Abstract
The goal of this study was to quantitatively assess the relationship linking vegetation and airborne pollen. For this, we established six sampling stations in the city of Thessaloniki, Greece. Once every week for 2 years, we recorded airborne pollen in them, at breast height, by use of a portable volumetric sampler. We also made a detailed analysis of the vegetation in each station by counting all existing individuals of the woody species contributing pollen to the air, in five zones of increasing size, from 4 to 40 ha. We found the local vegetation to be the driver of the spatial variation of pollen in the air of the city. Even at very neighbouring stations, only 500 m apart, considerable differences in vegetation composition were expressed in the pollen spectrum. We modelled the pollen concentration of each pollen taxon as a function of the abundance of the woody species corresponding to that taxon by use of a Generalized Linear Model. The relationship was significant for the five most abundantly represented taxa in the pollen spectrum of the city. It is estimated that every additional individual of Cupressaceae, Pinaceae, Platanus, Ulmus and Olea increases pollen in the air by approximately 0.7, 0.2, 2, 6 and 5%, respectively. Whether the relationships detected for the above pollen taxa hold outside the domain for which we have data, as well as under different environmental conditions and/or with different assemblages of species representing them are issues to be explored in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Airborne pollen is governed largely by local flora and vegetation (Rojo et al. 2015). In many cities, the pollen spectrum has changed over the recent years, one of the main reasons being the planting of wind-pollinated species in urban green spaces that produce large amounts of pollen (Alcázar et al. 2004; Charpin et al. 2005). Apart from their aesthetic value, urban parks provide essential ecosystem services, such as improving air quality (Beckett et al. 2000; Dzierzanowski et al. 2011) and contributing to the quality of life and the welfare of citizens (Latinopoulos et al. 2016; Livesley et al. 2016; Shackleton et al. 2015). Because of these highly appreciated effects and in view of the climate change, many cities have undertaken an accelerated greening process in recent decades (Jim 2013; Grant 2012). However, greening of the cities may have negative impacts on the quality of life and the health of the human population (von Döhren and Haase 2015), either resident or visiting, if not carefully planned. It is imperative to understand which factors may cause such disservices and control them as much as possible.
Pollen emission by higher plants during the flowering period may become an ecosystem disservice with a great negative impact. Allergenic pollen contributes to biotic air pollution (Cariñanos et al. 2007; Cariñanos et al. 2008) and has high direct (doctor costs, medications, allergy diagnostic tests), indirect (loss of work and school days, premature retirement) and intangible (social costs, lowering of the quality of life) cost implications (Green and Davis 2005; Reed et al. 2004). Approximately 10–30% of adults and 40% of children suffer worldwide from allergic rhinitis, a common reaction to allergenic pollen (Pawankar 2014). Much of the symptom-causing airborne pollen comes from the species most commonly used in urban forests and green areas (D’Amato et al. 2010; Hruska 2003; Nicolau et al. 2005). In many cities with a temperate climate, urban green spaces are often characterized by an overabundance of a low number of species, particularly poplars (Populus spp.), willows (Salix spp.), elms (Ulmus spp.), cypresses (Cupressus spp.) and planes (Platanus spp.), which release simultaneously large amounts of pollen into the air during the main pollen season (Cariñanos and Casares 2011). This is the reason why there is an increasing interest in evaluating the negative impact of urban green spaces to human health. For such an evaluation, information extracted from aerobiological research and vegetation studies together with the assessment of the allergenic risk of extant green spaces (e.g. Cariñanos and Casares 2011; Cariñanos et al. 2014; Weinberger et al. 2015; Werchan et al. 2017) are very important.
Airborne pollen monitoring can provide valuable information on the pollen-related air quality but also on the occurrence and abundance of taxa in the vegetation of the area where the sampler is located. However, as pollen in the air is influenced by several factors like time of the day, season, weather conditions, geographical location, type of vegetation dominating the area, proximity to pollen sources (Puc 2011; Grewling et al. 2012; Ziello et al. 2012), it is important to have a deeper knowledge of the drivers of airborne pollen variation. Pollen data at a fine temporal scale (e.g. as bi-hourly concentrations) are the first essential piece of information. Detailed mapping of vegetation and/or information on land use and its management (Skjøth et al. 2013), from which pollen sources can be identified, are also very important.
Many pollen observation stations are in operation worldwide providing basic information on the daily concentrations of airborne allergenic pollen. Most often they are equipped with a 7-day volumetric trap (Hirst 1952), located at a rooftop (10–30 m aboveground). However, such a setting provides information that is representative of a large area thus reflecting mainly the influences of regional rather than local sources (O’Rourke and Lebowitz 1984) and does not allow quantification of the relationship between vegetation and airborne pollen or assessment of the complexity of pollen distribution in cities (Werchan et al. 2017). According to Werchan et al. (2017), the main limitations of the studies so far that need to be overcome are related to pollen sampling only within part of a city or using only a few pollen traps per city, the non-uniform heights of pollen traps and the long intervals between changing traps. Others add that roof-level data do not address intra-urban spatial heterogeneity of pollen concentrations (Peel et al. 2013) and are thus less accurate for exposure assessment when studying potential human health effects, whereas the need of quantifying the relations between environmental determinants and allergenic pollen concentrations across urban gradients at local (≤ 100–300 m) scales has been also stressed (Haberle et al. 2014; WHO 2013).
In this study, we assess the sensitivity of the relationship between airborne pollen and the vegetation producing it at a local scale, in the urban environment. For this, we established a number of sampling stations in the city of Thessaloniki, Greece. In each station, we recorded pollen in the air and made a detailed analysis of vegetation. This allows us to (1) examine how accurately local vegetation is expressed in the local pollen spectrum, (2) quantify the relationship between the two parameters, thus enabling prediction of changes in airborne pollen concentration from changes in vegetation abundance and vice versa, and (3) examine how the quantitative aspects of this relationship differ among taxa.
2 Materials and methods
2.1 Pollen sampling
We selected six stations for sampling in the wider area of Thessaloniki (Fig. 1), at highly visited sites. These are: Aristotelous st. (Ari), at the heart of the city; Ktel Makedonia (Kte), the major intercity bus station, far from the city centre; the Zoological station (Zoo), at the edge of the peri-urban forest of Seih Sou on the hill surrounding the city; Ethnikis Aminis st. (EAm), close to the campus of the Aristotle University of Thessaloniki; Aretsou st. (Are), close to the seafront, and Chimeiou square (Uni), within the campus of the Aristotle University of Thessaloniki.
Map of Thessaloniki [image from Google Earth (2017)] with the location of the sampling stations. Numbers before their names indicate the order in which the stations were visited on sampling days. Given in parenthesis is the time of the day, when sampling took place in the stations, and in brackets the abbreviations of their names as used in the text
Air samples were collected once every week for two consecutive years (2012 and 2013). Sampling took place on the same day in all stations, at a specified time for each station, and in the order indicated in Fig. 1, always starting from downtown (station 1) and ending at the University (station 6); it lasted 15 min in each station. Samples were collected by use of a portable volumetric air sampler (Hirst-type, Burkard Manufacturing, Hertfordshire, England), which operates under battery, at a nominal air throughput of 10 L min−1. Records were taken at approximately 1.5 m above ground, while in motion on foot, at an average speed of 3 km hr1, within a 4 ha area (core zone of the station). Pollen grains were directly collected onto the glass slide coated with gelvatol inside the sampler; they were fixed with a mixture of glycerol–distilled water–gelatine–phenol (in proportions of 25:21:4:1) and 1% safranin (for staining) and stored under cover slips (22 × 22 mm). Pollen grains were counted and identified at x400 magnification using an optical microscope (Nikon Eclipse E200). Results are expressed as number of pollen grains per m3 of air (Hirst 1952; British Aerobiology Federation 1995). This is estimated as follows: Within the sampling time of 15 min, the volume of air sampled equals to 10 L min−1 (the throughput of the sampler) multiplied by 15 min, thus to 150 L or 0.15 m3. Hence, the number of pollen grains in 1 m3 equals the number in the sample divided by the volume of the sample (being 0.15 m3).
2.2 Vegetation sampling
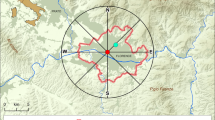
In each station, and in five areas of increasing size, the one surrounding the other, we recorded all individuals of woody species, known from previous studies to contribute pollen in the air (Damialis et al. 2007). In each station, a core zone was defined measuring 40,000 m2 (4 ha), where pollen sampling took place. Depending on the station, the shape of the core zone was square, if an open space, or rectangular, if sampling was conducted on a street. The four other zones were at a distance of 50, 100, 150 or 200 m from each side of the core zone. The following codes will be used hereafter for the five zones: core zone, zone 1 (core + area within a distance of 50 m from each side of the core zone), zone 2 (core + area within a distance of 100 m), zone 3 (core + area within a distance of 150 m) and zone 4 (core + area within a distance of 200 m). Zone 4 corresponding to the entire sampled area per station was divided into 50 m × 50 m squares, resulting in 144 squares for the square sampling design and in 160 for the rectangular one (Fig. 2). This division in squares was made by using panoramic Google Earth images, which were georeferenced and rectified, and the ArcMap 10.1 (ESRI 2011) software. The sizes of the sampled zones were 9(10) ha, 16(18) ha, 25(28) ha and 36(40) ha, for zones 1–4, respectively, for the two sampling designs (rectangular in parenthesis). With the help of a GPS device, we counted the existing woody individuals of the taxa contributing airborne pollen in each square of every station.
Sampling designs for the estimation of the abundance of woody species contributing airborne pollen. The design is that of a square, when sampling took place in open places like squares, or a rectangle, when in streets. The darker area in the middle is the core zone (where pollen sampling took place) corresponding to 4 ha. Surrounding it, there are four zones, corresponding to the core zone and the area up to a distance of 50 m (zone 1), or 100 m (zone 2), or 150 m (zone 3), or 200 m (zone 4) from each side of the core zone. The sampled area was divided into 50 m × 50 m squares (in total, 144 and 160, for the square and the rectangular sampling designs, respectively). Individuals representing woody taxa that contribute airborne pollen in the city were counted in every small square of every station
2.3 Data analysis
Two data sets were created. The vegetation data set contains the number of individuals per pollen taxon (consisting of one or more species), which were recorded in the five zones of each sampling station, whereas the pollen data set contains the sums of pollen concentrations (pollen grains per m3 of air) per taxon and station over the 2 years of sampling. The vegetation data set contains five subsets (one for each zone in each station), while the pollen data set is made of only one set per station. Stations are then compared regarding pollen taxa and their abundance on the ground and in the air.
All analyses were conducted with R (version 3.3.2; R Core Team 2016).
We applied Principal component analysis (PCA) to data of vegetation and airborne pollen in order to explore whether there are differences among sampling stations. PCA was conducted at the level of the entire sampled area (zone 4) in each station using the ‘vegan’ package (Oksanen et al. 2012). We further estimated the similarity of sampling stations; for this, we used the Jaccard (based on presence-absence) and the Bray–Curtis (based on abundance) indices.
By use of the Generalized Linear Model (GLM; Gaussian error distribution and identity link function), we modelled the pollen abundance of each pollen taxon as a function of the abundance of the woody species that belong to the pollen taxon, as estimated in the five zones of each station. Pollen values were log-transformed (natural logarithms) to meet normality. Then, we selected the best-fitting GLM model using the Akaike information criterion (AIC) to detect the optimal spatial scale. Using the package ‘boot’ (Canty and Ripley 2015), we applied the leave-one-out cross validation method to estimate the adjusted root mean squared error (RMSE) of prediction. Additionally, via GLM we modelled pollen at a station as a function of the vegetation of the six stations (sorted by distance from it); for this analysis, the data we used for each station are those corresponding to zone 4 (entire sampled area).
3 Results
3.1 Pollen in the air
A total of 27 woody taxa were represented in the airborne pollen that was sampled from all stations during the 2 years of study. Of these, Cupressaceae, Pinaceae, Quercus, Platanus and Olea were the most abundant (Table 1).
Total pollen concentration in the Zoo station (Fig. 1) is four to six times higher than in the other stations (Table 1). This is mainly due to Cupressaceae and Pinaceae pollen; particularly for the latter, pollen recorded in this station was from eight to 29 times higher than in the other stations. The quantitative aspects of the pollen spectrum differ greatly among stations, even between those that are very close to each other, as are the ‘EAm’ and ‘Uni’ stations (Fig. 1), which are located at a distance of only 500 m. For instance, Platanus pollen in ‘Uni’ is approximately eightfold that in ‘EAm’, whereas Ulmus pollen in ‘EAm’ is sevenfold that in ‘Uni’, in correspondence to the differing abundances of the two species in these two stations (Fig. 3). For these taxa, differences in pollen reflect differences in vegetation. In contrast, for several taxa present in the pollen spectrum of the stations, we did not find their representatives locally, not even in one of the stations; the latter holds true for Alnus, Carpinus, Castanea, Corylus, Ericaceae, Fagus and Salix (Table 1).
Relative abundance of pollen in the air (solid line) per station (y-axis) and of the woody plants contributing pollen in the air (bars) per zone of each station (x-axis). Zone 4, represented by the fifth bar, corresponds to the entire sampled area of each station. The value for a species’ abundance in a certain zone of a station corresponds to the number of trees in the zone of this station divided by the total number of trees recorded in zones of the same size from all stations. Ari: Aristotelous st., Kte: Ktel (bus station) Makedonia, Zoo: Zoological station, EAm: Ethnikis Aminis st., Are: Aretsou, Uni: Chimeiou Square-University; maxP is the value of the station with the highest pollen concentration for the taxon examined; maxV is the value of the station with the highest number of the taxon’s individuals
3.2 Vegetation
A total of 11,714 individuals of woody species, representing 15 pollen taxa, were counted in the six stations (Table 2). Of these, more than half belong to Pinaceae, with Cupressaceae following. The former family is represented by Pinus brutia and P. pinea and the latter by Cupressus arizonica, C. horizontalis, C. pyramidalis and Thuja orientalis. Next in abundance are Fabaceae (represented by four species), Platanus (two species) and Ulmus (two species). With only 195 individuals, ‘Kte’ is the poorest station in terms of vegetation, whereas ‘Zoo’, with 6978 individuals, is the richest.
3.3 Relationships of airborne pollen with vegetation at the local scale
The patterns of change in relative pollen concentrations and relative abundances of the taxa with woody representatives within the city of Thessaloniki are presented in Fig. 3. In the case of the taxa ensemble, the two patterns agree well. The same holds true for several of the individual taxa. This is the case of Cupressaceae, Pinaceae, Platanus, Ulmus and partially for Tilia. In the case of Acer, Fabaceae, Liquidambar, Moraceae, Olea, Populus and Rosaceae, the pollen concentration maxima do not always concur with the vegetation-abundance maxima. High values may even appear in stations, in which representatives of the pollen producing taxa are not recorded (e.g. Rosaceae). For Juglans and Quercus, pollen is traceable in many stations but their representatives are present in only one station (Quercus) or only one zone of a station (Juglans).
Principal component analysis that was conducted at the level of the entire sampled area per station showed similar ordination patterns for pollen (Fig. 4a) and vegetation (Fig. 4b). In both cases, the Zoo station is separated from the other stations along the first axis. For pollen, this is due to Pinaceae and Cupressaceae, whereas for vegetation to Pinaceae. This is because the number of Cupressaceae individuals does not differ as much among stations (Table 2) as it does for pollen (Table 1). Platanus also contributes to the ordination pattern separating stations after the pollen spectrum.
Results of Principal component analysis (PCA) for pollen and vegetation: a ordination of sampling stations with respect to pollen; b ordination of sampling stations with respect to woody species contributing pollen to the air at the spatial scale of the entire sampled area (zone 4) of each station. The insets are magnifications of the central parts of the plots, where points representing taxa overlap
Similarity estimations among stations showed that they are more similar with respect to pollen than to vegetation (Fig. 5). Also, when only presence-absence is taken into account (Jaccard index), the similarity of stations is higher than when abundance is also examined (Bray–Curtis index) for both pollen and vegetation. As with PCA, the Zoo station, having the lowest values of the similarity coefficients, differs clearly from all others with respect to pollen. Regarding vegetation, low values of the similarity coefficients are also associated with this station but not spectacularly so as when pollen is considered.
Similarity values of Bray–Curtis (upper plots) and Jaccard (lower plots) indices among the sampling stations with respect to concentration of airborne pollen and abundance of woody species contributing pollen to the air. Data analyzed correspond to the core zone (plots to the left) or to the entire sampled area per station (zone 4; plots to the right). Diamonds, bold lines and dots indicate means, medians and outliers, respectively. Ari: Aristotelous st., Kte: Ktel (bus station) Makedonia, Zoo: Zoological station, EAm: Ethnikis Aminis st., Are: Aretsou, Uni: Chimeiou Square-University
Results of the GLM that was used to explore the effect exerted by the vegetation of each of the six stations to the airborne pollen of a station showed that its airborne pollen is influenced by its own vegetation and that at the nearest station (Table 3). The average distance of the nearest stations is 2.1 km (range 0.55–5.77 km). The effect of the other stations was not significant.
The models describing the relationships between the concentration of pollen in the air and the number of individuals producing it on the ground, examined separately per zone sampled, were significant in the case of Cupressaceae, Pinaceae, Olea, Platanus and Ulmus. The best-fitting model was not always observed at the same spatial scale. For Cupressaceae, Olea and Pinaceae, this was at the scale of zone 1, for Platanus at zone 4, and for Ulmus at the core zone (Fig. 6). For these models, the slopes of the curves ranged from 0.002 (Pinaceae) to 0.056 (Ulmus). For the remaining taxa, we did not detect a significant relationship at any spatial scale. Highly significant was also the relationship of the sum of woody individuals and the sum of pollen grains over all pollen taxa. The summary statistics of models for taxa with at least one significant relationship and for the total are shown in Table 4, for all the spatial scales examined. Selection of the best-fitting model (Fig. 6) was based on AIC: it was the model with the minimum value. Models were cross validated by the adjusted RMSE of prediction. Results for the latter show good accordance with AIC (Table 4): in most cases, values minimize at the same scale.
Generalized Linear Model examining the relationship between the abundance of pollen [in ln(number of pollen grains + 1)] and the abundance of woody species (number of individuals) contributing pollen to the air for taxa with at least one significant relationship and for the total, at the spatial scale of the best-fitting model according to AIC
4 Discussion
The vegetation of the area surrounding a pollen sampling station has a decisive effect on the counts of airborne pollen locally (González and Candau 1997), but there are features of the urban environment that can act as obstacles or as enhancers to the transportation pathways and, hence, play an important role in pollen dispersal (Cariñanos et al. 2002b; Nazridoust and Ahmadi 2006; Rodriguez-Rajo et al. 2010). Results of our study that was conducted at a local scale, from 0.25 to 40 ha, indicate that the pollen spectrum reflects well the local vegetation in the city of Thessaloniki. Even at very neighbouring stations, considerable differences in the vegetation composition are expressed in the airborne pollen. For instance, this is the case for the ‘Uni’ and ‘EAm’ stations, only about 500 m apart, and the taxa Platanus and Ulmus. Areas further away may influence pollen richness but they do not play a decisive role in the pollen abundance of a station. For Thessaloniki, we found that the airborne pollen at a station is significantly affected by the vegetation of the station and of the one nearest to it, at an average distance of 2.1 km. Werchan et al. (2017) reported that vegetation data from the area at a radius of 100 m from the pollen sampler were not sufficient to explain the magnitude of pollen sedimentation locally, in the city of Berlin. Our study shows that vegetation data from an area up to 200 m from the volumetric pollen sampler could explain the magnitude of pollen in the air, locally, in the city of Thessaloniki. Our results support the emerging body of the literature suggesting that the amount of pollen at a particular site varies widely over small spatial scales within metropolitan areas (Weinberger et al. 2015).
The two most abundant taxa on the ground are Pinaceae and Cupressaceae; they are also the most abundantly represented in the pollen spectrum. For these, there is a complete match between presence on the ground and pollen in the air. Fairly consistent spatial patterns are also found for other taxa (e.g. Platanus, Ulmus), but not for Fabaceae, third in the rank with respect to vegetation, or Quercus that is third with respect to pollen. The discrepancy for Fabaceae may be explained by the fact that it is a primarily insect-pollinated taxon; also, it is represented not only by woody but also by herbaceous species in the stations. In the case of Quercus, the discrepancy is due to the fact that the taxon is present only in one station, at the edge of the peri-urban forest of Seih-Sou. Evidently, this area functions as a pollen source for some of the taxa represented in the pollen spectrum of the city but absent in its other green spaces (Krigas 2004). Nevertheless, pollen from some of the absent taxa may arrive from even further. For Carpinus, Fagus, Alnus, Castanea, we estimated the nearest sources (Karagiannakidou and Raus 1996) to be at a distance between 6 and 15 km. Another discrepancy was found for the dioecious taxon Populus. This is a rather widely planted taxon in the city, but the pattern of its airborne pollen does not agree well with that of its presence on the ground. As our specific search revealed, most of the individuals that are planted in the city are usually female, and this may be the reason for this discrepancy.
The micro-environmental conditions in a given urban district can affect the quality of life of its residents. Comparison of pollen counts in areas with different levels of urbanization reveals differences in the quantity and number of the pollen types recorded (Cariñanos et al. 2002a), the daily pollen cycles (Kasprzyk 2006; Šikoparija et al. 2006) and plant growth and productivity (Ziska et al. 2004). In Thessaloniki, the Zoo station is the most loaded with pollen. This station is located in a semi-natural environment, at the edge of the Seih Sou peri-urban forest that covers around 3000 ha. Evidently, the area around this station should be avoided during the pollen season by those who are sensitive to the pollen produced by species in fair quantities at this part of the city.
Several earlier studies provided evidence that local vegetation is a driver of spatial variation for airborne pollen. Gonzalo-Garijo et al. (2006) used information from a tree census in their study area and found that the site with the highest sycamore pollen concentration was closer to major stands of sycamore trees; however, exact distances and densities of the stands were not reported. Katelaris et al. (2004) conducted vegetation surveys in 2000 m circular areas around three sampling sites and found differences in local vegetation, which they paralleled with pollen concentrations; however, no taxa-specific information was reported. In a study by Nowak et al. (2012), one site was located at 100 m and another at 6.5 km from the closest sycamore trees; annual sycamore pollen sums were 10–20 times lower at the site that was farthest from the sycamore trees, suggesting that most of the sycamore pollen did not travel far from its source and that the local sources were the most important drivers of local pollen concentrations. Other studies also mentioned that local vegetation data may help explain pollen results but did not include systematic investigations of this relationship (Weinberger et al. 2015).
To address intra-urban spatial heterogeneity of pollen concentrations, we sampled pollen in several stations within the city of Thessaloniki and studied in a very detailed way the local vegetation. The results we took allow quantitative estimations of pollen changes to be expected once vegetation changes are planned or detected. For every additional individual of Cupressaceae, Pinaceae, Platanus, Ulmus, and Olea, we estimate concomitant increases of pollen in the air by approximately 0.7, 0.2, 2, 6 and 5%, respectively. Such quantified relationships allow us to evaluate the impact of increasing species abundances on the allergenic pollen that they produce and, hence, on the residents of an area. This is important to know especially for the urban and peri-urban environment that is usually humanly managed. Identification of the sources of airborne pollen and quantification of their contribution are essential for a more efficient design of urban parks and gardens, enabling specific recommendations to be made regarding the most appropriate ornamentals for future green spaces (Rojo et al. 2015; RNSA 2016). Such quantifications also provide insight on the type and magnitude of associated changes to be expected once pollen or vegetation changes are recorded. For instance, if increases in pollen abundance are not justified in a quantitative way by land use and vegetation changes, climate change could be considered a plausible cause for further exploration.
Pollen productivity, which is the primary factor influencing pollen abundance, differs among species. Differences may be large, as those detected at the level of flower between Corylus avellana (3.9 × 103 pollen grains) and Olea europaea (1.3 × 105) (Damialis et al. 2011). Large differences are also detected at higher levels, for instance at that of the individual, between unrelated species like Juglans regia (109) and Quercus rotundifolia (1011) (Tormo-Molina et al. 1996), but also between co-generic species like representatives of Cupressus, viz. C. sempervirens (6.4 × 1010) and C. marcocarpa (1012) (Hidalgo et al. 1999). In other cases, differences may not be pronounced. Charalampopoulos et al. (2013) found comparable amounts of pollen, approximately 104 grains per flower, 108–109 per m2 of crown surface and 1010–1011 per individual produced by Quercus coccifera, Q. ilex, Pinus pinea and P. heldreichii. Pollen productivity may also differ within species, for populations differing in elevation, exposure or year of study (Charalampopoulos et al. 2013; Damialis et al. 2011). Pollen production at higher levels (e.g. individual) is more variable than at lower levels (e.g. anther, flower) and, in general, of lower accuracy, because it requires collection of several data other than pollen (size of individuals, of the flower production, etc.), representative samplings at all required levels (at that of flower, inflorescence, crown, etc.), approximations (e.g. for the shape of the crown) and complex calculations. How well the sizes of pollen production at these levels match the patterns of airborne pollen concentrations is an open issue. Charalampopoulos et al. (2013) found Pinaceae to be under-represented in the airborne pollen spectrum of Mt Olympos. Similarly, in the study area of Thessaloniki, Pinaceae are represented by three times as many individuals as Cupressaceae but their pollen in the air is half that of Cupressaceae. Nevertheless, the amounts of pollen produced at the level of individual by Pinus and Cupressus species are of the same range (Charalampopoulos 2017; Charalampopoulos et al. 2013; Hidalgo et al. 1999). Several factors may be responsible for non-matching cases, both intrinsic and extrinsic, such as differences in size and age of individuals and, hence, in the amounts of pollen that they produce, in the species with differing production sizes making each time the same pollen taxon, in the environmental factors prevailing, and also in potentially differing distributions of the individual pollen types in the complex environment of a city.
In conclusion, we found that the local vegetation is the driver of the spatial variation of airborne pollen within the city of Thessaloniki and we could quantify the relationship between the number of pollen grains in the air and the number of individuals of the producing species on the ground for the five most abundantly represented taxa in the pollen spectrum of the city. Our study adds to previous attempts to quantify the vegetation–pollen relationships (Cariñanos et al. 2014; Charalampopoulos et al. 2013; Fotiou et al. 2011; González and Candau 1997; Rodriguez-Rajo et al. 2010) and thus get a better understanding of pollen patterns and dispersal. Further research is needed to explore how well the relationships detected hold outside the domain for which we have data, as well as under different environmental conditions and/or with different species assemblages making up the pollen taxa.
References
Alcázar, P., Cariñanos, P., De Castro, C., Guerra, F., Moreno, C., Domínguez-Vilches, E., et al. (2004). Airborne plane tree (Platanus hispanica) pollen distribution in the city of Córdoba, South-Western Spain, and possible implications on pollen allergy. Journal of Investigational Allergology and Clinical Immunology, 14, 238–243.
Beckett, K. P., Freer-Smith, P. H., & Taylor, G. (2000). Particulate pollution capture by urban trees: Effect of species and windspeed. Global Change Biology, 6, 995–1003.
British Aerobiology Federation. (1995). Airborne pollens and spores. A guide to trapping and counting. Rotherham: National Pollen and Hayfever Bureau.
Canty, A., & Ripley, B. (2015). Boot: Bootstrap R (S-Plus) Functions. R package version 1.3-17.
Cariñanos, P., Alcázar, P., Galán, C., & Dominguez, E. (2002a). Privet pollen (Ligustrum sp.) as potential cause of pollinosis in the city of Cordoba, southwest Spain. Allergy, 57, 1–7.
Cariñanos, P., & Casares, M. (2011). Urban green zones and related pollen allergy: A review. Guidelines for designing spaces of low allergy impact. Landscape and Urban Planning, 101, 205–214.
Cariñanos, P., Casares-Porcel, M., & Quesada-Rubio, J. M. (2014). Estimating the allergenic potential of urban green spaces: A case-study in Granada, Spain. Landscape and Urban Planning, 123, 134–144.
Cariñanos, P., Galán, C., Alcázar, P., & Dominguez, E. (2008). Classification, analysis and interaction of solid airborne particles in urban environments. In A. G. Kungolos, C. A. Brebbia, & M. Zamorano (Eds.), Environmental toxicology II (pp. 317–325). Southampton: WIT Press.
Cariñanos, P., Galán, C., Alcázar, P., & Domínguez, E. (2007). Analysis of the solid particulate matter suspended in the atmosphere of Córdoba, south-western Spain. Annals of Agricultural and Environmental Medicine, 14, 159–160.
Cariñanos, P., Sánchez-Mesa, J. A., Prieto-Baena, J. C., Lopez, A., Guerra, F., Moreno, C., et al. (2002b). Pollen allergy related to the area of residence in the city of Córdoba, south-west Spain. Journal of Environmental Monitoring, 4, 734–738.
Charalampopoulos, A. (2017). Pollen-scapes in natural and urban environments: Production and atmospheric circulation of pollen grains at different heights and elevations (Ph.D. thesis, in Greek). Thessaloniki: Aristotle University of Thessaloniki.
Charalampopoulos, A., Damialis, A., Tsiripidis, I., Mavrommatis, T., Halley, J. M., & Vokou, D. (2013). Pollen production and circulation patterns along an elevation gradient in Mt Olympos (Greece) National Park. Aerobiologia, 29, 455–472.
Charpin, D., Calleja, M., Lahoz, C., Pichot, C., & Waisel, Y. (2005). Allergy to cypress pollen. Allergy, 60, 293–301.
D’Amato, G., Cecchi, L., D’Amato, M., & Liccardi, G. (2010). Urban air pollution and climate change as environmental risk factors of respiratory allergy: An update. Journal of Investigational Allergology and Clinical Immunology, 20, 95–102.
Damialis, A., Fotiou, C., Halley, J. M., & Vokou, D. (2011). Effects of environmental factors on pollen production in anemophilous woody species. Trees, 25, 253–264.
Damialis, A., Halley, J. M., Gioulekas, D., & Vokou, D. (2007). Long-term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmospheric Environment, 41, 7011–7021.
Dzierzanowski, K., Popek, R., & Gawronska, H. (2011). Deposition of particulate matter of different size fraction on leaf surfaces and in waxes of urban forests species. International Journal of Phytoremediation, 13, 1037–1046.
ESRI. (2011). ArcGIS desktop: Release 10. Redlands, CA: Environmental Systems Research Institute.
Euro + Med (2006): Euro + Med PlantBase—the information resource for Euro-Mediterranean plant diversity. Published on the Internet http://ww2.bgbm.org/EuroPlusMed/. Accessed May 17, 2017.
Fotiou, C., Damialis, A., Krigas, N., Halley, J. M., & Vokou, D. (2011). Parietaria judaica flowering phenology, pollen production, viability and atmospheric circulation, and expansive ability in the urban environment: Impacts of environmental factors. International Journal of Biometeorology, 55, 35–50.
González, F. J., & Candau, P. (1997). Study on pollen content in the air of Seville (SW Spain): The pollen spectrum and its relation with vegetation and anthropogenic activity. Botanica Helvetica, 107, 221–237.
Gonzalo-Garijo, M. A., Tormo-Molina, R., Muñoz-Rodríguez, A. F., & Silva-Palacios, I. (2006). Differences in the spatial distribution of airborne pollen concentrations at different urban locations within a city. Journal of Investigational Allergology and Clinical Immunology, 16, 37–43.
Google Earth Pro v.7.1.7.2602 [April 20, 2017] Thessaloniki, Greece. 40°37’11.53”N, 22°55’36.78”E, Eye alt 13.12 km, Digital Globe, 2017, http://www.earth.google.com. Accessed April 30, 2017.
Grant, G. (2012). Ecosystem services come to town: Greening cities by working with nature. Chicester: Wiley.
Green, R. J., & Davis, G. (2005). The burden of allergic rhinitis. Current Allergy and Clinical Immunology, 18, 176–178.
Grewling, Ł., Šikoparija, B., Skjøth, C., Radišić, P., Apatini, D., Magyar, D., et al. (2012). Variation in Artemisia pollen seasons in Central and Eastern Europe. Agricultural and Forest Meteorology, 160, 48–59.
Haberle, S. G., Bowman, D. M., Newnham, R. M., Johnston, F. H., Beggs, P. J., Buters, J., et al. (2014). The macroecology of airborne pollen in Australian and New Zealand urban areas. PLoS ONE, 9, e97925.
Hidalgo, P. J., Galán, C., & Domínguez, E. (1999). Pollen production of the genus Cupressus. Grana, 38, 296–300.
Hirst, J. M. (1952). An automatic volumetric spore trap. Annals of Applied Biology, 39, 257–265.
Hruska, K. (2003). Assessment of urban allergophytes using and allergen index. Aerobiologia, 19, 107–111.
Jim, C. Y. (2013). Sustainable urban greening strategies for compact cities in developing and developed economies. Urban Ecosystems, 16, 741–761.
Karagiannakidou, V., & Raus, T. (1996). Vascular plants from Mount Chortiatis (Macedonia, Greece). Willdenovia, 25, 487–559.
Kasprzyk, K. I. (2006). Comparative study of seasonal and intradiurbnal variation in airborne pollen in urban and rural areas. Aerobiologia, 22, 185–195.
Katelaris, C. H., Burke, T. V., & Byth, K. (2004). Spatial variability in the pollen count in Sydney, Australia: Can one sampling site accurately reflect the pollen count for a region? Annals of Allergy, Asthma & Immunology, 93, 131–136.
Krigas, N. (2004). Flora and human activities in the area of Thessaloniki: Biological approach and historical considerations (Ph.D. thesis, in Greek). Thessaloniki: Aristotle University of Thessaloniki.
Latinopoulos, D., Mailios, Z., & Latinopoulos, P. (2016). Valuing the benefits of an urban park project: A contingent valuation study in Thessaloniki, Greece. Land Use Policy, 55, 130–141.
Livesley, S. J., McPherson, G. M., & Calfapietra, C. (2016). The urban forests and ecosystem services: Impacts on water, heat and pollution cycles at the tree, street and city scale. Journal of Environmental Quality, 45, 119–124.
Med-Checklist (2006). A critical inventory of vascular plants of the circum-mediterranean countries. Published on the Internet http://ww2.bgbm.org/mcl/. Accessed May 22, 2017.
Nazridoust, K., & Ahmadi, G. (2006). Airflow and pollutant transport in Street canyon. Journal of Wind Engineering and Industrial Aerodynamics, 94, 491–522.
Nicolau, N., Siddique, N., & Custovic, A. (2005). Allergic disease in urban and rural populations: Increasing prevalence with increasing urbanization. Allergy, 60, 1357–1360.
Nowak, M. A., Szymanska, L., & Grewling, L. (2012). Allergic risk zones of plane tree pollen (Platanus sp.) in Poznan. Postepy Dermatologii I Alergologii, 29, 156–160.
O’Rourke, M. K., & Lebowitz, M. D. (1984). A comparison of regional atmospheric pollen with pollen collected at and near homes. Grana, 23, 55–64.
Oksanen, J., Blanchet, G., Kindt, R., Minchin, P. R., Legendre, P., O’Hara, B., & Suggests, M. A. S. S. (2012). Vegan: Community Ecology Package. R package Version 2.0–3. Available at: http://cran.r-project.org/.
Pawankar, R. (2014). Allergic diseases and asthma: A global public health concern and call to action. World Allergy Organization Journal, 7, 12.
Peel, R. G., Hertel, O., Smith, M., & Kennedy, R. (2013). Personal exposure to grass pollen: Relating inhaled dose to background concentration. Annals of Allergy, Asthma & Immunology, 111, 548–554.
Puc, M. (2011). Threat of allergenic airborne grass pollen in Szczecin, NW Poland: The dynamics of pollen seasons, effect of meteorological variables and air pollution. Aerobiologia, 27, 191–202.
R Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reed, S. D., Lee, T. A., & McCrory, D. C. (2004). The economic burden of allergic rhinitis: A critical evaluation of the literature. Pharmacoeconomics, 22, 345–361.
RNSA (2016). Vegétation en ville: Guide d’ information. http://www.vegetation-en-ville.org/wp-content/themes/vegetationenville/PDF/Guide-Vegetation.pdf.
Rodriguez-Rajo, F. J., Fernández-sevilla, D., Stach, A., & Jato, V. (2010). Assessment between pollen seasons in areas with different urbanization level related to local vegetation sources and differences in allergen exposures. Aerobiologia, 26, 1–4.
Rojo, J., Rapp, A., Lara, B., Fernández-González, F., & Pérez-Badia, R. (2015). Effect of land uses and wind direction on the contribution of local sources to airborne pollen. Science of the Total Environment, 538, 672–682.
Shackleton, S., Chinyimba, A., Hebinck, P., Shackleton, C., & Kaoma, H. (2015). Multiple benefits and value of trees in urban landscapes in two towns in northern South Africa. Landscape and Urban Planning, 136, 76–86.
Šikoparija, B., Radisik, P., Pejak, T., & Simié, S. (2006). Airborne grass and ragweed pollen in the southern pannonian valley: Consideration of rural and urban environments. Annals of Agricultural and Environmental Medicine, 13, 263–266.
Skjøth, C. A., Ørby, P. V., Becker, T., Geels, C., Schlünssen, V., Sigsgaard, T., et al. (2013). Identifying urban sources as cause of elevated grass pollen concentrations using GIS and remote sensing. Biogeosciences, 10, 541–554.
Tormo-Molina, R., Rodríguez, A. M., Palaciso, I. S., & López, F. G. (1996). Pollen production in anemophilous tree. Grana, 35, 38–46.
von Döhren, P., & Haase, D. (2015). Ecosystem disservices research: a review of the state of the art with a focus on cities. Ecological Indicators, 52, 490–497.
Walters, S. M., Alexander, J. C. M., Brady, A., Brickell, C. D., Cullen, J., Green, P. S., Heywood, V. H., Matthews, V. A., Robson, N. K. B., Yeo, P. F., & Knees, S. G. (Eds) (1989). The European Garden Flora volume III. Dicotyledons (Part I). Cambridge: Cambridge University Press.
Weinberger, K. R., Kinney, P. L., & Lovasi, G. S. (2015). A review of spatial variation of allergenic tree pollen within cities. Arboriculture & Urban Forestry, 41, 57–68.
Werchan, B., Werchan, M., Mücke, H. G., Gauger, U., Simoleit, A., Zuberbier, T., et al. (2017). Spatial distribution of allergenic pollen through a large metropolitan area. Environmental Monitoring and Assessment, 189, 169.
WHO (World Health Organization) (2013). Review of Evidence on Health Aspects of Air Pollution - REVIHAAP. First Results. Copenhagen, Denmark:WHO Regional Office for Europe.
Ziello, C., Sparks, T. H., Estrella, N., Belmonte, J., Bergmann, K. C., Bucher, E., et al. (2012). Changes to airborne pollen counts across Europe. PLoS ONE, 7, e34076.
Ziska, L. H., Bunce, J. A., & Goins, E. W. (2004). Characterization of an urban–rural CO2/temperature gradient and associated changes in initial plant productivity during secondary succession. Oecologia, 139, 454–458.
Acknowledgements
This project was funded by the programs ‘Aristeia Scholarship 2014’ and ‘Action C: Supporting Research activity of Basic Research 2013’ of the Aristotle University of Thessaloniki (AUTH), Greece.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Charalampopoulos, A., Lazarina, M., Tsiripidis, I. et al. Quantifying the relationship between airborne pollen and vegetation in the urban environment. Aerobiologia 34, 285–300 (2018). https://doi.org/10.1007/s10453-018-9513-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-018-9513-y