Abstract
Altamira Cave (north of Spain) contains one of the world's most prominent Paleolithic rock art paintings, which are threatened by a massive microbial colonization of ceiling and walls. Previous studies revealed that exchange rates between the cave and the external atmosphere through the entrance door play a decisive role in the entry and transport of microorganisms (bacteria and fungi) and nutrients to the interior of the cave. A spatial-distributed sampling and measurement of carrier (CO2) and trace (CH4) gases and isotopic signal of CO2 (δ13C) inside the cave supports the existence of a second connection (active gas exchange processes) with the external atmosphere at or near the Well Hall, the innermost and deepest area of the cave. A parallel aerobiological study also showed that, in addition to the entrance door, there is another connection with the external atmosphere, which favors the transport and increases microorganism concentrations in the Well Hall. This double approach provides a more complete knowledge on cave ventilation and revealed the existence of unknown passageways in the cave, a fact that should be taken into account in future cave management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Altamira Cave (Spain) is listed as a World Heritage UNESCO site due to its world famous collection of Paleolithic paintings and engravings. The Paleolithic rock art was preserved due to the special environmental conditions, characterized by low rates of water infiltration and precipitation of mineral deposits, and the maintenance of very stable microclimate due to the limited air exchange with the external atmosphere.
Before its discovery, Altamira Cave was likely an oligotrophic environment with a limited air exchange with the outside atmosphere. Nevertheless, agricultural and livestock uses of the top soil, archaeological digs in the cave sediments, the numerous conditioning works to facilitate visits, as well as the massive visits transformed this once pristine ecosystem into one with an abundance of available nutrients (Saiz-Jimenez et al. 2011). In fact, in the past, cave visitors produced a strong environmental impact on the cave ecosystem by transporting, releasing, and causing re-suspension of aerosols within the cave (Dredge et al. 2013).
Entry and dispersion of microorganisms and nutrients into the interior of the cave depended on the exchange rates between the cave atmosphere and exterior through the entrance (Cuezva et al. 2009). These exchange rates were higher during the summer season (June–September) (Cuezva et al. 2011). Therefore, one of the main objectives of the corrective measures implemented in recent years to manage and protect this site was focused on minimizing the exchange rates through the entrance and reinforcing the isolation of the cave area with the highest presence of rock art (Polychrome Hall), limiting thus the dispersion of microorganisms and the supply of nutrients by airflow (Saiz-Jimenez et al. 2011). The cave ventilation model in Altamira Cave was proposed based on the assumption that there was only a single known cave entrance. However, the existence of another possible point of entry of microorganisms and nutrients in the cave was suspected in light of some aerobiological analyses (Porca 2011), and this knowledge would be essential to take the most suitable measures for ensuring the conservation of rock art.
Here, we have used a double analytical approach that included a multiparametric survey of atmospheric gases which was developed for determining the spatial distribution of carrier (CO2) and trace (CH4) gases and isotopic signal of CO2 (δ13C), and an aerobiological study conducted to quantify the concentration and diversity of airborne microorganisms and their spatial distribution inside the cave. Both analyses were performed during summer season when the exchange rate between the cave and exterior atmospheres are maximal according to Cuezva et al. (2011). It was expected that this approach will shed some light on the cave atmosphere dynamics and will contribute to the management and conservation of this geological and cultural heritage site.
Material and methods
Site
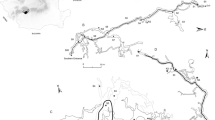
Altamira is a well-studied cave in Cantabria, northern Spain (43°22′40″ N, 4°7′6″ W) (Fig. 1). It is one of many caves in the upper vadose area of the tabular polygenic karstic system developed within Cretaceous bedrock. The cavity (270 m in length) is situated at 152 m a.s.l., and has a depth of 3–22 m (averaging 8 m) below the surface. The cavity has a single entrance in a topographically higher position and includes a number of main halls having a downward slope from the outside access to the deepest part of the cave. At present, the cave is permanently closed to visitors. The main entrance is closed by a metal gate (slotted surface <4 %; thermally insulated) acting as the initial barrier to stop the exchange of matter with the outside. In addition, a second door isolates the entrance hall of the crossing and the rest of the cavity (mainly Polychrome Hall and Walls Hall, Fig. 1).
a Altamira Cave map with location of the aerobiological and atmospheric gases sampling points inside the cavity and sampling path considered for Fig. 3. b Geographic location of the cave
Distribution of atmospheric gases
Cave air parameters are influenced by the interaction processes with the soil and the exterior atmosphere. In order to characterize soil, atmospheric, and cave levels of CO2, δ13CO2 and CH4, 38 air samples were successively taken during a 3-day long campaign in July 2012, comprising the following locations: exterior air (7), soil air (8), and cave air along the whole inner space (23) (Fig. 1; Table 1). Air samples were obtained by filling Tedlar sampling bags with a capacity of 1 L and with lock valves, especially designed to ensure the inertness and impermeability of air samples. Both cave and outside air bags were filled by using a hand air pump and a micro-diaphragm gas pump of 1.8 L/min at atmospheric pressure was used for soil air sampling. The samples were immediately analyzed after sampling by using a laser-based analyzer (Picarro G2101-i) that employs cavity ring-down spectroscopy (CRDS-WS) (Crosson 2008). This is a field-deployable CRDS analyzer capable of performing in situ and real-time measurements of atmospheric levels of carbon dioxide (12CO2 and 13CO2), CH4, and water vapor. Isotopic relationship δ13CO2 is automatically measured as a calculation from 12CO2 and 13CO2 concentrations and considering the correction due the presence of other target species (CH4, H20v). Precisions of 200 ppb, 10 ppb, and 0.3 % are guaranteed for 12CO2, 13CO2 and δ13CO2, respectively. Precision of 0.1 ppm was obtained for CH4 in the studied level range (0–2 ppm).
Aerobiological study
An extensive aerobiological sampling all over the cave was conducted to quantify the concentration of airborne microorganisms (bacteria and fungi). Some specific applications of these aerobiological techniques in subterranean environments have been previously published (Jurado et al. 2010b; Fernandez-Cortes et al. 2011; Porca et al. 2011). Briefly, a Duo SAS (Surface Air System) model 360 sampler (International PBI, Milan, Italy) containing Petri dishes with dichloran rose bengal chloramphenicol agar was used for the sampling of fungi. The antifungal agent, dichloran, was added to the medium to reduce colony diameters of spreading fungi. The presence of rose bengal in the medium suppresses the growth of bacteria so does chloramphenicol and restricts the size and height of colonies of the more rapidly growing fungi (King et al. 1979). For bacteria, the medium trypticase–soy agar was used. Duplicate samples of 100 L in air volume were selected as the most appropriate method for easy counting. The plates were incubated (fungi at 25 °C for 5 days and bacteria at 28 °C for 2–4 days) and counted. After counting, all morphologically distinct colonies were isolated in pure culture. The isolates were grouped based on sequence similarity. Representative sequences of each cluster were assigned GenBank numbers HF952623-HF952709.
Fungal mycelium and bacterial biomass were scraped from the plates and transferred to a 1.5-mL Eppendorf tube containing 500 μL lysing buffer and 200 μL glass beads. The mixture was shaken in a cell disrupter (Fast Prep-24, Solon, OH) at full speed for 3 min. Amplification of DNA was performed with fungi-specific primers, ITS1 (5´-TCC GTA GGT GAA CCT GCG G-3´) and ITS4 (5´-TCC TCC GCT TAT TGA TAT GC-3´) described White et al. (1990) for the internal transcribed spacer regions (ITS), and bacteria-specific primers, 616 F (5´-AGA GTT TGA TYM TGG CTC AG) (Zimmermann et al. 2005) and 1522R (5´-AAG GAG GTG ATC CAG CCG CA-3´) (Edwards et al. 1989) for prokaryotic 16S rRNA gene. Amplification protocol was performed using the following thermal conditions: 94 °C for 2 min; 35 cycles of 94 °C for 15 s, 55 °C for 16S rRNA gene or 55 °C for ITS regions during 1 min; 72 °C for 2 min, and a final step of 72 °C for 10 min. Amplification products were sequenced in an ABI 3700 sequencer (Applied Biosystems). A similarity search was performed using the BLAST algorithm at the NCBI database (National Centre for Biotechnology Information, http://www.ncbi.nlm.nih.gov/).
Results and discussion
Spatial distribution of atmospheric gases
The atmosphere of Altamira Cave is characterized by a high thermo-hygrometric stability, relatively high levels of air CO2 with light isotopic signatures and CH4 concentration lower than the external atmosphere (Garcia-Anton et al. 2012). Data obtained during the survey carried out in July 2012 (Table 1) were in agreement with previous studies (Sanchez-Moral et al. 2010). Mean values of cave air samples were of 1,274 ppm for CO2, −21.8‰ for δ13C isotopic signal and 0.76 ppm for CH4. Soil air showed mean values of 1,211 ppm of CO2, 20.6‰ of δ13C and 1.7 ppm of CH4. The exterior air sampled during the fieldwork registered mean values of 433 ppm for CO2, −10.7‰ for δ13C and 1.8 ppm for CH4. Exterior and cave air samples fit the Keeling model (Keeling 1958; Pataki et al. 2003) with a very high correlation degree (R 2 = 0.99 in Fig. 2a). Variation of the isotopic signal is linearly dependent on the inverse of CO2 concentration for atmospheric and cave air samples (expressed as parts per millions) and δ13C decrease as CO2 concentration reached higher values (Fig. 2a). The y-intercept value (−28.3‰) points to the soil as the source of CO2. According to Keeling model, CO2 concentration of cave air is the result of mixing the background atmospheric CO2 with soil-produced CO2. CH4 and CO2 values for air samples from the external and cave environments showed a logarithmic inverse relationship reaching the lowest values when air presented a higher CO2 concentration (Fig. 2b).
a Isotope ratio (δ13C) plotted against the inverse of CO2 concentration for atmospheric and cave air samples. The linear fitting is based on a two-endpoint Keeling plot model (Keeling 1958; Pataki et al. 2003). b Plotted relationship between the target gases (CO2 and CH4). The cave air samples are shown by solid squares and the exterior air by open triangles. Arrows indicate the prevailing renewal of cave air during the 3-day-long monitoring period
The entire set of results obtained corresponds to the summer season during which the prevailing and highest air exchange between the cavity and the external atmosphere is established. The external air inlet causes changes in cave micro-environmental conditions. A comparison of the data obtained on the 23rd and 26th of July precisely showed that the ventilation process was active during the sampling campaign. On these days, there was a decrease in the concentration of CO2 and a significant increase in air CH4 (Fig. 2b). These changes were very pronounced in areas not directly connected to the main entrance and even higher than those recorded at or near the entrance hall (e.g. Grave Hall, Well Hall, Polychrome Hall). These data indicated a process of ventilation likely through the system of interconnected fissures and pores of the host rock and not only through the cave entrance.
Overall, cave areas influenced by the external atmosphere presented lower CO2 concentration, lighter heavier isotopic signal and higher CH4, while the opposite pattern characterized the zones with a greater isolation level. Both opposite patterns in relation to the gas exchange degree with the exterior have been marked by dashed line circles (Fig. 2), corresponding to the most connected and isolated zones (cave entrance and Grave Hall, respectively). Inside the cave, the lowest CO2 concentration and heaviest δ13C values corresponded to the entrance hall and identified this area as the one most directly connected to the exterior (Fig. 3). The influence of external atmosphere was also pronounced in the Polychrome Hall while internal parts of the cave showed lower isotope signal values, maximum CO2, and minimum CH4 concentrations in the innermost areas. Surprisingly, the most isolated zone identified by the target gases relationship (CO2–CH4) did not correspond to the area farthest from the entrance (Well Hall) but, however, it coincided with an intermediate and deeper area (Grave Hall). In the Grave Hall, the lowest CH4 concentration, highest CO2 concentration, and lighter isotopic signal of air were registered (Figs. 3 and 4). However, in the innermost part of the cave (Well Hall), a change of trend was noticed despite being roughly 200 m far from the only known human accessible entrance. The Well Hall is the farthest hall from the entrance (except for Horse-tail zone, an inactive meander completely filled by sediments) but a slight increase in CH4 together with a decrease in CO2 concentration with heavier isotope values suggested an additional process of ventilation likely through a second external connection near or in the Well Hall area.
Aerobiological study
Usually, airborne fungi exist as spores while most airborne bacteria are attached to dust particles (Che 2004). We found that the concentration of fungal spores outdoors was higher than inside the cave (Table 2). Inside the cave, the concentration of fungal spores was higher in the halls and galleries close to the entrance door (Kitchen Hall, Crossing, Polychrome Hall) and it was reduced towards the interior of the cave (Walls Hall) (Figs. 3 and 4). The concentration of fungal spores reached minimum in the Grave Hall and Great Hall, where it ranged between 10 and 21 colony forming units (CFU) per cubic meter. However, fungal distribution changed in the Well Hall, which showed 50 CFU/m3. This discontinuity is likely a consequence of a direct connection of this hall with the exterior, as indicated by the gas concentrations (CO2 and CH4) and the carbon isotopic signature data.
Upon identification we found that the spores of the genus Cladosporium represented 80.3 % of the total abundance in the cave, followed by the genus Epicoccum with 14.7 % (Table 2). Minor percentages were obtained for Acremonium (2 %), Penicillium (1.3 %), Aspergillus (0.4 %), etc. In the halls near the entrance (Kitchen Hall, Crossing, Polychrome Hall, and Walls Hall) Cladosporium spores attained a concentration of 79.3 %, followed by Epicoccum (15.9 %).
The genera Cladosporium (72 %) and Epicoccum (14 %) accounted for 86 % of the fungal taxa found in an outdoor air study (Flannigan et al. 2001). These genera comprise cosmopolitan air-borne and soil-borne fungi. Cladosporium spores are present in Europe and North America outdoor environment throughout the year (Cooley et al. 1998; Aira et al. 2012). This genus represented from 40 to 80 % of the total spores in the air of many European cities (Spieksma 1995).
The growth, sporulation, and dispersal of Cladosporium seem to be very sensitive to environmental changes. Aira et al. (2012) found that many of the stations in north Spain presented a peak of spore concentrations in summer, the same season of our cave sampling. Clearly, concentrations of Cladosporium spores in indoor air are influenced by outdoor concentrations and indoor growth sources. In accordance with this last fact, the air of Nerja Cave, southern Spain, showed 50.2 % of Aspergillus/Penicillium and 17.1 % of Cladosporium. This high Aspergillus/Penicillium concentration was promoted by the installation of a wooden stage for a festival of music and dance which, together with the high relative humidity reached in this season, favoured the development of these fungi (Docampo et al. 2011); likewise, the comparatively high abundance of Cladosporium in Altamira Cave likely denotes an external influence (see Table 2). We suggest that the abundance of Cladosporium and Epicoccum all over Altamira Cave can be a consequence of an outdoor air influence reflected in the existence of two connections with the exterior, one, the cave entrance, and the second at the end of the cave, likely in the Well Hall, which promoted air exchange and ventilation. In fact, 84 % of the fungal spores present in the Well Hall were identified as Cladosporium and the remaining were identified as Trichoderma gamsii, a cosmopolitan soil-borne fungus (Kubicek et al. 2008). In addition, the Cladosporium sp. and Epicoccum nigrum sequences from isolates collected outdoor are identical to those collected inside the cave, which confirms that the presence of these two genera in the air of Altamira Cave is due to transport from the exterior due to ventilation and wind currents.
The concentration of airborne bacteria inside the cave was higher than that registered outside (Table 3). This spatial pattern could be attributed to the massive presence of bacteria on the walls and ceiling of the halls and galleries close to the entrance (Kitchen Hall, Crossing, Polychrome Hall, and Walls Hall; Fig. 1), which form distinguishable colonizations or microbial mats coating the rocks (Schabereiter-Gurtner et al. 2002; Saiz-Jimenez et al. 2011). Wind currents favour the entry and transport of bacteria into the cave. In addition, wind currents promote aerosols detachment from rock surfaces with condensed water, which can also remove and suspend bacteria from the cave walls and ceiling. The higher concentrations of airborne bacteria were found in the Crossing and the entrance gallery to Polychrome Hall (Figs. 3 and 4). Considering the samplings in the transect entrance–Polychrome Hall, the most abundant bacterium in the air of Altamira Cave was Micrococcus luteus (85.3 %), followed by different species of Pseudomonas (6.8 %) and Bacillus (1.8 %). It is noteworthy to mention that the outdoor abundance of M. luteus (36.4 %), Bacillus spp. (36.3 %), and Rhodococcus corynebacterioides (27.2 %), also denotes the influence of the transportation of external bioaerosols inside the cave. The dissemination of M. luteus even reached the Walls Hall, although it CFU per cubic meter is nine times lower than the concentration registered in the Polychrome area. After the Walls Hall, M. luteus disappeared or was present at a low abundance (Great Hall) but reached in the Horse Tail gallery contents similar to those of the Walls Hall. Again the influence of a possible second connection with the exterior in the Well Hall is suggested by the increasing number of bacteria in this hall (740 CFU/m3, from which 89.2 % belonged to Bacillus weihenstephanensis, a soil-dwelling bacterium) in comparison with the samplings obtained in the nearby halls (Great Hall: 360 CFU/m3 and Horse Tail Gallery: 280 CFU/m3). Outdoor abundance of B. weihenstephanensis was 27.2 %. However, we were not surprised to observe lower counts (150 CFU/m3) in the Grave Hall than in the other parts of the cave. The Grave Hall is located in a lower topographical level and is partially isolated from the rest of the cave. The sequences from isolates of M. luteus and B. weihenstephanensis collected outdoor and those from isolates collected inside the cave revealed to be identical, which confirm the strong influence of outdoor air on Altamira Cave.
A plethora of potentially pathogenic bacteria and fungi were isolated from the cave air. Cladosporium has been recognized as a human pathogen, although infections are not very common. Cladosporium cladosporioides can cause pulmonary and cutaneous infections (De Hoog et al. 2000). In addition, Cladosporium species have the ability to trigger allergic reactions to sensitive individuals. Prolonged exposure to elevated spore concentrations can provoke chronic allergy and asthma. Concentrations of 3,000 Cladosporium spores per cubic meter of air are generally taken as the threshold concentrations for clinical significance. However, individuals may react at lower concentrations depending on their sensitivity. Ballero et al. (2000) suggested that 100 spores per cubic meter is sufficient to trigger early allergic symptoms, a figure lower than that found in the air of different Altamira Cave halls. Similar allergy was reported for Epicoccum nigrum (Bisht et al. 2008).
To mention only a few relevant bacteria in the cave, M. luteus can produce bacteremia, pneumonia, and endocarditis (von Eiff et al. 1996; Salar et al. 1997; Usó et al. 2003; Jurado et al. 2010a), P. putida causes bacteremia and wound infections (Yoshino et al. 2011), B. weihenstephanensis produces enterotoxins associated with a diarrhoeal type of food poisoning (Stenfors et al. 2002). Infections associated with Stenotrophomonas maltophilia most commonly include respiratory tract infections (pneumonia and acute exacerbations of chronic obstructive pulmonary disease), bacteremia, infections of the bones, urinary tract and soft tissues, eye infections, endocarditis, and meningitis (Brooke 2012). Last but not least, although not isolated from air, Altamira Cave walls includes among other pathogenic bacteria Aureimonas (=Aurantimonas) altamirensis. This emerging pathogen was isolated from cystic fibrosis, keratitis, and corneal ulcer (Luong et al. 2008), bacteremia (Mendes et al. 2009), pleural effusions (Téllez-Castillo et al. 2010), and from a catheter (García-Lozano et al. 2012).
To summarize, the entry of airborne microorganisms by transportation of aerosols is possible only in areas with direct communication with the exterior. During the summer season the door of Altamira Cave favours the direct communication with the external atmosphere, where warm and wet air entering to the cave results in the formation of clouds of water microparticles (hydroaerosols) that move towards the interior of the cave. Each hydroaerosol acts as potential adherence nucleus for dust particles (solid aerosols) and microorganisms in the air. The displacement of the aerosols increases liquid water and organic matter availability towards the interior of the cave which favours the development and dissemination of microbial colonies. Thus, in the case of Altamira Cave, one would expect that the area near the cave entrance has the highest level of airborne microorganisms in relation to deeper halls. However, a change in the trend of trace gases and a positive anomaly in bioaerosols in the Well Hall in relation to nearby halls and galleries reveal a second connection with the external atmosphere at this cave site.
The Well Hall is the farthest site from the cave entrance but it is close to the well-developed drained network at surface, located towards southeast at lowest karst levels (Elez et al. 2013). There, a big doline, aligned and genetically related to existing fractures, entails that the sub-horizontal host and soil cover is thinner. Consequently, not only transport gas but also allochthonous matter may easily reach the interior of the cave through fractures and rock discontinuities due to its proximity to the doline.
A few years ago, the current closure system of the cavity was installed to minimize cave ventilation process (Saiz-Jimenez et al. 2011). However now there is evidence that cave and karst ventilation processes occur not only through the cave entrance door. During the warm, dry season there is an active process of ventilation through a second small entry or by the network system of fissures and pores of the host rock, which effects are noticeable in certain areas far from the cave entrance.
Conclusions
In Altamira Cave, the main threat for the conservation of Paleolithic rock art is the opening of the entrance door, which reinforces the role of the air as a vehicle for the transport and dispersion of airborne microorganisms and nutrients inside the cave. Most of the frequent microorganisms inside and outside the cave are the same.
The study of atmospheric gases (CO2, CH4, and the isotopic signal of CO2) combined with aerobiology provided very useful information on the cave atmosphere dynamics and can help in the management of any show cave. We have shown that the data are consistent with the existence of a second connection favouring the entry and transport of airborne microorganisms in the innermost area of the cave. In fact, an increased level of airborne microorganisms in the Well Hall in relation to nearby halls and galleries reveals a clear connection with the external atmosphere at this cave site, far from the original entrance. On the other hand, the spatial distribution of carrier (CO2) and trace (CH4) gases and isotopic signal of CO2 (δ13C) also support this assumption and suggests a slight but direct connection (active gas exchange processes) with the external atmosphere at this cave site. The discovery of a second and unknown access to the cave is a threat for the conservation of the rock art and reinforces the need to assess the impact of the second external connection, which should be taken into account in future cave management and preservation guidelines. Also cave managers should be aware of the potential risks for human health due to the presence of abundant airborne microorganisms inside the cave.
References
Aira MJ, Rodríguez-Rajo F-J, Fernández-González M, Seijo C, Elvira-Rendueles B, Gutiérrez-Bustillo M, Abreu I, Pérez-Sánchez E, Oliveira M, Recio M, Morales J, Muñoz-Rodríguez A-F (2012) Cladosporium airborne spore incidence in the environmental quality of the Iberian Peninsula. Grana 51:293–304
Ballero M, Piu G, Ariu A (2000) The impact of the botanical gardens on the aeroplankton of the city of Cagliari, Italy. Aerobiologia 16:143–147
Bisht V, Singh BP, Arora N, Sridhara S, Gaur SN (2008) Allergens of Epicoccum nigrum grown in different media for quality source material. Allergy 55:274–280
Brooke JS (2012) Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41
Che F (2004) The principle and application of airborne microbiology. Science Press, Beijing, pp 1–41
Cooley JD, Wong WC, Jumper CA, Straus DC (1998) Correlation between the prevalence of certain fungi and sick building syndrome. Occup Environ Med 55:579–584
Crosson ER (2008) A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor. Appl Physics B 92:403–408
Cuezva S, Sanchez-Moral S, Saiz-Jimenez C, Cañaveras JC (2009) Microbial communities and associated mineral fabrics in Altamira Cave, Spain. Community structure in oligotrophic cave environments. Int J Speleol 38:83–92
Cuezva S, Fernandez-Cortes A, Benavente D, Serrano-Ortiz P, Kowalski AS, Sanchez-Moral S (2011) Short-term CO2(g) exchange between a shallow karstic cavity and the external atmosphere during summer: role of the surface soil layer. Atmos Environ 45:1418–1427
Docampo S, Trigo MM, Recio M, Melgar M, García-Sánchez J, Cabezudo B (2011) Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): diversity and origin. Sci Total Environ 409:835–843
Dredge J, Fairchild IJ, Harrison RM, Fernandez-Cortes A, Sanchez-Moral S, Jurado V, Gunn J, Smith A, Spotl C, Mattey D, Wynn PM, Grassineau N (2013) Cave aerosols: distribution and contribution to speleothem geochemistry. Quat Sci Rev 63:23–41
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853
Elez J, Cuezva S, Fernandez-Cortes A, Garcia-Anton E, Benavante D, Cañaveras JC, Sanchez-Moral S (2013) A GIS based methodology to quantitatively define an adjacent protected area in a shallow karst cavity: the case of Altamira cave. J Environ Manage 118:122–134
Fernandez-Cortes A, Cuezva S, Sanchez-Moral S, Porca E, Jurado V, Martin-Sanchez PM, Saiz-Jimenez C (2011) Detection of human-induced environmental disturbances in a show cave. Environ Sci Poll Res 18:1037–1045
Flannigan B, Samson RA, Miller JD (2001) Microorganisms in home and indoor work environments: diversity, health impacts, investigation and control. Taylor and Francis, London
Garcia-Anton E, Cuezva S, Fernandez-Cortes A, Sanchez-Moral S, Benavente D (2012) Daily variations of CO2, δ13CO2 and CH4 of cave air controlled by external weather conditions: example of rapid survey in Altamira cave (north of Spain). Geophys Res Abstracts 14:4859–4862
García-Lozano T, Aznar Oroval E, Juan Bañón JL (2012) First isolation in Spain of Aurantimonas altamirensis in a blood culture from a port-a-cath in a patient with Bence-Jones type multiple myeloma (in Spanish). Enferm Infec Microbiol Clin 30:217–218
Hoog GS de, Guarro J, Gené J, Figueras MJ (2000) Atlas of Clinical Fungi, 2nd edn. CBS, Utrecht and Universitat Rovira i Virgili, Reus.
Jurado V, Laiz L, Rodriguez-Nava V, Boiron P, Hermosin B, Sanchez-Moral S, Saiz-Jimenez C (2010a) Pathogenic and opportunistic microorganisms in caves. Int J Speleol 39:15–24
Jurado V, Porca E, Cuezva S, Fernandez-Cortes A, Sanchez-Moral S, Saiz-Jimenez C (2010b) Fungal outbreak in a show cave. Sci Total Environ 408:3632–3638
Keeling CD (1958) The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Acta 13:322–334
King AD, Hocking AD, Pitt JI (1979) Dichloran-rose bengal medium for enumeration and isolation of molds from foods. App Environ Microbiol 37:959–964
Kubicek CP, Komon-Zelazowska M, Druzhinina IS (2008) Fungal genus Hypocrea/Trichoderma: from barcodes to biodiversity. J Zhejiang Univ Sci B 9:753–763
Luong M-L, Békal S, Vinh DC, Lauzon D, Leung V, Al-Rawahi GN, Ng B, Burdz T, Bernard K (2008) First report of isolation and characterization of Aurantimonas altamirensis from clinical samples. J Clin Microbiol 46:2435–2437
Mendes RE, Denys GA, Fritsche TR, Jones NR (2009) Case report of Aurantimonas altamirensis bloodstream infection. J Clin Microbiol 47:514–515
Pataki DE, Ehleringer JR, Flanagan LB, Yakir D, Bowling DR, Still CJ, Buchmann N, Kaplan JO, Berry JA (2003) The application and interpretation of Keeling plots in terrestrial carbon cycle research. Glob Biogeochem Cycle 17:1022. doi:10.1029/2001GB001850
Porca E (2011) Aerobiología: mecanismos de dispersión de los microorganismos en cuevas turísticas. Ph.D. Thesis, University of Seville.
Porca E, Jurado V, Martin-Sanchez PM, Hermosin B, Bastian F, Alabouvette C, Saiz-Jimenez C (2011) Aerobiology: an ecological indicator for early detection and control of fungal outbreaks in caves. Ecol Indic 11:1594–1598
Saiz-Jimenez C, Cuezva S, Jurado V, Fernandez-Cortes A, Porca E, Benavente D, Cañaveras JC, Sanchez-Moral S (2011) Paleolithic art in peril: policy and science collide at Altamira Cave. Science 334:42–43
Salar A, Carratalà J, Fernández-Sevilla A, Marín D, Grañena A (1997) Pneumonia caused by Micrococcus species in a neutropenic patient with acute leukemia. Eur J Clin Microbiol Infect Dis 16:546–548
Sanchez-Moral S, Cuezva S, Fernández-Cortés A, Benavente D, Cañaveras JC (2010) Effect of ventilation on karst system equilibrium (Altamira Cave, N Spain): an appraisal of karst contribution to the global carbon cycle balance. In: Andreo B, Carrasco F, Duran JJ, LaMoreaux JW (eds) Advances in research in karst media. Springer, Berlin, pp 469–474
Schabereiter-Gurtner C, Saiz-Jimenez C, Piñar G, Lubitz W, Rolleke S (2002) Altamira Cave paleolithic paintings harbour partly unknown bacterial communities. FEMS Microbiol Lett 211:7–11
Spieksma FTHM (1995) Outdoor atmospheric mould spores in Europe. XVIth European Congress of Allergology and Clinical Immunology. Monduzzi, Bologna, pp 625–630
Stenfors LP, Mayr R, Scherer S, Granum PE (2002) Pathogenic potential of fifty Bacillus weihenstephanensis strains. FEMS Microbiol Lett 215:47–51
Téllez-Castillo CJ, González Granda D, Bosch Alepuz M, Jurado Lobo V, Saiz-Jimenez C, Juan JL, Millán Oria J (2010) Isolation of Aurantimonas altamirensis from pleural effusions. J Med Microbiol 59:1126–1129
Usó J, Gil M, Gomila B, Tirado MD (2003) Endocarditis by Micrococcus luteus (in Spanish). Enferm Infec Microbiol Clin 21:116–120
von Eiff C, Kuhn N, Herrmann M, Weber S, Peters G (1996) Micrococcus luteus as a cause of recurrent bacteremia. Pediatr Infect Dis J 15:711–713
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Yoshino Y, Kitazawa T, Kamimura M, Tatsuno K, Ota Y, Yotsuyanagi H (2011) Pseudomonas putida bacteremia in adult patients: five case reports and a review of the literature. J Infect Chemother 17:278–282
Zimmermann J, Gonzalez JM, Ludwig W, Saiz-Jimenez C (2005) Detection and phylogenetic relationships of a highly diverse uncultured acidobacterial community on paleolithic paintings in Altamira Cave using 23S rRNA sequence analyses. Geomicrobiol J 22:379–388
Acknowledgments
This research was supported by the Spanish Ministry of Sciences and Innovation, project CGL2010-17108/BTE. EG-A is supported by a CSIC JAE-Predoctoral grant. SC benefits of a postdoctoral fellowship from the Spanish Ministry of Science and Innovation, research programme Juan de la Cierva. AF-C was funded by a postdoctoral fellowship the JAE-Doc Program (CSIC). AZM was supported by FCT grant SFRH/BPD/63836/2009. Altamira Cave Research Centre and Museum staffs are acknowledged for their collaboration throughout the research period. This is a TCP-CSD 2007–00058 paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Garcia-Anton, E., Cuezva, S., Jurado, V. et al. Combining stable isotope (δ13C) of trace gases and aerobiological data to monitor the entry and dispersion of microorganisms in caves. Environ Sci Pollut Res 21, 473–484 (2014). https://doi.org/10.1007/s11356-013-1915-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1915-3