Abstract
The present study characterizes the fish assemblage in the surf zone of Cassino Beach, Rio Grande, Brazil, and analyzes temporal fluctuations in richness and abundance of these species in medium (months) and long terms (years), associating them also with abiotic covariates. Data were collected monthly between 1996 and 2012 at two locations. Bayesian generalized additive models (GAMs) were used as statistical tool, placing this study among few that have used Bayesian GAMs in Ecology. Our results show a decrease in both species richness and abundance of the most representative species, over the last 16 years, but no significant distinction between locations. Water temperature and salinity along with seasonality were the statistically most influential explanatory covariates to describe fluctuations in richness and abundance. Higher discharge rates of the three main rivers that flow into Patos Lagoon (Jacuí, Taquari, Camaquã) were associated with increased richness and abundance of some species in the assemblage. Hence, our findings show that medium- and long-term fluctuations in richness and species abundance are controlled by abiotic factors related to seasonal cycles (temperature) and productivity of the ecosystem. Long-term changes seem to be also related to man-induced factors and climate change; but further research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species richness, defined as the total number of species in a unit of area (Brown et al. 2007), is a parameter of some concern in conservation biology, biogeography and community ecology (Royle and Dorazio 2008). It is the simplest way to describe community and regional diversity (Magurran 2004). A vast amount of ecological research has been undertaken using species richness as a measure to understand what affects, and is affected by, biodiversity. At the small spatial scale, species richness is generally used as a measure of diversity within a single ecological community, habitat or microhabitat (Brown et al. 2007). These authors cite as main factors of small-scale variation in species richness: (1) geographic factors; (2) biotic factors such as competition or predation; and (3) abiotic factors, such as temperature and salinity. Since probability of detection differs among species, all measures of species richness have a positive correlation with sample size (Silvy 2012); therefore, it is important to also take the effects of abundance into account (Magurran 2004).
Among the main ways to analyze species richness are diversity indices (Hubálek 2000) and numerical modeling (Royle and Dorazio 2008). The trouble with diversity indices is that there are several to choose from (Margalef 1958; Menhinick 1964; McIntosh 1967; etc.). Alternatively, in numerical modeling, species richness can be characterized by a Poisson distribution and the variation in the latent ecological process described statistically by covariates (Royle and Dorazio 2008; Kinas and Andrade 2010). This is the approach used here.
Much of contemporary ecological theory, conservation biology and natural resource management are further concerned with variation in the abundance of species (Royle and Dorazio 2008). While richness shows which individual species are present in space and time (Brown et al. 2007), the abundance of individuals associated with other parameters governs the dynamics of this process (Royle and Dorazio 2008). The typical distribution assumed for modeling abundance is also Poisson. Other widely used discrete distributions are Binomial and Negative Binomial. Among continuous distributions, the Normal, log-Normal and Gamma can be cited (Kéry 2010).
Statistical analysis of ecological data with generalized additive models (GAMs) has been recognized as a powerful statistical tool for exploratory data analysis (Schoeman and Richardson 2002; Venables and Dichmont 2004; Guisan and Thuiller 2005; Dalla Rosa et al. 2012). Despite the versatility of linear models (LMs) and generalized linear models (GLMs) for data analysis and prediction, the restriction to a linear structure in the covariates is, however, a limitation, circumvented by GAMs. This is so because GAMs go a step further and use smoothed functions (splines) to model nonlinear relationships for some or all the explanatory variables (Hastie and Tibshirani 1990; Wood 2006a).
The statistical analyses of LMs, GLMs and GAMs can be made by conventional maximum likelihood inference (Edwards 1972) or, alternatively, by Bayesian methods (Gelman et al. 2013). For LMs and GLMs, both approaches (classical and Bayesian) are already widely used in ecology and environmental science (Faraway 2006; Royle and Dorazio 2008). Comparatively, Bayesian GAMs are still rare and are, therefore, a methodological highlight in the present work.
The growing interest in Bayesian statistics in many fields of applied sciences, particularly ecology, is partially explained by (the Bayesian) definition of probability as a coherent metric for uncertainty, combined with the conceptually rigorous structure of statistical inference defined plainly as decision-making in the presence of uncertainty—in contrast to the largely ad hoc procedures of conventional frequentist statistics (Lindley 2000). The popularization of specialized software to perform complex mathematical operations required in some Bayesian numerical calculations further helps explain this expansion in ecology (McCarthy 2007; Kinas and Andrade 2010).
Sandy beaches constitute most of the coastal areas of the word, with surf zones characterized as the area between the outer limit of the breaking waves and the beach shoreline (Brown and McLachlan 1990). Studies of the ichthyofauna in marine surf zones have reported that these fish assemblages are highly variable and dominated by a small number of species made up largely of juveniles (Ayvazian and Hyndes 1995; Gibson et al.1996; Vasconcellos et al. 2007). Similar to estuarine beaches, marine surf zones are recognized as important nursery areas for several species (Bell et al. 2001; Strydom and d’Hotman 2005), and the recruitment variability in these habitats is related to a wide range of biotic and abiotic factors (e.g., food supply, predation risk, temperature and salinity) (Taylor et al. 2007; Haynes et al. 2010; Able et al. 2011).
Systematic long-term sampling programs contain valuable information to characterize most assuredly the community structure and simultaneously monitor changes in medium term (months) and long term (years). Rare or irregular events that affect fish populations, but are spread over large time intervals, can only be evaluated by long-term studies. This study uses a 16-year long database of the ichthyofauna inhabiting the surf zone of a sandy beach in southern Brazil. Our working hypothesis is that variations in the surf-zone fish assemblage richness as well as changes in abundance of selected groups of species show associations, not necessarily linear, with environmental factors and that these features can be captured by GAMs.
Materials and methods
Study area
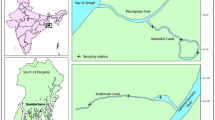
This study focuses on Cassino Beach, located in southern Brazil (32°10′S, 52°20′W) (Fig. 1). Cassino is a subtropical sandy beach strongly influenced by seasonal variation in physico-chemical parameters. In general, observed temperatures and salinities are significantly higher in summer than in winter (Lima and Vieira 2009). Along this beach, the morphodynamic stages vary between dissipative and reflective and are seasonally influenced by the action of winds and waves, which affect the dynamics of the surf zone (Calliari et al. 2001); additionally, freshwater flow from the Patos Lagoon into the adjacent coastal marine area that may increase during periods of rain (Moresco and Bemvenuti 2006) and El Niño (Garcia and Vieira 2001).
Data description
Samples were collected monthly in shallow waters (depth <20 m) from January 1997 to August 2012 at two fixed stations (1) CB1 (near Molhe Oeste) and (2) CB2 (near Estação Marinha de Aquacultura—EMA/FURG) (Fig. 1). From August to December of 1996, samples were collected only at station CB1. The sampling procedure is part of a long-term ecological research project (ICTIO/PELD CNPq-MCT, 1996–2012). The beach morphologies at both stations are the same; but CB1 is situated close to the outlet of the Patos Lagoon, being more sheltered in comparison with CB2 situated about 10 km south of this outlet.
Biological samplings (n = 5 hauls(site)−1(month)−1) were performed at each site using a 9-m beach seine (13-mm bar mesh in the wings and 5 mm in the center). All applicable institutional and national guidelines for the care and use of animals were followed. Samplings had the “authorization for scientific purpose activities” n° 17762-1 of IBAMA-SISBIO. Specimens were fixed in formalin in the field, due to long time gap between sampling and the identification process to the lowest possible taxonomic level conducted in the laboratory. Along with biological sampling, measurements on water conditions (temperature, salinity and transparency) were made in situ. Data on flow of the three main rivers of the Patos–Mirim system (Jacuí, Taquari, Camaquã) for the study period (1996-2012) were obtained with the National Water Agency (ANA—hidroweb.ana.gov.br). The monthly discharges of these three rivers combined correspond to 70 % of flow throughout the watershed (Vaz et al. 2006). Therefore, it was used as an indicator of the flow of the Patos Lagoon Estuary into the surf zone of Cassino Beach. Monthly values of zonal (east–west) and meridional (north–south) wind components at the sea surface (32°S; 52°W) were obtained from the Division of Environmental Sciences Research Centre for Fisheries of NOAA (“National Oceanic and Atmospheric Administration”—http://las.pfeg.noaa.gov/las6_5/servlets/dataset).
Data analysis
Fluctuations in total abundance and species richness of fish in the surf zone of Cassino Beach over the past 16 years were associated with the covariates month, year, location, surface water temperature in °C (temperature), water salinity (salinity), water transparency in cm (transparency) (Secchi), zonal wind component in m(s)−1 (zonal), meridional wind component in m(s)−1 (meridional) and flow of the three main rivers of the Patos-Mirim system in m3(s)−1: Jacuí (jacui), Taquari (taquari), Camaquã (camaqua); totaling 11 covariates. The model for species richness had additionally added the covariate abundance (monthly total number of collected specimens).
GAMs with a linear predictor composed of q linear components and k smooth functions are defined as.
where μ i = E(Y i ) and Y i has some distribution from the exponential family with associated known link function \( {\text{g}} \). While Y i denotes some response variable (in our context species richness and abundance), \( z_{1i} , \ldots , z_{qi} \) and \( x_{1i} , \ldots ,\, x_{qi} \) are \( q + k \) covariates, the former (z) being modeled with a strictly parametric model and the latter (x) with smooth functions.
The Bayesian fits of the GAMs were conducted in the free software BayesX, version 2.0.1 (Belitz et al. 2009), which uses penalized regression splines (P-splines) as smooth functions (Eilers and Marx 1996). In this approach, it is assumed that the unknown smooth functions f j can be approximated by a spline of degree l with equally spaced knots \( X_{j,\hbox{min} } = \zeta_{j0} < \,\zeta_{j1} < \, = \, < \,\zeta_{j,r - 1} \, < \,\zeta_{jr} \, = \,X_{j,\hbox{max} } \) This spline can be written in terms of a linear combination of m = r + l B-spline basis functions \( B_{jp} \), which in turn are known and calculable functions for each value in the domain x j , that is,
Following the Bayesian approach, second-order random walks were used as priors for the coefficients, i.e., for \( p > 2, \;\beta_{jp} \) are given by: \( \beta_{jp} = 2\beta_{jp - 1} - \beta_{jp - 2} + u_{jp} \) with Gaussians errors \( u_{jp} \sim N (0, \tau_{j}^{2} ) \) and diffuse priors \( p\left( {\beta_{1} } \right) \propto {\text{const}} \), \( p\left( {\beta_{2} } \right) \propto {\text{const}} \) and \( p\left( {\tau_{j} } \right)\sim {\text{IG}}\left( {0.001, 0.001} \right) \) with \( {\text{IG}}\,(a,b) \) denoting an inverse-gamma distribution. The degree of smoothness is controlled by the variances \( \tau_{\text{j}}^{2} \), which correspond to the inverse of the parameter λ 2 that is used in the cross-validation technique of the classical approach (Lang and Brezger 2004). The joint posterior distribution for the set of parameters \( \left( {\alpha_{*} ,\;\beta_{**} ,\;\tau_{*} } \right) \) was obtained with Markov Chain Monte Carlo (MCMC) stochastic simulation by the Metropolis–Hastings algorithm. For a chain of 12,000 values, the first 2,000 were discarded to eliminate the dependence of the initial value (burn-in period) after which each tenth value was retained (thinning) to achieve independence between sampled values (Kinas and Andrade 2010). Standard diagnostic tools were applied in preliminary analyses to check for MCMC convergence (Gelman et al. 2013).
For model comparison, the Deviance information criterion (DIC) was used (Spiegehalter et al. 2002). By this criterion, regarding predictive power, models with smaller value of DIC are better. A difference in DIC of at least two units can be considered indication of superior fit for the model with the lowest DIC (Spiegehalter et al. 2002).
Species richness
Species richness was analyzed by a Poisson model for the count data of number of species per area \( (Y_{i} ) \), with the natural logarithm as link function.
Models with different combinations of covariates were fitted. The adequacy of each variable to a spline function or a linear structure was evaluated visually at first. When for some covariate x j a linear structure was suspected, another fit after replacing f j (x ji ) by α j x ji was performed and both alternatives compared by DIC.
Species abundance
For the analysis of abundance with all species combined, there is a chance that abundance remains nearly constant, while species composition keeps changing. Therefore, to analyze abundance fluctuations more carefully, we adopted the following strategy: (1) work only with species that had at least 10 % frequency of occurrence in the total database; (2) calculate some matrix of dissimilarity among the species; (3) identify subsets of species characterized by a low dissimilarity; and (4) perform abundance modeling for each identified subset of species.
The aim was to create groups of species that have a tendency to have occurred together over time during the period of data collection. We then hypothesized that, because of their persistent co-occurrence, they should display similar responses to factors that may influence their abundance.
Cluster analysis of species
Cluster analysis was performed using the multivariate ordination method of metric multidimensional scaling (MDS) and the Jaccard similarity index based on a presence (1) and absence (0) matrix (i.e., the similarity between two species increases with growing numbers of co-occurrences) (Hardle and Simar 2007). The Jaccard similarity index has been widely used in studies of presence–absence (Romesburg 1984). The metric MDS finds the spatial configuration of points by algebraic reconstruction, taking into account the dissimilarities between these points, where the dissimilarities are Euclidean distances (Chatfield and Collins 1980). Calculations were performed with the free software Past (Hammer et al. 2001), version 2.17b.
Models for abundance
For each group of species identified by MDS, abundance was modeled by GAMs. A Gaussian distribution with identity link function was used to model the total number of individuals (Y i ). Because the number of individuals is sometimes very large, the Gaussian distribution combined with the identity link serves here as an approximation to the Poisson and showed a numerically more stable performance at fitting.
Models with different combinations of variables were tested. The adequacy of each covariate to either a spline function or a linear structure was evaluated as previously described.
Results
List of species
During the study period, 171,737 individuals were collected at 343 sampling occasions. Sixty-four taxa were found, nine identified only to the family level, and 55 identified to the species level. The eight most abundant species, Mugil liza, Trachinotus marginatus, Mugil curema, Brevoortia pectinata, Mugil sp, Odontesthes argentinensis, Menticirrhus littoralis and Atherinella brasiliensis, comprise 97.1 % of all individuals captured. Regarding the frequency of occurrence, 13 species were present in at least 10 % of the samples (Table 1).
Species richness
The best GAM for species richness models richness as a function of month, year, transparency, temperature, salinity, jacui, taquari, camaqua and abundance (Model 1—Table 2). The results show that, on a monthly scale, the number of species displays seasonal variation, being higher especially in the austral summer months, with a decrease starting in April and increasing again from October onwards. In the long term (annual scale), species richness shows a decreasing trend (Fig. 2).
Among the abiotic covariates, only temperature affects variations in species richness, with peak of species richness occurring between 20 and 25 °C (Fig. 2). Flows jacui and camaqua display a positive linear association with the number of species. Variables salinity, transparency and taquari also have a linear relationship but with slope close to zero, indicating that individually their influence is nonsignificant. Nevertheless, they were maintained in the model because, in combination with other covariates, they improved the predictive power of the model decreasing the DIC (Table 2). Species richness was positively associated with abundance until about 1,000 individuals after which in flattens out (Fig. 2).
The sampling site (location), as well as the zonal and meridional wind components, was not included in the model, because they increased the DICs. This indicates that sampling stations as well as values of wind components do not help explain variations in species richness.
Species abundance
Cluster analysis
Two distinct groups were formed by MDS (Fig. 3), with the first two axes synthesizing about 40 % of the data variation. Group A is composed of species Lycengraulis grossidens, Menticirrhus americanus, Micropogonias furnieri, Oncopterus darwinii and Platanichthys platana, and Group B of A. brasiliensis, B. pectinata, M. littoralis, M. curema, M. liza, M. sp, O. argentinensis and T. marginatus.
Group A
On a monthly scale, the abundance of species that composes Group A is higher in warmer months and lower during colder autumn–winter months, with the decrease starting in April, remaining stable with low abundance until August, when abundance increases again (Fig. 4). The covariate year enters the best model as a linear component, but with slope close to zero (Model 2—Table 2), indicating that the abundance of this group of species has remained stable over the years (1996–2012).
Regarding temperature, a slight oscillation around an optimum of 20 °C was found, with abundances decreasing toward the extremes. Abundance decreases with increasing salinity (Fig. 4), which is associated negatively with transparency and positively with the jacui river discharge (Model 2—Table 2).
Group B
For species of group B, the covariate month has a negative linear relationship with negative slope, indicating that in the first months of the year abundance is higher (Model 3—Table 2). In the long term (year), Group B shows a decreasing trend in abundance (Fig. 5).
This group further displays an increase in mean abundance at salinities from 25 upwards (Fig. 5). However, we point out that the variances in both tails are large; this is due to the fact that there were few sampled points with extreme salinities (≤5; 35≥). Group B also has a positive association with temperature. The variables transparency, taquari and camaqua were included in the best fitted model because they impacted reduction of DIC, although individually their influence on changes in mean abundance is unclear (Model 3—Table 2).
Discussion
To understand which factors drive the spatial and temporal patterns in communities is a central goal of ecology. This study emerges as one of few to investigate this phenomenon with the use of Bayesian generalized additive models. To our knowledge, this is the first such approach to be published with data from Brazil. While there are several papers describing the formal development of these models (Denison et al. 1998; Lin and Zhang 1999; Fahrmeir and Lang 2001; Spiegehalter et al. 2002; Lang and Brezger 2004; Wood 2006b), there are still only a few with applications. Examples are Guisan and Zimmermann (2000) and Phillips et al. (2006), who applied these models to describe geographic distribution of species.
The models, fitted here with P-splines (Eilers and Marx 1996; Lang and Brezger 2004), proved very amenable to the study of species richness and abundance because direct visual inspection of nonlinear relationships and predictive power of covariates can be evaluated simultaneously. The nontrivial influence of sample size up to about 1,000 specimens on the observed species richness was handled easily by simply including abundance as a covariate into the model. The rarefaction technique is the most consecrated method in the comparison of the number of taxa in samples of different size (Krebs 1989). However, only the sampling effort (usually expressed as the total abundance of species) is considered as a covariate by this technique. Until recently, the comparison of the richness-to-sampling effort ratio considering environmental variables was only performed for linear models. However, since this relationship is clearly nonlinear, GAMs are an excellent replacement for the rarefaction technique when comparing species richness between different sampling units.
One fundamental pattern concerning life on Earth is the decrease of biological diversity from the equator toward the poles (Willig et al. 2003). This helps to explain why tropical oceans are in general biologically diverse, while coastal regions of temperate and polar zones are highly productive but less diverse (Caddy and Sharp 1986). In Brazil, there are higher numbers of species recorded in the surf zones on tropical beaches (Itamaracá (PE)—95 species (Santana and Severi 2009); Cabuçu (BA)—63 species (Silva et al. 2008); São Francisco do Itabapoana (RJ)—68 species (Gomes et al. 2003)) than on the subtropical beach of the present study (55 species). However, all share the same striking feature of only a few dominant species. This peculiarity had already been recognized by Modde and Ross (1981), when they say that “surf-zones are dominated by a small group of species, and this organization remains constant over wide geographic areas.”
Surf zones, especially for sandy beaches, are uniform habitats characterized by low primary productivity (Knox 2001; McLachlan and Brown 2006). Thus, the abiotic factors seem to be the main components that drive the structure and composition of the communities in these habitats. This similarity in abiotic conditions explains the absence of sampling station effect in our study, corroborating Rodrigues and Vieira (2012), who also reported insignificant differences in the abundance and diversity of fish between these two locations, using another analytic approach.
Groups A and B of species were characterized on their co-occurrence. The pattern that emerged seems to be associated mainly with salinity and seasonality. While species in group A were shown to be more abundant in less salty waters, those in Group B had their abundance increased with increasing salinity. Species of Group A can be further described as occasional, only appearing in specific seasons of the year (M. furnieri and L. grossidens in Summer and Autumn, and M. americanus and O. darwinii, in Spring and Summer (Lima and Vieira 2009)); in contrast, species in Group B (A. brasiliensis, B. pectinata, M. littoralis, M. curema, M. liza, M. sp, O. argentinensis, T. marginatus) are usually present in the surf zone all year long (Lima and Vieira 2009).
Monteiro-Neto et al. (2003) for samples conducted from March 1980 to February 1982 found seven groups (A to G) in this surf zone using inverse cluster analysis and taking into account 24 species. Group C of their study has resemblance to our Group A, and their groups F and G together resemble our Group B. The time lag between both studies suggests that these associations tend to occur with some consistency in the surf zone of Cassino Beach, even in the long term.
The results obtained here show the strong influence of seasonality on the ichthyofauna structure in the surf zone of Cassino Beach. The increase of marine productivity driven by the seasonality of temperatures and sunlight in subtropical habitats favors the survival of larvae and juveniles during warmer months, especially due to increase of food supply (Castillo-Rivera et al. 2010; Martino and Houde 2010; Kristiansen et al. 2011). Thus, the seasonal dynamics of the spawning activity and recruitment in subtropical areas is closely associated with the temperature regime. According to Moraes et al. (2012), the time required for the fish larvae produced in the spring reproductive period to grow and reach the shallow areas of the surf zone explains the lag of 1 month observed in the response of the species abundance to water temperature in Cassino beach. Similar to what happens in other subtropical environments, biological cycles of reproduction and recruitment of fishes associated with the increased productivity of the system during spring and summer also occur in this study site (Monteiro-Neto et al. 2003; Lima and Vieira 2009; Moraes et al. 2012; Rodrigues and Vieira 2012). Monteiro-Neto et al. (2003) emphasized that the occurrence and seasonal abundance of fish species in the surf zone reflect patterns of recruitment of juveniles determined by both the seasonality of reproduction and the seasonal variations in circulation patterns. These ecological mechanisms explain the strong positive relationships of temperature and seasonality with abundance and species richness that were detected by our models.
Salinity and surface water temperature appeared as important factors in the variation of abundance and in defining distinct groups A and B for the ichthyofauna. The river discharges were important components in all proposed models contributing with increased predictive power for both richness and abundance of the species that compose Group A. A hypothesis to help explain this result is the fertilization of the continental shelf promoted by the estuarine plume (Miranda et al. 2002). With more nutrients on the coast, there is an increase in marine productivity, which could attract more species into the assemblage and increase the abundance of at least some of them.
The unique possibility of the present study to glimpse at the fish assemblage over the long period of 16 years allows the identification of a decreasing trend in both species richness and in the overall abundance in Group B. Other studies in the region, covering shorter periods, had been unable to identify these concerning trends.
Over the past decades, natural and anthropogenic factors, and their conjunction, have been modifying the dynamics of the Patos Lagoon Estuary and adjacent coastal waters, which includes the Cassino Beach (Odebrecht et al. 2010a). For instance, positive anomalies of estuarine freshwater outflow associated with choked morphology of the Patos Lagoon Estuary can generate seaward currents that block the entrance of fish and crustaceans recruits of marine origin into the estuary. Additionally, the input of the strong freshwater outflow into the adjacent coastal zone, especially those observed during El Niño events, forces the drop in salinity and changes the structure and composition of the communities at Cassino Beach (e.g., Garcia et al. 2004, Odebrecht et al. 2010b). The estuarine plume of the Patos Lagoon also plays an important role with the input of fine sediments (silt and clay) into the coastal zone. These sediments form natural mud deposits that can reach the Cassino Beach during climatic events, changing the surf-zone hydrodynamics, especially in the attenuation of waves (Calliari et al. 2007), altering the concentrations of the dissolved inorganic nutrient (Odebrecht et al. 2010b) and causing significant changes in biotic components (e.g., phytoplankton species—Odebrecht et al. 2003; benthic invertebrates—Silva et al. 2008; and ichthyofauna—Mont’Alverne et al. 2012). Although it is a natural phenomenon, dredging operations on poorly sited dumpsites seems to amplify the production of fluid mud (Calliari et al. 2001) and this activity has become more frequent during the last century (Calliari et al. 2009).
Commercially important species, such as O. argentinensis, T. marginatus and, especially, M. liza (Fischer et al. 2011), are present in Group B. This fact allows us to restate the hypothesis that the increase in fishing effort has led to a decrease in the reproductive stock and consequent decrease in the recruitment of those species that are directly related to the surf zone. The long-term changes that have been observed in Cassino Beach, as well as in the Patos Lagoon Estuary, still need further investigation. Causes appear to be complex and are the fruit of an intricate network of natural and anthropogenic factors.
Species richness as response variable showed a positive association with the covariate abundance. Such relation between number of species and number of collected individuals had already been reported in other studies (Gotelli and Colwell 2001; Silvy 2012). But, because our fitted GAM curve showed an asymptote, the number of collected specimens seems to have been sufficient to characterize the assemblies’ richness. While abundance has been decreasing over the last years, its effect over species richness has become part of the explanation for the observed reduction in richness. However, year remains an important covariate in the model, showing that species richness is declining in time, irrespective of the effect induced by changes in abundance.
Finally, we emphasize the importance of continued monitoring of this fish assemblage in order to deepen the investigation of the long-term causes for changes in the abundance of individuals and species richness, perhaps associating these changes also with data from fisheries landing and proxies that serve as evidence of climate change. This is especially attractive due to the location of our study site near the boundary between tropical and temperate regimes.
Conclusions
The Bayesian generalized additive models (GAMs) proved to be an appropriate exploratory tool in ecological studies of species richness and abundance. No significant difference was found between the sampled sites (1) near Molhe Oeste and (2) near Marine Aquaculture Station—EMA/FURG. Seasonality, influenced by changes in water temperature and salinity, is the most influential in the oscillations of richness and abundance of the ichthyofauna in the surf zone of Cassino Beach, RS. Higher discharges of the three main rivers that flow into the Lagoa dos Patos (Jacuí, Taquari, Camaquã) were associated with an increase in species richness and in abundance of some occasional species of the assemblage (Group A). There has been a decrease in the total species richness and abundance of the most representative species in the surf zone of Cassino Beach over the past 16 years.
References
Able KW, Grothues TM, Rowe PM, Wuenschel MJ, Vasslides JM (2011) Near-surface larval and juvenile fish in coastal habitats: comparisons between the inner shelf and an estuary in the New York Bight during summer and fall. Estuar Coasts 34:726–738
Ayvazian SG, Hyndes GA (1995) Surf-zone fish assemblages in south-western Australia: do adjacent nearshore habitats and the warm Leeuwin Current influence the characteristics of the fish fauna? Mar Biol 122:527–536
Belitz C, Brezger A, Kneib T, Lang S (2009) BayesX—Bayesian inference in structured additive regression models. http://www.stat.uni-muenchen.de/~bayesx. Accessed March 2012
Bell KNI, Cowley PD, Whitfield AK (2001) Seasonality in frequency of marine access to an intermittently open estuary: implications for recruitment strategies. Estuar Coast Shelf Sci 52:327–337
Brown AC, McLachlan A (1990) Ecology of Sandy shores. Elsevier, New York
Brown RL, Jacobs LA, Peet RK (2007) Species richness: small scale, encyclopedia of life sciences, vol 26. Wiley, New Jersey
Caddy JF, Sharp GD (1986) An ecological framework for marine fishery investigations. FAO fisheries technical paper, Rome
Calliari LJ, Speranski N, Torronteguy M, Oliveira MB (2001) The mud banks of Cassino Beach, southern Brazil: characteristics, processes and effects. J Coast Res 34:318–325
Calliari LJ, Holland KT, Pereira PS, Guedes RMC, Santo RE (2007) the influence of mud on the Inner Shelf, Shoreface, Beach and Surf Zone Morphodynamics—Cassino, Southern Brazil. Coast Sedim 07:1–11
Calliari LJ, Winterwerp JC, Fernandes E, Cuchiara D, Vinzon SB, Sperle M, Holland KT (2009) Fine grain sediment transport and deposition in the Patos Lagoon—Cassino Beach sedimentary system. Cont Shelf Res 29:515–529
Castillo-Rivera M, Zárate-Hernandez R, Ortiz-Burgos S, Zavala-Hurtado J (2010) Diel and seasonal variability in the fish community structure of a mud-bottom estuarine habitat in the Gulf of Mexico. Mar Ecol 31:633–642
Chatfield C, Collins AJ (1980) Introduction to multivariate analysis. CRC Press, London
Dalla Rosa L, Ford JKB, Trites AW (2012) Distribution and relative abundance of humpback whales in relation to environmental variables in coastal British Columbia and adjacent waters. Cont Shelf Res 36:89–104
Denison DGT, Mallick BK, Smith AFM (1998) Automatic Bayesian curve fitting. J R Stat Soc B 60:333–350
Edwards AWF (1972) Likelihood. Cambridge Academic Press, Cambridge
Eilers P, Marx B (1996) Flexible smoothing using B-splines and penalizes likelihood. Stat Sci 11:89–121
Fahrmeir L, Lang S (2001) Bayesian inference for generalized additive mixed models based on Markov random field prioris. Appl Stat 50:201–220
Faraway JJ (2006) Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. CRC Press, Boca Raton
Fischer LG, Pereira LED, Vieira JP (2011) Peixes estuarinos e costeiros. Conscientia, Rio Grande
Garcia AM, Vieira JP (2001) O aumento da diversidade de peixes no estuário da Lagoa dos Patos durante o episódio El Niño 1997–1998. Atlantica 23:85–96
Garcia AM, Vieira JP, Winemiller KO, Grimm AM (2004) Comparison of 1982–1983 and 1997–1998 El Niño effects on the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil). Estuar Coasts 27:905–914
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin D (2013) Bayesian data analysis. CRC Press, Boca Raton
Gibson RN, Robb L, Burrows MT, Ansell AD (1996) Tidal, diel and longer term changes in the distribution of fishes on a Scottish sandy beach. Mar Ecol Prog Ser 130:1–17
Gomes MP, Cunha MS, Zalmon IR (2003) Spatial and Temporal Variations of Diurnal Ichthyofauna on Surf-Zone of São Francisco do Itabapoana Beaches, Rio de Janeiro State, Brazil. Braz Arch Biol Technol 46:653–664
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. http://folk.uio.no/ohammer/past/. Accessed March 2012
Hardle W, Simar L (2007) Applied multivariate statistical analysis. Springer, Berlin
Hastie TG, Tibshirani RJ (1990) Generalized additive models. CRC Press, Boca Raton
Haynes PS, Brophy D, McGrath D, O’Callaghan R, Comerford S, Casburn P (2010) Annual and spatial variation in the abundance length and condition of juvenile turbot (Psetta maxima L.) on nursery grounds on the west coast of Ireland: 2000–2007. J Sea Res 64:494–504
Hubálek Z (2000) Measures of species diversity in ecology: an evaluation. Folia Zool 49:241–260
Kéry M (2010) Introduction to WinBUGS for ecologists. Elsevier, London
Kinas PG, Andrade HA (2010) Introdução à Análise Bayesiana (com R). Mais Que Nada, Porto Alegre
Knox GA (2001) The Ecology of Sea Shores. CRC Press, Boca Raton
Krebs CJ (1989) Ecological methodology. Harper and Row, New York
Kristiansen T, Drinkwater KF, Lough RG, Sundby S (2011) Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS One 6:e17456
Lang S, Brezger A (2004) Bayesian P-splines. J Comput Gr Stat 13:183–212
Lima MSP, Vieira JP (2009) Variação espaço-temporal da ictiofauna da zona de arrebentação da Praia do Cassino, Rio Grande do Sul, Brasil. Zoologia 26:499–510
Lin X, Zhang D (1999) Inference in generalized additive mixed models by using smoothing splines. J R Stat Soc B 61:381–400
Lindley D (2000) The philosophy of statistics. Statistician 49:233–337
Magurran AE (2004) Measuring biological diversity. Blackwell, Malden
Margalef DR (1958) Information theory in ecology. Gen Syst 3:36–71
Martino EJ, Houde ED (2010) Recruitment of striped bass in Chesapeake Bay: spatial and temporal environmental variability and availability of zooplankton prey. Mar Ecol Prog Ser 409:213–228
McCarthy MA (2007) Bayesian methods for ecology. Cambridge University Press, New York
McIntosh RP (1967) An index of diversity and the relation of certain concepts to diversity. Ecology 48:392–404
McLachlan A, Brown AC (2006) The ecology of sandy shores. Academic Press, Burlington
Menhinick EF (1964) A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 45:859–861
Miranda LB, Castro BM, Kjerfve B (2002) Princípios de Oceanografia Física de Estuários. Editora Universidade de São Paulo, São Paulo
Modde T, Ross ST (1981) Seasonality of fishes occupying a surf zone habitat in the Northern Gulf of Mexico. Fish Bull 78:911–922
Mont’Alverne R, Moraes LE, Rodrigues FL, Vieira JP (2012) Do mud deposition events on sandy beaches affect surf zone ichthyofauna? A southern Brazilian case study. Estuar Coast Shelf Sci 102–103:116–125
Monteiro-Neto C, Cunha LPR, Musick JA (2003) Community structure of surf-zone fishes at Cassino Beach, Rio Grande do Sul, Brazil. J Coast Res 35:492–501
Moraes LE, Paes E, Garcia A, Moller JRO, Vieira J (2012) Delayed response of fish abundance to environmental changes: a novel multivariate time-lag approach. Mar Ecol Prog Ser 456:159–168
Moresco A, Bemvenuti MA (2006) Biologia reprodutiva do peixe-rei Odontesthes argentinensis (Valenciennes) (Atherinopsidae) da região marinha costeira do sul do Brasil. Revista Brasileira de Zoologia 23:1168–1174
Odebrecht C, Abreu PC, Fujita CCY, Bergesch M (2003) The impact of mud deposition on the long term variability of the surf-zone diatom Asterionellopsis glacialis (Castracane) round. J Coastal Res 35:493–498
Odebrecht C, Abreu PC, Bemvenuti CE, Coppertino M, Muelbert JH, Vieira JP, Seeliger U (2010a) Coastal lagoons: critical habitats of environmental change. CRC Press, Boca Raton
Odebrecht C, Bergesch M, Rorig LR, Abreu PC (2010b) Phytoplankton interannual variability at Cassino Beach, Southern Brazil (1992–2007), with emphasis on the surf zone diatom Asterionellopsis glacialis. Estuar Coasts 33:570–583
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Rodrigues FL, Vieira JP (2012) Surf zone fish abundance and diversity at two sandy beaches separated by long rocky jetties. J Mar Biol Assoc U. K. 93:867–875
Romesburg HC (1984) Cluster analysis for researchers. Lifetime Learning Publications, California
Royle JA, Dorazio RM (2008) Hierarchical modeling and inference in ecology. Academic Press, San Diego
Santana FMS, Severi W (2009) Composition and structure of fish assemblage of the surf zone at Jaguaribe beach, Itamaracá (PE), Brazil. Bioikos 23:3–17
Schoeman DS, Richardson AJ (2002) Investigating biotic and abiotic factors affecting the recruitment of an intertidal clam on an exposed sandy beach using a generalized additive model. J Exp Mar Biol Ecol 276:67–81
Silva JTO, Aguiar MCP, Lopes PRD (2008) Ictiofauna das praias de Cabuçu e Berlinque: uma contribuição ao conhecimento das comunidades de peixes na Baía de Todos os Santos, Bahia, Brasil. Biotemas 21:105–115
Silvy NJ (2012) The wildlife techniques manual: volume 1: research, volume 2: management. Johns Hopkins University Press, Maryland
Spiegehalter DJ, Best NJ, Carlin BP, Van der Linde A (2002) Bayesian measure of model complexity and fit. J R Stat Soc B 64:583–639
Strydom NA, d’Hotman BD (2005) Estuary-dependence of larval fishes in a non-estuary associated South African surf zone: evidence for continuity of surf assemblages. Estuar Coast Shelf Sci 63:101–108
Taylor DA, Nichols RS, Able KW (2007) Habitat selection and quality for multiple cohorts of young-of-the-year bluefish (Pomatomus saltatrix): comparisons between estuarine and ocean beaches in southern New Jersey. Estuar Coast Shelf Sci 73:667–679
Vasconcellos RM, Santos JMS, Silva MA, Araujo FG (2007) Efeito do grau de exposição às ondas sobre a comunidade de peixes juvenis em praias arenosas do Município do Rio de Janeiro, Brasil. Biota Neotropica 7:93–100
Vaz AC, Möller OO, Almeida TL (2006) Análise Quantitativa da Descarga dos Rios Afluentes da Lagoa dos Patos. Atlântica 28:13–23
Venables WN, Dichmont CM (2004) GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fish Res 70:319–337
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal Gradients of Biodiversity: pattern, Processes, Scale and Synthesis. Annu Rev Ecol Evol Syst 34:273–309
Wood SN (2006a) On confidence intervals for Generalized additive models based on Penalized regression splines. Aust N. Z. J Stat 48:445–464
Wood SN (2006b) Generalized additive models: an introduction with r. CRC Press, Boca Raton
Acknowledgments
This work was developed as part of the Master of Science dissertation by the first author working under the guidance of the second and with a scholarship provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). While any errors that may be present are our own, we would like to thank three excellent reviewers along with Luciano Dalla Rosa and Henrique N. Cabral for their helpful comments and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Thomas Mehner.
Rights and permissions
About this article
Cite this article
Martins, A.C.B., Kinas, P.G., Marangoni, J.C. et al. Medium- and long-term temporal trends in the fish assemblage inhabiting a surf zone, analyzed by Bayesian generalized additive models. Aquat Ecol 49, 57–69 (2015). https://doi.org/10.1007/s10452-015-9504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-015-9504-9