Abstract
Man-made reservoirs are important freshwater ecosystems as they are globally common and share features of both standing and running waters. In streams and lakes, crayfish are an important component of freshwater ecosystems due to their habitat-modifying behaviour, substantial size, omnivorous feeding and often high abundance; however, their trophic role in reservoirs is not known. We evaluated the distribution and diet of noble crayfish (Astacus astacus) in the canyon-shaped, oligotrophic Nýrsko reservoir in West Bohemia region, Czech Republic. Using stable isotope analysis, we quantified the trophic level of all major components of the reservoir food web and investigated seasonal isotopic variation and how the trophic role of noble crayfish varied with habitat and ontogeny. Crayfish were an important food source for both predatory and omnivorous fish and consumed food sources from multiple trophic levels, including detritus, algae, zoobenthos and other crayfish. Throughout ontogeny, crayfish had similar levels of carnivory, but cannibalism was more prevalent in adult crayfish, while juveniles and sub-adults fed more on other zoobenthos. Moreover, crayfish had high feeding plasticity in time, as the relative importance of dominant food sources varied with season. Their feeding plasticity was especially evident in crayfish populations from different habitats, which adapted their feeding strategy to local resources. In addition, pelagic source usage increased with the depth as detritus and algae usage decreased. Proportion of females increased with the depth, while population density showed a unimodal response to the depth gradient. These findings indicate that crayfish are indeed ecologically important species with both direct and indirect roles in the trophic web of this reservoir ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trophic webs in almost every ecosystem contain many units which link together (Vander Zanden and Rasmussen 2001), and in aquatic systems crayfish, if present, are one of the most important (Momot 1995). Crayfish are abundant omnivores and can represent up to 85% of benthic biomass (Neveu 2009; Nyström et al. 2001). They are often considered ecosystem engineers in freshwaters due to their ability to change inhabited ecosystems to suit their requirements (Collier et al. 1997; Crandall and Buhay 2007; Nyström and Granéli 1996). Their influence on trophic webs is both direct and indirect: they feed on species belonging to various trophic levels and are an important prey item for a wide range of predators, thus it can affect cycling of nutrients across trophic levels (Dorn and Wojdak 2004; Lodge et al. 2012; Pringle and Hamazaki 1998; Twardochleb et al. 2013).
Omnivory has been recognized as an important aspect of both aquatic and terrestrial food webs affecting their length and quality and availability of food source for higher trophic levels (Arts et al. 2009; Polis and Strong 1996). Compared to specialist feeders (predators, herbivores), the omnivorous diet of crayfish makes evaluation of their exact trophic position and role in the food web difficult. Crayfish have the capability to exploit a large variety of food sources including detritus, algae, macrophytes, zoobenthos and dead fish (Twardochleb et al. 2013); therefore, their trophic niche width (Post 2002) could vary, from narrow to wide, according to food source availability and season (Alcorlo et al. 2004; Hollows et al. 2002). For example, Alcorlo et al. (2004) found detritus as dominant food source for red swamp crayfish (Procambarus clarkii; Girard, 1852) in winter period, and then they increase consumption of zoobenthos and fish in other seasons, while Paranephrops zealandicus (White 1842) consumes more allochthonous detritus in the autumn‐winter period, in winter period increases consumption of zoobenthos, and lately in spring and summer switches to more autochthonous sources (Hollows et al. 2002). In some cases, when food resources are limited, crayfish could disperse over land to new areas (Herrmann et al. 2018) or exploit terrestrial food sources (Grey and Jackson 2012). Moreover, increase in depth can also change food source preferences from littoral to pelagic food sources (Ruokonen et al. 2012). In addition to dietary changes with resource availability, crayfish feeding preferences can change during ontogenetic development. Momot (1995) found that juvenile crayfish are more carnivorous than older specimens. On the other hand, Stenroth et al. (2006) found no significant ontogenetic diet shift.
Although lakes and reservoirs share some features (presence of pelagic zone, reduced flow), reservoirs cannot be considered as equivalent to natural lakes (Irz et al. 2002; Kalff 2002). Compared to lakes, man-made reservoirs have shorter retention times, higher water level fluctuation, distinct longitudinal gradients (inlet, transitional and lake part) and, typically, large organic loads due to their usual position down in watersheds (Irz et al. 2002; Kalff 2002). Canyon-shaped reservoirs, which are common worldwide, have steep banks and therefore limited littoral areas and diversity of habitats. For these reasons, reservoirs are more like hybrid systems between rivers and lakes (Gido et al. 2009; Kalff 2002). Although previous studies have explored the trophic role of crayfish in lakes (Dorn and Wojdak 2004; Ercoli et al. 2014; Lipták et al. 2019; Ruokonen et al. 2012) and streams (Creed 1994; Creed and Reed 2004; Usio and Townsend 2000, 2002), there is a lack of information about their role in reservoirs. There are few studies dealing with bioaccumulation of heavy metals in crayfish occurring in reservoirs (Kuklina et al. 2014; Stewart et al. 2008). Also, there are studies of predator–prey interaction where crayfish were important prey item for predatory fish population (Hepworth and Duffield 1987; Saiki and Ziebell 1976; Winters and Budy 2015). In addition, some studies described establishment of invasive crayfish in reservoirs (Ahyong and Yeo 2007; Light 2003). Yet, Duriš and Smutný (2000) explored depth distribution of crayfish in a reservoir in the Czech Republic where they found that sex ratio varies with the depth. However, more comprehensive studies dealing with trophic role of crayfish in reservoirs are missing. Thus, there are gaps in our basic knowledge of crayfish trophic niches and dietary plasticity in reservoirs.
In lotic as well as lentic environments, crayfish are important units of the ecosystem, although their role can differ among these ecosystems (Bronmark et al. 1992; Mather and Stein 1993; Nyström et al. 2006). For example, in lentic ecosystems the abundance of crayfish population can be driven by presence of specific substratum together with predator biomass. However, in lotic ecosystems the type of substrate was important only if biomass of predatory fish was low, suggesting combined effect of substratum and predator biomass on crayfish abundance (Nyström et al. 2006). Moreover, in contrast to lotic ecosystems, crayfish in lentic habitats can inhabit often only littoral areas, while deepest zones cannot be inhabited due to oxygen depletion (Momot 1995; Reynolds et al. 2013). Last but not least, predator pressure in shallow lotic ecosystems is higher in comparison with lakes, due to lower protection of shelters in substratum. This is probably given by higher encounter rate of predatory fish in lotic ecosystems than in large and deep lentic ecosystems where predatory fish have to exploited larger area to find a prey and spend more time in pelagic zone (Nyström et al. 2006). On the other hand, there are also similarities between lotic and lentic ecosystems regarding to crayfish population. Generally, there is agreement among studies suggesting that crayfish populations in lentic and lotic ecosystems are driven by predation, resource and shelter availability as well as physico-chemical parameters and their interactions (Englund 1999; Jones and Momot 1981; Lodge 1994; Usio and Townsend 2000). Based on previous studies, we can expect that in reservoirs, the role and habits of crayfish will be more similar to lentic ecosystems as the reservoirs are more similar to lentic than lotic environment.
In this study, we investigated the role of noble crayfish Astacus astacus (L., 1758) in the trophic web of Nýrsko reservoir in Czech Republic. We hypothesized that in reservoirs (1) crayfish use food sources at multiple trophic levels, (2) crayfish transfer energy from lower to higher trophic levels, (3) zoobenthos and algae are their dominant food sources in the shallow parts of the reservoir due their higher biomass, (4) food composition varies with crayfish ontogeny, (5) and with season and habitats and (6) size and sex ratio vary with depth gradient. To address these expectations, we sampled crayfish populations in different habitats, at different depths and in different seasons. To quantify the trophic position of crayfish and other major components of the reservoir trophic web, we analysed the ratio of the stable isotopes carbon (δ13C) and nitrogen (δ15N) in their tissues and estimated their putative food source usage.
Materials and methods
Site description
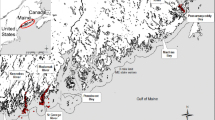
Nýrsko reservoir is an oligotrophic water supply reservoir (A = 1.48 km2, max depth = 34 m) in the West Bohemia region of Czech Republic (49° 15′ 27″ N, 13° 8′ 46″ E) with a well-established population of noble crayfish Astacus astacus (L., 1758), a species native to the watershed. The left side of the canyon-shaped reservoir gradually slopes to the bottom which is composed of fine particles and covered by macrophytes (hereafter referred to as ‘beach habitat’). The right side is steeper, with stony shores (cobble and boulders) in the lower section of the reservoir, and a mix of sand and stony spots in the middle section (abbreviated as ‘stony slopes habitat’). The reservoir also has an inlet section, with muddy bottoms on both shores (Fig. 1).
Field sampling
Trophic web of Nýrsko reservoir
To gain insight into the trophic web of the reservoir and to reveal the position of noble crayfish in the web, all potential food sources of each trophic level were sampled in the first week of August 2015. Fish were collected by angling. Crayfish were caught manually with handheld nets as well as by scuba diving, and with traps baited with fresh fish meat placed along the shoreline in late afternoon and collected the following morning. Bulk zooplankton samples were collected using a net (mesh size 250 µm) pulled vertically through the water column. Zoobenthos was collected down to 1 m depth using a hand net (mesh size 500 µm). Macrophytes, periphyton and detritus were collected by hand from the shoreline. The collecting methods are common in mentioned biota, but like all collecting method it can bring selectivity of the sampling and thus it can also produce bias in samples. However, it is important evaluate whether bias produced by given sampling method can affect key results. In this study, we did not identify such risk resulted from sampling method, and thus these methods were used in this study. All samples were kept on dry ice immediately after collection and then transferred to the laboratory freezer (− 30 °C) until further processing for stable isotope analysis (SIA) of carbon (δ13C) and nitrogen (δ15N). Fish and crayfish were measured and weighed to the nearest 0.1 mm and 0.1 g (Table S1 and S2). A piece of white dorsal muscle tissue of fish and a piece of abdominal muscle tissue of crayfish were used for SIA as recommended by Stenroth et al. (2006). Samples of zoobenthos, terrestrial detritus and macrophytes were identified to species or genus level for analysis. Later, to analyse energy flow in the food web, fish and zoobenthos species were assigned into functional groups (Table S1, Table S4 and Table S5).
Habitat and ontogeny differences and seasonal isotopic variation in noble crayfish population
To quantify seasonal variation in the isotopic composition of crayfish, samples of them were collected three times (beginning of May, beginning of August and mid October 2015). Crayfish were trapped at 3 m depth. For seasonal comparisons, we only used crayfish collected from stony slopes habitat, which had higher abundances of crayfish even in the coldest season. Only sub-adult and adult crayfish were compared across seasons and habitats due to the insufficient number of juveniles caught across the seasons. In 2015, the water level fluctuation was low and thus all habitats in reservoir were similarly available over the season (Figure S1).
The samples collected in August were also used to investigate differences in isotopic composition with habitat and ontogeny. To study differences in crayfish diets between habitats, beach habitat and stony slopes habitat in the dam part of the reservoir were selected (Fig. 1). These habitats were internally homogenous, at a similar distance from the dam and of sufficient size (300 m long) not to be influenced by neighbouring habitats. To reveal any differences in diet during ontogeny, juveniles, sub-adults and adults were sampled. Life stage was determined by carapace length (CL; juvenile range 23.2–25.2 mm, sub-adult range 26.2–33.9 mm, adult range 31.8–58.9 mm), combined with the presence or absence of secondary sexual characteristics. Due to the insufficient number of juvenile crayfish in the stony slopes habitat, only crayfish caught from beach habitat were used. Adult and sub-adult crayfish were trapped at 3 m depth, and juvenile crayfish, for which traps were ineffective, were caught at the same depth by scuba diving at night.
Depth gradient
To investigate the diet of noble crayfish along a depth gradient, in the first week of August we sampled six different depths (3, 6, 9, 12, 15, 20 m) by scuba diving. The thermocline was at ~ 8 m depth, but there was no oxygen deficiency zone. In the surface layer, oxygen varied between 7 and 9 mg/L and at 20 m between 4.5 and 6.5 mg/L. At each depth, ten traps were placed 3–5 m apart (total transect length 50 m) in the late afternoon and collected the following morning. This approach presumably limited any potential bias from mixing crayfish from different depths. According to Abrahamsson and Goldman (1970) similar traps as we used to attract crayfish from a range of 12–13 m2, or distance of ca. 2 m. Crayfish from each depth were divided by sex and size class and then measured (carapace length; CL) and weighed to the nearest 0.1 mm and 0.1 g, respectively (Table S3). In addition to crayfish sampling, all available food sources from each depth were collected for SIA. For analysis, we focused on stony slopes habitat due to the low abundance of crayfish in beach habitat where no specimens were caught deeper than 3 m. Only sub-adults and adults were found in the stony slopes habitat, so the juvenile stage was not included.
Stable isotope analyses
All samples for stable isotope analysis were dried at 50 °C for 48 h to constant weight and ground to a fine homogenous powder. Approximately 0.6 mg of animal samples and 1.5 mg of plant and detritus samples were weighed (at the precision of 0.001 mg) into tin cups. Stable isotope analyses were performed at the University of Jyväskylä using a Carlo Erba Flash EA 1112 elemental analyser connected to Thermo Finnigan DELTAplus and Advantage continuous-flow isotope ratio mass spectrometer (Thermo Electron Corporation, Waltham, MA, USA).
Vienna Pee Dee belemnite and atmospheric N2 were used as reference standards for carbon and nitrogen. To control for instrument stability after every 6 samples, northern pike Esox lucius L., 1758 muscle tissue and birch Betula pendula R. leaves of known isotopic compositions were run as internal working standards for animal and plant samples. Results are expressed using the conventional δ notation as parts per thousand difference from the international standards. Analytical precision was < 0.1‰ for δ13C and < 0.3‰ for δ15N.
Trophic position of each species/functional group was calculated using the formula of Anderson and Cabana (2007):
where Tp is the trophic position of organism, δ15N sample represents the nitrogen isotope value of a given organism, δ15N baseline is the isotopic ratio from several individuals of filter feeders (the pea mussel Pisidium sp.), 3.23 is the nitrogen isotope fractionation between trophic levels (Vander Zanden and Rasmussen 2001), and EP is the trophic position of the organism selected as baseline. According to the recommendation by Anderson and Cabana (2007), we used a long-lived filter feeder (Pisidium) considered a first level consumer (EP = 2) as the baseline organism, which integrates stable isotope signals over the season.
Statistical analyses
Trophic web of Nýrsko reservoir
To assess the contribution of the different food sources to the isotopic signature of each target organism or functional group, a separate Bayesian mixing model (Moore and Semmens 2008) with a specific number of putative sources was run in MixSIAR package (Stock and Semmens 2016) in R (R Core Team 2016).
For predatory fish (Esox lucius; Leuciscus aspius (L., 1758); Perca fluviatilis L., 1758), a six-source mixing model was produced (zoobenthos, crayfish (sub-adult and adult), juvenile crayfish, juvenile perch, rudd (Scardinius erythrophthalmus; L., 1758) and roach (Rutilus rutilus; L., 1758). For omnivorous fish (roach and rudd), a six-source mixing model including algae, macrophytes, detritus, zooplankton, zoobenthos and juvenile crayfish was applied. For crayfish (adult), a six-source mixing model including algae, detritus, macrophytes, pelagic sources (zooplankton), zoobenthos and juvenile crayfish was run. Zooplankton were used as a pelagic source due to their sedimentation on the bottom. Lastly, predatory zoobenthos was divided into two groups due to their clear separation in carbon signal (Table S5), with predatory zoobenthos 1 consisting of Erpobdella octoculata and Helobdella stagnalis, and predatory zoobenthos 2 consisting of Laccophilus hyalinus, Nebrioporus elegans, Sympetrum sp., Haemopis sanguisuga, Hygrotus versicolor and Nepa cinerea. For both groups of predatory zoobenthos, we applied a three-source mixing model including juvenile crayfish, zoobenthos and zooplankton. As recommended by Vander Zanden and Rasmussen (2001), our models assumed the fractionation factors 3.23 ± 0.41‰ for δ15N and 0.47 ± 1.23‰ for δ13C for animals, and 2.4 ± 0.42‰ for δ15N and 0.40 ± 0.28‰ for δ13C for detritus and macrophytes (McCutchan et al. 2003). Although these fractionation factors values were not established specifically for crayfish, we used fractionation factors from literature sources as many previous studies did.
Habitat and ontogeny differences and seasonal isotopic variation in noble crayfish population
We calculated the trophic niche width of noble crayfish over season, habitat and ontogeny using standard ellipse area in carbon and nitrogen isotope space with the SIBER package. The overlap between the ellipses from different depths was calculated as a proportion of the non-overlapping area of ellipses (Jackson et al. 2011). Additionally, we ran a six-source Bayesian mixing model (algae, detritus, macrophytes, pelagic sources, zoobenthos and juvenile crayfish) to test for differences in putative food source usages between noble crayfish life stages.
Depth gradient
To assess the contribution of the putative food sources along the depth gradient, we used a Bayesian mixing model with depth as a continuous factor (Francis et al. 2011; Stock and Semmens 2016). Algae, detritus and pelagic source (zooplankton) were used in the three-source mixing model. Additionally, the standard ellipse area was calculated and the overlap between the ellipses was estimated in the same manner as for seasonal, habitat and ontogeny differences.
To test for any differences in size, weight and sex of crayfish among different depths, we used generalized linear model (GLM) with the gamma distribution. The final model was determined by sequential deletion of the least significant explanatory parameters (or interaction terms) from the full model. Parameter significance was evaluated using F-tests from analysis of deviance.
Results
Trophic web of Nýrsko reservoir
The food web of Nýrsko reservoir consists of four trophic levels, with predatory fish at the apex position and collectors at the bottom position together with primary sources (Fig. 2; Table S1). Omnivorous roach, rudd and crayfish occupied the central part of the trophic pyramid, while predatory zoobenthos, grazers and filterers were situated in lower trophic positions between collectors and roach and rudd.
Results from MixSIAR models suggest that crayfish were an important food source for both predatory fish and omnivorous fish (Table 1). The diet of predatory fish, here presented as mean proportions of putative food source usage (%), consisted of mixture of adult and sub-adult crayfish (28%), juvenile perch, (18%), juvenile crayfish (18%), rudd (17%), zoobenthos (13%) and roach (4%). The diet of omnivorous species was also largely composed of crayfish. For roach, juvenile crayfish were the most important food source (42%), but they also fed on macrophytes (14%), algae (13%), zooplankton (11%), zoobenthos (10%) and detritus (10%) (Table 1). In rudd, juvenile crayfish were the most important food source (23%), followed by zooplankton (20%) and detritus (23%), while zoobenthos (11%), macrophytes (12%) and algae (12%) were less utilised.
For crayfish, detritus and juvenile crayfish accounting for 21 and 20%, respectively, seemed to be an important food source (Table 2). Other putative food sources (algae, macrophytes, pelagic sources, zoobenthos) had a similar contribution ranging from 14 to 17% of their diet. In the predatory zoobenthos 1, 85% of their diet consisted of other zoobenthos (Table 2). Juvenile crayfish and zooplankton were also used to a lesser extent (8 and 8%, respectively). In the second predatory zoobenthos group (predatory zoobenthos 2), zoobenthos was still important (47%; Table 2), but there was increased use of zooplankton (35%) and juvenile crayfish (18%).
Habitat and ontogeny differences and seasonal isotopic variation in noble crayfish population
The estimated crayfish diet changed throughout ontogeny (Table 3). While in juvenile and sub-adult crayfish zoobenthos accounted for 38% and 30% of their diet, respectively, this decreased to 14% in adults. Instead, the cannibalism of juvenile crayfish was an important component of adult diets (20%), a phenomenon far less prevalent in sub-adults (8%) and juveniles (5%). For the other putative sources (macrophytes, algae, detritus and pelagic source), the changes between the life stages were marginal. Juvenile and adult crayfish had the smallest and most distinct trophic niches, (Fig. 3 and Fig. S4), overlapping each other by only 3%. Sub-adult crayfish had the widest niche, substantially shared with both juveniles (46%) and adults (22%).
Trophic niche also differed with habitat. The width and standard ellipse area of crayfish trophic niche were greater in beach habitats than in stony slope (Fig S5, Fig S6), with a relatively small niche overlap (25%) between the habitats.
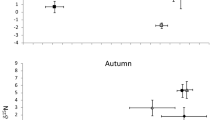
Trophic niche width differed among the seasons (Fig. S7 and Fig. S8). In spring, crayfish relied more on carbon-depleted sources (detritus), while in summer and autumn there was a slight shift to less carbon-depleted sources. However, in spring and summer trophic niche width was almost the same, with slightly higher values recorded in autumn. The overlap between the trophic niches among seasons ranged from 32 to 45% (Table S8).
Depth gradient
In the stony slope habitat, the average number of crayfish individuals per trap increased with depth from 6.3 in 3 m depth to 8.2 in 12 m depth, but then declined to 2.9 in 15 m depth, and no crayfish were caught from the deepest transect monitored (20 m). The proportion of females increased with depth (Table S3), but independent of sex, there were no differences in weight or CL among the depth zones (p > 0.05). There was only significant differences in CL (F1, 322 = 17.61, p < 0.001) and weight (F1, 322 = 27.11, p < 0.001) between males and females.
The three-source mixing model suggested that with increasing depth, the basal sources of crayfish diet changes from littoral autotrophy (algae) and detritus to pelagial production (zooplankton) (Fig. 4). The trophic niche width was greatest at 6 m depth, followed by 3 m depth. Other depths had similar and more restricted trophic niche widths (Figs. S2 and S3). Trophic niche overlap from different depths ranged from 37 to 77% (Table S7).
Discussion
Our study indicates that, as in other freshwater systems (Darrel 2003; Ercoli et al. 2014; Lipták et al. 2019), reservoir populations of noble crayfish can exploit a wide range of food sources at multiple trophic levels, but their diet has high levels of carnivory. However, despite wide variation of diet proportion estimates, our results suggest that crayfish at every life stage were an important food source for many consumers in the reservoir, especially predatory and omnivorous fish, thus transferring energy from lower to higher trophic levels. Our results are in line with those of Rabeni (1992), Dorn and Mittelbach (1999), Englund (1999), Nyström et al. (2006) and Lipták et al. (2019), who found that crayfish were an important food source for consumers from higher trophic levels in both lotic and lentic environments. It seems that detritus was an important food source for all developmental stages, and other food sources such as algae, macrophytes or pelagic sources (sedimeted zooplankton) were utilized to a lesser extent, with their usage dependent on crayfish size. For juvenile and sub-adult crayfish, it seems that zoobenthos is the most important source of prey. However, in adult crayfish, zoobenthos consumption was largely replaced by cannibalism of juvenile crayfish.
Cannibalism is a common phenomenon in crayfish populations (Kouba et al. 2011), and the rate of cannibalism depends mainly on population density, habitat diversity, presence of shelters and crayfish predators (Englund 1999; Englund and Krupa 2000; Usio and Townsend 2002). Previously reported rates of cannibalism vary across species and ecosystems from almost zero (Lipták et al. 2019; Whitmore 1997) to 17–24% (Guan and Wiles 1999; Houghton et al. 2017) with a maximum around 47% (Alcorlo et al. 2004). Hence, our estimated 20% contribution of cannibalism to the diet of adult crayfish is high. We suggest that such a high cannibalism rate can be caused by the combination of high population density together with a lack of shelter in beach habitat.
Each of diet tracing methods has some limitations which can lead to bias in data interpretation (Nielsen et al. 2018). In stable isotopes analysis, fractionalization factor (trophic discrimination factors: TDF = δtissue − δdiet) can be considered as the most problematic part leading to possible errors in data interpretation (Martínez del Rio et al. 2009; Nielsen et al. 2018) as it influences many variables such temperature, taxonomic group, tissue selection and others (Martínez del Rio et al. 2009; McCutchan et al. 2003). Therefore, using fractionalization factors from the literature can lead to result bias. However, current version of Bayesian mixing models is accounting for uncertainty associated with multiple sources, fractionation factors and isotope signature, which decrease bias in data interpretation (Moore and Semmens 2008; Stock and Semmens 2016). Nonetheless, in case of using fractionalization factor from literature, a caution is needed (Bastos et al. 2017).
In addition, our Bayesian mixing models results indicate large credible intervals of relative contribution of putative food sources to consumer diets, which might limit result interpretation. MixSIAR models are accounting for variation in several factors (consumers, putative food sources, fractionation factors) which often leads to wide variation in food source use estimates. In addition, crayfish and fish (roach, rudd) have omnivorous feeding behaviour making diet estimation difficult. Moreover, when isotopic values of putative food sources tend to overlap, results might show large credible intervals making precise food source proportions difficult to be discernible as our model outcomes indicate.
Habitat and ontogeny differences and seasonal isotopic variation in noble crayfish population
Omnivory is an important functional trait of animal ecosystems (Begon et al. 2006). However, throughout ontogeny, an omnivorous species can be either more carnivorous or more herbivorous (Parkyn et al. 2001; Polis and Strong 1996; Pringle and Hamazaki 1998). Using gut content analysis, Momot (1995) found that juvenile crayfish are primarily carnivorous in contrast to older specimens which are more omnivorous. This is likely due to the rapid growth and thus increased protein requirements of juvenile crayfish compared to adult crayfish (Hill et al. 1993; Momot 1995). Conversely, Stenroth et al. (2006), using isotope analysis, suggested that there is no significant shift in diet between size categories of signal crayfish (Pacifastacus leniusculus; (Dana, 1852)); however, this could be an artefact of their omnivorous feeding habits. Similarly, Bondar et al. (2005) found almost no differences in putative source usages among the life stages of signal crayfish. In line with this, our results suggest that the total amount of carnivorous feeding was similar across the life stages (Table S3). However, unlike in signal crayfish, the different life stages of noble crayfish focused on different prey. While juvenile and sub-adults used zoobenthos as an animal source, adult crayfish substituted other zoobenthos with juvenile crayfish in their diet. This mechanism probably prevents competition between life stages and it helps the surviving young crayfish by reducing intraspecific competition for zoobenthos. In addition, the high level of cannibalism can be explained by the high proportion of crayfish in the zoobenthos community in this reservoir (Veselý et al. personal observation). Sub-adult crayfish had the widest trophic niche, suggesting use of the prey typical of both juveniles and adults. This might be a relic of the juvenile life habits, because adult crayfish share almost nothing with the juvenile trophic niche. These results are concordant with previous findings on rusty crayfish Faxonius rusticus; (Girard, 1852)) whose sub-adults have the widest trophic niche, overlapping the much narrower trophic niches of juveniles and adults (Roth et al. 2006).
Habitat diversity is an important feature of ecosystems, increasing species richness and ecosystem stability (Begon et al. 2006; Kalff 2002). Generally, each habitat can provide different food sources which can be utilized by consumers (Begon et al. 2006; Schälicke et al. 2019). We found that the trophic niche width of crayfish in beach habitat was greater than in stony slopes habitat. This might be due to a wider range of available food sources in the beach habitat (Veselý et al. personal observation), in line with Alcorlo et al. (2004), who found that food item diversity in crayfish stomachs correlates with prey availability. Furthermore, Alcorlo et al. (2004) found differences in the diet of red swamp crayfish between human modified and natural habitats. In both habitats, red swamp crayfish behaved as a generalist, but in the natural habitat, in contrast to the modified habitat, insect larvae formed an important part of its diet. On the other hand, Hollows et al. (2002) found that P. zealandicus exploited similar food sources in both native bush and pasture streams. Generally, these findings confirm our hypothesis about high diet plasticity in crayfish, especially if they are faced with different habitat types (Beatty 2006; Johnston et al. 2011).
Seasonal changes in food preferences and availability of food sources are common in many organisms. These changes can be caused by physiological processes (mating, pregnancy, hibernation) or environmental factors such as temperature, day length, drought, seasonal floods and spatial heterogeneity of a given ecosystem (Begon et al. 2006). It is well known that crayfish can change food source with the season; however, this is dependent on the environmental context and the species. For example, in New Zealand streams P. zealandicus consumes more terrestrial detritus in the autumn–winter period, and more zoobenthos in winter, but switches to more autochthonous sources in spring and summer (Hollows et al. (2002). Conversely, red swamp crayfish prefer detritus in the winter period and then increase their consumption of zoobenthos and fish in other seasons (Alcorlo et al. (2004). Our results suggest that in the winter-spring period noble crayfish are resource-limited and prefer relatively C13 depleted resources (detritus), while in the spring–summer and summer-autumn period, they use a much wider array of resources. These results are in line with common findings that in temperate climates food sources are limited from winter to spring, and summer serves as period for crayfish to gain energy for reproduction and overwintering (Holdich and Crandall 2002; Kawai et al. 2016; Kozák et al. 2015).
Depth gradient
Depth is an important environmental factor shaping biota dynamics and composition in ecosystems (Begon et al. 2006; Kalff 2002). In comparison with lakes, canyon-shaped reservoirs have quite steep banks with a limited proportion of littoral areas and a low diversity of habitats (Kalff 2002). This condition can be critical for most of species inhabiting bottom part of reservoirs. Generally, with increasing depth, there are less food sources as well as light (Kalff 2002; Ruokonen et al. 2012). Moreover, under thermocline, there is a rapid change in temperature and oxygen level (Kalff 2002).
In Nýrsko reservoir, crayfish were found to a depth of 15 m, and while thermocline was established at 8 m depth during the summer season, oxygen was at acceptable levels from the surface to the bottom of reservoir without a layer with oxygen deficiency. The overlap of trophic niche suggests that crayfish move across different depths. Thus crayfish are likely geomorphic agents in reservoirs (Statzner et al. 2000), their movements transporting and mixing organic and inorganic matter between different depths. Moreover, our results suggest that with increasing depth, noble crayfish food preference changes from detritus and algae to pelagic sources (sedimented zooplankton). These results are in line with Ruokonen et al. (2012) who found a similar trend in signal crayfish in Lake Päijänne, Finland, although the estimated usage of pelagic sources by noble crayfish in Nýrsko reservoir was much higher in the deeper layer than for signal crayfish at their locality. Additionally, in Nýrsko reservoir, more females were found in deeper parts, suggesting that females avoid the upper layers to escape increased food competition, or higher predation pressure due to their generally smaller size. They may also go deeper to avoid the action of waves, or for a period of moulting after the onset of independence in their offspring. These results are similar to Flint (1977) who found female signal crayfish at greater depths than males in Lake Tahoe. However, in the oligotrophic reservoir Šance, in Czech Republic the distribution of noble crayfish females was irregular and males were more common with increasing depth (Ďuriš and Smutný 2000).
In contrast to our study, Abrahamsson and Goldman (1970) found signal crayfish in Lake Tahoe up to 200 m depth with the highest population density between 10 and 20 m depth. There, 0–10 m depth had a lower population density due to predation pressure, light intensity and scarcity of food resources due to the heavy wave action in shallow areas. Abrahamsson and Goldman (1970) also found that with increasing depth crayfish size increased while population density decreased. This observation is in line with Ďuriš and Smutný (2000) who also observed larger animals in deeper zones. However, it is contrast to our study, which found no size differences with depth. The inconsistency among all mentioned studies indicate species-specific as well as ecosystem-related variability.
Conclusion
Crayfish utilized food sources from several trophic levels in oligotrophic Nýrsko reservoir. Moreover, cannibalism was an important feeding habit for adult crayfish, while sub-adult and juvenile crayfish foraged on conspecifics to a lesser extent. The proportion of carnivorous feeding behaviour was similar throughout crayfish ontogeny; nevertheless, the food sources used varied between crayfish age classes. In Nýrsko reservoir, crayfish are not only consumers but also important prey for variety of consumers. Additionally, use of pelagic food sources increased with depth. Thermocline did not limit the distribution of the crayfish population as they were abundant even in deeper parts of the reservoir. Lastly, food source preferences changed with habitat and season and crayfish had a high feeding plasticity in this ecosystem. Finally, we can conclude that crayfish are important prey as well as important consumers of lower trophic levels, with the ability to consume conspecifics, and the ability to mix organic and inorganic matter along a depth gradient. To sum up, these findings indicate that crayfish are indeed ecologically important species with both direct and indirect roles in the trophic web of in this reservoir ecosystem.
Data accessibility
Primary data used in this study will be uploaded as online supporting information if and when the manuscript is accepted for publication.
References
Abrahamsson SA, Goldman CR (1970) Distribution, density and production of the crayfish Pacifastacus leniusculus Dana in Lake Tahoe, California-Nevada. Oikos 21:83–91
Ahyong ST, Yeo DC (2007) Feral populations of the Australian red-claw crayfish (Cherax quadricarinatus, von Martens) in water supply catchments of Singapore. Biol Invasions 9:943–946
Alcorlo P, Geiger W, Otero M (2004) Feeding preferences and food selection of the red swamp crayfish, Procambarus clarkii, in habitats differing in food item diversity. Crustaceana 77:435–453
Anderson C, Cabana G (2007) Estimating the trophic position of aquatic consumers in river food webs using stable nitrogen isotopes. J N Am Benthol Soc 26:273–285
Arts MT, Brett MT, Kainz M (2009) Lipids in aquatic ecosystems. Springer, Berlin
Bastos RF, Corrêa F, Winemiller KO, Garcia AM (2017) Are you what you eat? Effects of trophic discrimination factors on estimates of food assimilation and trophic position with a new estimation method. Ecol Indic 75:234–241
Beatty SJ (2006) The diet and trophic positions of translocated, sympatric populations of Cherax destructor and Cherax cainii in the Hutt River, Western Australia: evidence of resource overlap. Mar Freshw Res 57:825–835
Begon M, Townsend CR, Harper JL (2006) Ecology: from individuals to ecosystems. Wiley-Blackwell, Oxford
Bondar CA, Bottriell K, Zeron K, Richardson JS (2005) Does trophic position of the omnivorous signal crayfish (Pacifastacus leniusculus) in a stream food web vary with life history stage or density? Can J Fish Aquat Sci 62:2632–2639
Bronmark C, Klosiewski SP, Stein RA (1992) Indirect effects of predation in a freshwater, benthic food chain. Ecology 73:1662–1674
Collier KJ, Parkyn SM, Rabeni C (1997) Koura: a keystone species. Water Atmos 5:18–20
Crandall KA, Buhay JE (2007) Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae—Decapoda) in freshwater. In: Balian EV (ed) Freshwater animal diversity assessment. Springer, Berlin, pp 295–301
Creed RP Jr (1994) Direct and indirect effects of crayfish grazing in a stream community. Ecology 75:2091–2103
Creed RP Jr, Reed JM (2004) Ecosystem engineering by crayfish in a headwater stream community. J N Am Benthol Soc 23:224–236
Darrel ES (2003) Invited overview: conclusions from a review of electrofishing and its harmful effects on fish. Rev Fish Biol Fish 13:445–453
Dorn N, Mittelbach G (1999) More than predator and prey: a review of interactions between fish and crayfish. Vie et Milieu 49:229–237
Dorn NJ, Wojdak JM (2004) The role of omnivorous crayfish in littoral communities. Oecologia 140:150–159
Ďuriš Z, Smutný M (2000) On depth distribution of crayfish. Freshw Crayfish 12:925–926
Englund G (1999) Effects of fish on the local abundance of crayfish in stream pools. Oikos 87:48–56
Englund G, Krupa JJ (2000) Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshw Biol 43:75–83
Ercoli F, Ruokonen TJ, Hämäläinen H, Jones RI (2014) Does the introduced signal crayfish occupy an equivalent trophic niche to the lost native noble crayfish in boreal lakes? Biol Invasions 16:2025–2036
Flint RW (1977) Seasonal activity, migration and distribution of the crayfish, Pacifastacus Ieniusculus, in Lake Tahoe. Am Midl Nat 97:280–292
Francis TB, Schindler DE, Holtgrieve GW, Larson ER, Scheuerell MD, Semmens BX, Ward EJ (2011) Habitat structure determines resource use by zooplankton in temperate lakes. Ecol Lett 14:364–372
Gido KB, Schaefer JF, Falke JA (2009) Convergence of fish communities from the littoral zone of reservoirs. Freshw Biol 54:1163–1177
Grey J, Jackson MC (2012) ‘Leaves and eats shoots’: direct terrestrial feeding can supplement invasive red swamp crayfish in times of need. PLoS ONE 7:e42575
Guan R-Z, Wiles PR (1999) Growth and reproduction of the introduced crayfish Pacifastacus leniusculus in a British lowland river. Fish Res 42:245–259
Hepworth DK, Duffield DJ (1987) Interactions between an exotic crayfish and stocked rainbow trout in Newcastle Reservoir. Utah N Am J Fish Manag 7:554–561
Herrmann A, Schnabler A, Martens A (2018) Phenology of overland dispersal in the invasive crayfish Faxonius immunis (Hagen) at the Upper Rhine River area. Knowl Manag Aquat Ecosyst 419:30
Hill AM, Sinars DM, Lodge DM (1993) Invasion of an occupied niche by the crayfish Orconectes rusticus: potential importance of growth and mortality. Oecologia 94:303–306
Holdich DM, Crandall K (2002) Biology of freshwater crayfish. Blackwell Science, Oxford
Hollows JW, Townsend CR, Collier KJ (2002) Diet of the crayfish Paranephrops zealandicus in bush and pasture streams: insights from stable isotopes and stomach analysis. N Z J Mar Freshwat Res 36:129–142
Houghton R, Wood C, Lambin X (2017) Size-mediated, density-dependent cannibalism in the signal crayfish Pacifastacus leniusculus (Dana, 1852) (decapoda, astacidea), an invasive crayfish in Britain. Crustaceana 90:417–435
Irz P, Laurent A, Messad S, Pronier O, Argillier C (2002) Influence of site characteristics on fish community patterns in French reservoirs. Ecol Freshw Fish 11:123–136
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Johnston K, Robson BJ, Fairweather PG (2011) Trophic positions of omnivores are not always flexible: evidence from four species of freshwater crayfish. Austral Ecol 36:269–279
Jones P, Momot W (1981) Crayfish productivity, allochthony, and basin morphometry. Can J Fish Aquat Sci 38:175–183
Kalff J (2002) Limnology: inland water ecosystems. Prentice Hall, New Jersey
Kawai T, Faulkes Z, Scholtz G (2016) Freshwater crayfish: a global overview. CRC Press, New York
Kouba A, Buřič M, Policar T, Kozák P (2011) Evaluation of body appendage injuries to juvenile signal crayfish (Pacifastacus leniusculus): relationships and consequences. Knowl Manag Aquat Ecosyst 401:04
Kozák P, Ďuriš Z, Petrusek A, Buřič M, Horká I, Kouba A, Kozubíková-Balcarová E, Policar T (2015) Crayfish biology and culture. Faculty of Fisheries and Protection of Waters, University of South Bohemia in České Budějovice, Vodňany
Kuklina I, Kouba A, Buřič M, Horká I, Ďuriš Z, Kozák P (2014) Accumulation of heavy metals in crayfish and fish from selected Czech reservoirs. Biomed Res Int 2014:1–9
Light T (2003) Success and failure in a lotic crayfish invasion: the roles of hydrologic variability and habitat alteration. Freshw Biol 48:1886–1897
Lipták B, Veselý L, Ercoli F, Bláha M, Buřič M, Ruokonen TJ, Kouba A (2019) Trophic role of marbled crayfish in a lentic freshwater ecosystem. Aquat Invasions 14:299–309
Lodge DM (1994) Factors governing species composition, population size, and productivity of cool-water crayfishes. Nordic J Freshw Res 69:111–136
Lodge DM, Deines A, Gherardi F, Yeo DC, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA (2012) Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annu Rev Ecol Evol Syst 43:449–472
Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ (2009) Isotopic ecology ten years after a call for more laboratory experiments. Biol Rev 84:91–111
Mather ME, Stein RA (1993) Direct and indirect effects of fish predation on the replacement of a native crayfish by an invading congener. Can J Fish Aquat Sci 50:1279–1288
Mccutchan JH, Lewis WM, Kendall C, Mcgrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390
Momot WT (1995) Redefining the role of crayfish in aquatic ecosystems. Rev Fish Sci 3:33–63
Moore JW, Semmens BX (2008) Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett 11:470–480
Neveu A (2009) A functional approach to patch suitability using biomass dynamics: application to a residual population of the white-clawed crayfish. Fundam Appl Limnol Arch für Hydrobiol 175:185–202
Nielsen JM, Clare EL, Hayden B, Brett MT, Kratina P (2018) Diet tracing in ecology: Method comparison and selection. Methods Ecol Evol 9:278–291
Nyström P, Granéli W (1996) The effect of food availability on survival, growth, activity and the number of mature females in crayfish populations. Freshw Crayfish 11:170–181
Nyström P, Svensson O, Lardner B, Brönmark C, Granéli W (2001) The influence of multiple introduced predators on a littoral pond community. Ecology 82:1023–1039
Nyström P, Stenroth P, Holmqvist N, Berglund O, Larsson P, Granéli W (2006) Crayfish in lakes and streams: individual and population responses to predation, productivity and substratum availability. Freshw Biol 51:2096–2113
Parkyn SM, Collier KJ, Hicks BJ (2001) New Zealand stream crayfish: functional omnivores but trophic predators? Freshw Biol 46:641–652
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Pringle CM, Hamazaki T (1998) The role of omnivory in a neotropical stream: separating diurnal and nocturnal effects. Ecology 79:269–280
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rabeni CF (1992) Trophic linkage between stream centrarchids and their crayfish prey. Can J Fish Aquat Sci 49:1714–1721
Reynolds J, Souty-Grosset C, Richardson A (2013) Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw Crayfish 19:197–218
Roth BM, Hein CL, Vander Zanden MJ (2006) Using bioenergetics and stable isotopes to assess the trophic role of rusty crayfish (Orconectes rusticus) in lake littoral zones. Can J Fish Aquat Sci 63:335–344
Ruokonen T, Kiljunen M, Karjalainen J, Hämäläinen H (2012) Invasive crayfish increase habitat connectivity: a case study in a large boreal lake. Knowl Manag Aquat Ecosyst 407:8
Saiki MK, Ziebell CD (1976) Some trophic relationships of the largemouth bass, Micropterus salmoides (Lacepede), in a southwestern impoundment. J Ariz Acad Sci 11:99–104
Schälicke S, Sobisch LY, Martin-Creuzburg D, Wacker A (2019) Food quantity–quality co-limitation: interactive effects of dietary carbon and essential lipid supply on population growth of a freshwater rotifer. Freshw Biol 64:903–912
Statzner B, Fievet E, Champagne JY, Morel R, Herouin E (2000) Crayfish as geomorphic agents and ecosystem engineers: biological behavior affects sand and gravel erosion in experimental streams. Limnol Oceanogr 45:1030–1040
Stenroth P, Holmqvist N, Nyström P, Berglund O, Larsson P, Granéli W (2006) Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Can J Fish Aquat Sci 63:821–831
Stewart AR, Saiki MK, Kuwabara JS, Alpers CN, Marvin-DiPasquale M, Krabbenhoft DP (2008) Influence of plankton mercury dynamics and trophic pathways on mercury concentrations of top predator fish of a mining-impacted reservoir. Can J Fish Aquat Sci 65:2351–2366
Stock B, Semmens B (2016) MixSIAR GUI user manual. Version 3.1. https://github.com/brianstock/MixSIAR
Twardochleb LA, Olden JD, Larson ER (2013) A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382
Usio N, Townsend CR (2000) Distribution of the New Zealand crayfish Paranephrops zealandicus in relation to stream physico-chemistry, predatory fish, and invertebrate prey. N Z J Mar Freshw Res 34:557–567
Usio N, Townsend CR (2002) Functional significance of crayfish in stream food webs: roles of omnivory, substrate heterogeneity and sex. Oikos 98:512–522
Vander Zanden M, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Whitmore N (1997) Population ecology of the freshwater crayfish Paranephrops zealandicus and its effect on the community structure of a lowland bush stream. Master thesis, University of Otago
Winters LK, Budy P (2015) Exploring crowded trophic niche space in a novel reservoir fish assemblage: how many is too many? Trans Am Fish Soc 144:1117–1128
Acknowledgements
This study was supported by the Ministry of Education, Youth, and Sports of the Czech Republic (Projects CENAKVA – CZ.1.05/2.1.00/01.0024, and CENAKVA II – LO1205 under the NPU I Programme). We also thank two anonymous reviewers for helpful comments. The authors declare no potential sources of conflict of interest.
Author information
Authors and Affiliations
Contributions
LV proposed the study. LV, AK, MB, MB and JK conducted field sampling. LV, TR and FE prepared and did analyses of samples on mass spectrometry. LV wrote the first draft of the manuscript and conducted data analyses. All authors provided comments and additional revisions of the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Veselý, L., Ercoli, F., Ruokonen, T.J. et al. The crayfish distribution, feeding plasticity, seasonal isotopic variation and trophic role across ontogeny and habitat in a canyon-shaped reservoir. Aquat Ecol 54, 1169–1183 (2020). https://doi.org/10.1007/s10452-020-09801-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09801-w