefore be classified as “non-self-purifying caves.” The presence of an airlock could buffer the influence of outdoor environment, as observed in Cap Blanc shelter. Finally, this study also confirms that the fungal airborne rates proposed in past studies for healthy caves should be reviewed in greater detail to take the individual characteristics of caves into account.
References
Adetutu, E. M., Thorpe, K., Bourne, S., Cao, X., Shahsavari, E., Kirby, G., et al. (2011). Phylogenetic diversity of fungal communities in areas accessible and not accessible to tourists in Naracoorte Caves. Mycologia, 103(5), 959–968. https://doi.org/10.3852/10-256.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2.
Angelova, R. G., & Groudeva, V. I. (2014). Microbial diversity on the rock paintings in Magoura Cave, Bulgaria. Journal of BioScience and Biotechnology (Special Edition) 51–54.
Armstrong, J. S., & Collopy, F. (1992). Error measures for generalizing about forecasting methods: Empirical comparisons. International Journal of Forecasting, 8(1), 69–80. https://doi.org/10.1016/0169-2070(92)90008-W.
Bastian, F., Alabouvette, C., & Saiz-Jimenez, C. (2009). The impact of arthropods on fungal community structure in Lascaux Cave. Journal of Applied Microbiology, 106(5), 1456–1462. https://doi.org/10.1111/j.1365-2672.2008.04121.x.
Bastian, F., Jurado, V., Nováková, A., Alabouvette, C., & Saiz-Jimenez, C. (2010). The microbiology of Lascaux cave. Microbiology, 156(3), 644–652. https://doi.org/10.1099/mic.0.036160-0.
Bercea, S., Năstase-Bucur, R., Mirea, I. C., Măntoiu, D. Ş., Kenesz, M., Petculescu, A., et al. (2018). Novel approach to microbiological air monitoring in show caves. Aerobiologia, 34(4), 445–468. https://doi.org/10.1007/s10453-018-9523-9.
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution, 24(3), 127–135. https://doi.org/10.1016/j.tree.2008.10.008.
Booth, J. G., Casella, G., Friedl, H., & Hobert, J. P. (2003). Negative binomial loglinear mixed models. Statistical Modelling, 3(3), 179–191. https://doi.org/10.1191/1471082X03st058oa.
Bretz, F., Hothorn, T., & Westfall, P. (2016). Multiple comparisons using R. Boca Raton: CRC Press.
Chambers, J. M., & Hastie, T. J. (1992). Linear models. In J. M. Chambers & T. J. Hastie (Eds.), Statistical models in S. Wadsworth & Brooks/Cole: Pacific Grove.
Cigna, A. A. (1993). Environmental management of tourist caves. Environmental Geology, 21(3), 173–180. https://doi.org/10.1007/BF00775302.
David, K., & Hadley, W. (2013). ggmap: Spatial visualization with ggplot2. The R Journal, 5(1), 144–161. https://doi.org/10.32614/RJ-2013-014.
Davison, A. C., & Hinkley, D. V. (1997). Bootstrap methods and their application. Cambridge: Cambridge University Press.
Docampo, S., Trigo, M., Recio, M., Melgar, M., García-Sánchez, J., & Cabezudo, B. (2011). Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): Diversity and origin. Science of the Total Environment, 409(4), 835–843. https://doi.org/10.1016/j.scitotenv.2010.10.048.
Domsch, K. H., Gams, W., & Anderson, T.-H. (1980). Compendium of soil fungi (Vol. 2). London: Academic Press Ltd.
Dredge, J., Fairchild, I. J., Harrison, R. M., Fernandez-Cortes, A., Sanchez-Moral, S., Jurado, V., et al. (2013). Cave aerosols: Distribution and contribution to speleothem geochemistry. Quaternary Science Reviews, 63, 23–41. https://doi.org/10.1016/j.quascirev.2012.11.016.
Dupont, J., Jacquet, C., Dennetière, B., Lacoste, S., Bousta, F., Orial, G., et al. (2007). Invasion of the French Paleolithic painted cave of Lascaux by members of the Fusarium solani species complex. Mycologia, 99(4), 526–533. https://doi.org/10.3852/mycologia.99.4.526.
Edel, V., Steinberg, C., Gautheron, N., Recorbet, G., & Alabouvette, C. (2001). Genetic diversity of Fusarium oxysporum populations isolated from different soils in France. FEMS Microbiology Ecology, 36, 61–71. https://doi.org/10.1016/S0168-6496(01)00119-2.
Fernandez-Cortes, A., Cuezva, S., Sanchez-Moral, S., Cañaveras, J. C., Porca, E., Jurado, V., et al. (2011). Detection of human-induced environmental disturbances in a show cave. Environmental Science and Pollution Research, 18(6), 1037–1045. https://doi.org/10.1007/s11356-011-0513-5.
Fox, J. (2015). Applied regression analysis and generalized linear models. Thousand Oaks: Sage Publications.
Garcia-Anton, E., Cuezva, S., Jurado, V., Porca, E., Miller, A. Z., Fernandez-Cortes, A., et al. (2013). Combining stable isotope (δ13C) of trace gases and aerobiological data to monitor the entry and dispersion of microorganisms in caves. Environmental Science and Pollution Research International, 21(1), 473–484. https://doi.org/10.1007/s11356-013-1915-3.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Molecular Ecology, 2(2), 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x.
Griffin, D. W., Gray, M. A., Lyles, M. B., & Northup, D. E. (2014). The transport of nonindigenous microorganisms into caves by human visitation: A case study at Carlsbad Caverns National Park. Geomicrobiology Journal, 31(3), 175–185. https://doi.org/10.1080/01490451.2013.815294.
Houillon, N., Lastennet, R., Denis, A., Malaurent, P., Minvielle, S., & Peyraube, N. (2017). Assessing cave internal aerology in understanding carbon dioxide (CO2) dynamics: implications on calcite mass variation on the wall of Lascaux Cave (France). Environmental Earth Sciences, 76(4), 170. https://doi.org/10.1007/s12665-017-6498-8.
Husson, F., Lê, S., & Pagès, J. (2017). Exploratory multivariate analysis by example using R. Boca Raton: CRC Press.
Jurado, V., Fernandez-Cortes, A., Cuezva, S., Laiz, L., Cañaveras, J. C., Sanchez-Moral, S., et al. (2009). The fungal colonisation of rock-art caves: Experimental evidence. Naturwissenschaften, 96(9), 1027–1034. https://doi.org/10.1007/s00114-009-0561-6.
Jurado, V., Sanchez-Moral, S., & Saiz-Jimenez, C. (2008). Entomogenous fungi and the conservation of the cultural heritage: A review. International Biodeterioration and Biodegradation, 62(4), 325–330. https://doi.org/10.1016/j.ibiod.2008.05.002.
Kuzmina, L., Galimzianova, N., Abdullin, S., & Ryabova, A. (2012). Microbiota of the Kinderlinskaya cave (South Urals, Russia). Microbiology, 81(2), 251–258. https://doi.org/10.1134/S0026261712010109.
Leplat, J., François, A., Touron, S., Galant, P., & Bousta, F. (2019). Aerobiological behavior of Paleolithic decorated caves: A comparative study of five caves in the Gard Department (France). Aerobiologia, 35, 105–124. https://doi.org/10.1007/s10453-018-9546-2.
Leroi-Gourhan, A. (1984). L’art des cavernes: atlas des grottes ornées paléolithiques françaises. Paris: Imprimerie Nationale.
Man, B., Wang, H., Yun, Y., Xiang, X., Wang, R., Duan, Y., et al. (2018). Diversity of fungal communities in Heshang Cave of central China revealed by mycobiome-sequencing. Frontiers in Microbiology, 9, 1400. https://doi.org/10.3389/fmicb.2018.01400.
Martin-Sanchez, P. M., Jurado, V., Porca, E., Bastian, F., Lacanette, D., Alabouvette, C., et al. (2014). Airborne microorganisms in Lascaux Cave (France). International Journal of Speleology, 43(3), 295–303. https://doi.org/10.5038/1827-806X.43.3.6.
Mulec, J., Oarga-Mulec, A., Šturm, S., Tomazin, R., & Matos, T. (2017). Spacio-temporal distribution and tourist impact on airborne bacteria in a cave (Škocjan Caves, Slovenia). Diversity, 9(3), 28. https://doi.org/10.3390/d9030028.
Ogórek, R. (2018). Speleomycology of air in Demänovská Cave of Liberty (Slovakia) and new airborne species for fungal sites. Journal of Cave and Karst Studies, 80(3), 153–160. https://doi.org/10.4311/2018mb0104.
Ogórek, R., Lejman, A., & Matkowski, K. (2014). Influence of the external environment on airborne fungi isolated from a cave. Polish Journal of Environmental Studies, 23(2), 435–440.
Out, B., Boyle, S., & Cheeptham, N. (2016). Identification of fungi from soil in the Nakimu caves of Glacier National Park. Microbiology and Immunology, 2, 26–32.
Porca, E., Jurado, V., Martin-Sanchez, P. M., Hermosin, B., Bastian, F., Alabouvette, C., et al. (2011). Aerobiology: An ecological indicator for early detection and control of fungal outbreaks in caves. Ecological Indicators, 11, 1594–1598. https://doi.org/10.1016/j.ecolind.2011.04.003.
Pusz, W., Grzeszczuk, J., Zagożdżon, P. P., & Kita, W. (2018). Aeromycological monitoring of disused mines in Poland. Polish Journal of Environmental Studies, 27(1), 1–10. https://doi.org/10.15244/pjoes/75201.
Pusz, W., Król, M., & Zwijacz-Kozica, T. (2017). Airborne fungi as indicators of ecosystem disturbance: An example from selected Tatra Mountains caves (Poland). Aerobiologia, 34, 111–118. https://doi.org/10.1007/s10453-017-9498-y.
Pusz, W., Ogórek, R., Knapik, R., Kozak, B., & Bujak, H. (2015). The occurrence of fungi in the recently discovered Jarkowicka cave in the Karkonosze Mts. (Poland). Geomicrobiology Journal, 32(1), 59–67. https://doi.org/10.1080/01490451.2014.925010.
R Core Team. (2014). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Saiz-Jimenez, C. (2012). Microbiological and environmental issues in show caves. World Journal of Microbiology & Biotechnology, 28(7), 2453–2464. https://doi.org/10.1007/s11274-012-1070-x.
Saiz-Jimenez, C., Cuezva, S., Jurado, V., Fernandez-Cortes, A., Porca, E., Benavente, D., et al. (2011). Paleolithic art in peril: Policy and science collide at Altamira Cave. Science, 334(6052), 42–43. https://doi.org/10.1126/science.1206788.
Schabereiter-Gurtner, C., Saiz-Jimenez, C., Piñar, G., Lubitz, W., & Rölleke, S. (2004). Phylogenetic diversity of bacteria associated with Paleolithic paintings and surrounding rock walls in two Spanish caves (Llonin and La Garma). FEMS Microbiology Ecology, 47(2), 235–247. https://doi.org/10.1016/S0168-6496(03)00280-0.
Seifert, K. A., Morgan-Jones, G., Gams, W., & Kendrick, B. (2011). The genera of Hyphomycetes. Utrecht: CBS-KNAW Fungal Biodiversity Centre.
Shah, P., & Pell, J. (2003). Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology, 61(5–6), 413–423. https://doi.org/10.1007/s00253-003-1240-8.
Taylor, E. L. S., Lopes, R., & de Resende Stoianoff, A. (2014). Cave entrance dependent spore dispersion of filamentous fungi isolated from various sediments of Iron Ore Cave in Brazil: A colloquy on human threats while caving. Ambient Science. https://doi.org/10.21276/ambi.2014.01.1.ra02.
Taylor, E. L. S., Resende Stoianoff, M. A. D., & Lopes Ferreira, R. (2013). Mycological study for a management plan of a neotropical show cave (Brazil). International Journal of Speleology, 42(3), 267–277. https://doi.org/10.5038/1827-806X.42.3.10.
UNESCO. (1979). Prehistoric sites and decorated caves of the Vézère Valley. Retrieved June 03, 2019 from http://whc.unesco.org/fr/list/85/.
Vanderwolf, K. J., Malloch, D., McAlpine, D. F., & Forbes, G. J. (2013). A world review of fungi, yeasts, and slime molds in caves. International Journal of Speleology, 42(1), 77–96. https://doi.org/10.5038/1827-806X.42.1.9.
Wang, W., Ma, X., Ma, Y., Mao, L., Wu, F., Ma, X., et al. (2010a). Seasonal dynamics of airborne fungi in different caves of the Mogao Grottoes, Dunhuang. China. International Biodeterioration & Biodegradation, 64(6), 461–466. https://doi.org/10.1016/j.ibiod.2010.05.005.
Wang, W., Ma, Y., Ma, X., Wu, F., Ma, X., An, L., et al. (2010b). Seasonal variations of airborne bacteria in the Mogao Grottoes, Dunhuang, China. International Biodeterioration and Biodegradation, 64, 309–315. https://doi.org/10.1016/j.ibiod.2010.03.004.
White, T. J., Bruns, T., Lee, S. J. W. T., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). San Diego: Academic Press Inc.
Wolfinger, R., & O’connell, M. (1993). Generalized linear mixed models a pseudo-likelihood approach. Journal of Statistical Computation and Simulation, 48(3–4), 233–243. https://doi.org/10.1080/00949659308811554.
Acknowledgements
We thank Joanna Lignot for English language editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leplat, J., François, A., Touron, S. et al. Aerobiological behavior of Paleolithic rock art sites in Dordogne (France): a comparative study in protected sites ranging from rock shelters to caves, with and without public access. Aerobiologia 36, 355–374 (2020). https://doi.org/10.1007/s10453-020-09637-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-020-09637-9