Abstract
The objective of this study was to evaluate the interactions between green tide-forming macroalgae Ulva linza and red macroalgae Gracilaria lemaneiformis in the laboratory. The results demonstrated that the presence of U. linza can restrict growth (9–31 %) and photosynthesis (25–85 %) of G. lemaneiformis. In contrast, G. lemaneiformis had little apparent effect on the growth of U. linza. Culture medium experiments confirmed that allelochemicals may be released by both the tested macroalgae. The causative mechanism for the growth and photosynthesis inhibition of G. lemaneiformis was not light limitation nor increase of pH, but a combination of allelopathic effects of U. linza and nutrient competition between the two macroalgae. Moreover, the “green tide” macroalga U. linza was a stronger competitor for nutrient than G. lemaneiformis. The results from this study provide evidence for the mechanisms of “green tide” formation by U. linza: potent allelopathic effects on G. lemaneiformis and faster nutrients uptake than its competitors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The structure, functioning, and dynamics of marine algal communities are not merely an assemblage of species populations in a given area, but governed by various positive, negative, or indifferent interactions between species and by interactions of species with the environment. A number of previous studies demonstrated that both positive and negative interactions exist among primary producers (Gross 2003; Vermeij et al. 2011). Aquatic macrophytes may produce toxic substances to defend themselves against herbivores or other phototrophic organisms in competition for light and nutrients (Mulderij et al. 2005; Wang et al. 2007; Huo et al. 2011). This phenomenon of interactions among living plants was named allelopathy by Molish (1937). The allelopathic relationships between microalgae and macroalgae have been investigated previously, and macroalgae have been found to be feasible and efficient biological inhibitors of harmful algal blooms (HABs) because of their environmentally friendly features (Jeong et al. 2000; Wang et al. 2007, 2009; Nan et al. 2008; Tang and Gobler 2011).
“Green tides” are a special type of “HABs” which have been increasing in severity and geographic range and are now a growing concern globally (Ye et al. 2011). Researchers have shown that the spread and decomposition of “green tide” macroalgae have a negative impact on other species and/or the environment. For example, they can lead to the uncoupling of biogeochemical cycles in sediments from those in the water column (Valiela et al. 1997), have a negative effect on sea grass beds due to shading and disruption of feeding by wading birds (Raffaelli et al. 1998), cause the development of a lethal environment due to oxygen deficiency (Charlier et al. 2007), and lead to a shift from a high-diversity mixture to low-diversity assemblages of fast-growing annuals (Worm et al. 2001; Ye et al. 2011). To date, the mechanisms underlying the negative impacts of green tide-forming macroalgae during their coexistence with other species remain unclear except for clues from a few studies. Svirski et al. (1993) found that Gracilaria spp. cultured in the presence of Ulva lactuca exhibited growth inhibition, but it was not due to shading or nutrient depletion but rather seemed to be caused by competition for inorganic carbon or some type of allelopathy. Friedlander et al. (1996) later confirmed that extracts derived from seawater in which U. cf. lactuca was cultured in advance had an allelopathic effect on Gracilaria spp.
Ulva linza is a “green tide” macroalga distributed naturally along the coast from the Liaodong peninsula to Hainan Island, China, and it grows well from March to May in northern China (Mu et al. 2010). U. linza reportedly can cause rapid lysis of Prorocentrum micans (Jin et al. 2005). Gracilaria lemaneiformis is an economic red macroalgae distributed naturally along the coast of Shandong Province and grows well from spring to late autumn in northern China. Thus, U. linza and G. lemaneiformis overlap in geographical distribution and growth periods for about 3 months (Brawley and Fei 1988; Zhou et al. 2006).
The purpose of this study was to provide a new insight into the ecological impacts of the “green tide” macroalga U. linza on its community and seaweed cultivation, and into the control of “green tides” by understanding the ecological relationship between U. linza and G. lemaneiformis in the co-culture system, and by determining if there are positive or negative effects on each other. To examine these effects, manipulative experiments were conducted in the laboratory, and physiological parameters, such as algal growth, algal photosynthesis, nutrient assimilation, and change in pH in the culture medium, were measured.
Materials and methods
Sampling and culture conditions
Ulva linza and G. lemaneiformis were collected in May 2011 from the intertidal zone of Sungo Bay, Weihai, Shandong Province of China. In the laboratory, the intact and healthy samples were washed several times with sterile seawater, and treated with 1 % sodium hypochlorite for 2 min, and then rinsed with autoclaved seawater (McLachlan 1979; Garcia-Jimenez et al. 1999; Zhang et al. 2010). Both U. linza and G. lemaneiformis were pre-cultured in f/2 medium in an incubator without additional N or P supplementation for 48 h before running the experiments. The temperature was maintained at 15 °C. Illumination was provided by cool-white fluorescent lamps at 100 μmol photons m−2 s−1 on a 12:12-h light:dark cycle. All cultures were shaken manually at fixed times twice every day. The pH and salinity of the seawater used for the experiments were 8.0 ± 0.01 and 30 ± 0.01, respectively.
Effects of fresh U. linza thalli on G. lemaneiformis and fresh G. lemaneiformis thalli on U. linza
In order to determine the effects of the presence of fresh U. linza thalli on the growth of G. lemaneiformis, samples (1.25 g wet weight L−1) of the latter were co-cultured with four different amounts of U. linza (0, 1.25, 3.75, and 6.25 g wet weight L−1, marked as 1G (control), 1G1U, 1G3U, and 1G5U, respectively). The experiments were conducted in 500-mL flasks containing 400 mL of f/2 culture medium supplemented with 240 μmol L−1 NaNO3 and 30 μmol L−1 KH2PO4 at 15 °C, and illuminated at 100 μmol photons m−2 s−1. Correspondingly, to determine the effects of the presence of G. lemaneiformis thalli on the growth of U. linza, samples (1.25 g wet weight L−1) of the latter were co-cultured with four different amounts of G. lemaneiformis (0, 1.25, 3.75, and 6.25 g wet weight L−1, marked as 1U (control), 1U1G, 1U3G, and 1U5G, respectively) under the same culture conditions as described above. All experiments in this study were conducted in triplicate, and sterile techniques were used in all experimental steps. These experiments lasted for 96 h. Macroalgae growth was estimated by monitoring changes in algal wet weight at 0, 48, and 96 h. The detailed procedures included: (1) collecting the thalli samples from culture medium; (2) drying the thalli samples with 5 layers of filter papers three times, 10 min each time; (3) measuring the fresh wet of thalli samples. All procedures were performed on clean benches, and filter papers were sterilized by ultraviolet light.
Nutrient analysis
During the experiments, water samples (5 mL) were collected at 0, 12, 24, 48, and 96 h of incubation and filtered immediately through acetate cellulose filters, then frozen in polyethylene centrifuge tubes for storage until analysis. Concentrations of nitrate (NO3–N) and phosphate (PO4–P) were analyzed photometrically using an AutoAnalyzer (BRAN and LUEBBE AA3, Germany).
Assessment of chlorophyll fluorescence
The efficiency of photosystem II [Y(II)] is the most important one among chlorophyll fluorescence parameters used to assess the ability of photosynthesis. It represents the proportion of light absorbed by PSII that is used in photochemistry and hence indicates overall photosynthesis. And its change trend can signify the degree of damage of algal cells (Scebba et al. 2006; Gao et al. 2012, 2013). Photosynthetic efficiency Y(II) of algal samples was measured using the pulse–amplitude modulated method on a Dual-PAM-100 (Walz, Effeltrich, Germany) connected to a PC running WinControl software. Values were calculated as follows: Y(II) = (F m′ − F t)/F m′. The real-time fluorescence yield F t was obtained by averaging the fluorescence readings within 0.2 s and the maximum fluorescence yield (F m′) was detected when the samples were illuminated by actinic light at 100 μmol photons m−2 s−1. The procedures were according to directions for the instrument and Bilger and Björkman (1990), Krause and Weis (1991), and Gao et al. (2012; 2013).
pH monitoring
Culture medium of samples was also monitored for pH levels during the experiments. Measurements were performed using a pH meter (Thermo Scientific Orion Star Series™ Benchtop pH meter, ±0.01 unit, calibrated prior to each use with NIST traceable standards).
Effects of culture filtrate of U. linza on G. lemaneiformis and of G. lemaneiformis on U. linza
Macroalgae culture medium was prepared by separately culturing G. lemaneiformis and U. linza in sterilized seawater at amount of 10 g wet weight L−1 for 48 h without nutrient enrichment. Thereafter, the macroalgae thalli were removed and the macroalgae-free culture medium was filtered through 0.45-μm acetate cellulose filters and the filtrates with f/2 mother solution was the medium for experiments to study the effects of culture filtrates on fresh algal thalli at a concentration of 1.25 g wet weight L−1. The media containing culture filtrates were resupplied with supplemented macronutrients every 24 h, and the pH was adjusted to 8.0 ± 0.01 using 0.2 mol L−1 HCl or 0.2 mol L−1 NaOH every day. The culture system was kept at 15 °C with a light intensity of 100 μmol photons m−2 s−1 and a 12:12-h light:dark cycle. The experiments lasted for 96 h, and parameters including algal growth and photosynthesis were monitored using the methods described above.
Statistical analysis
The significance of the variance between treatments was analyzed using two-way ANOVA by the software SPSS 17.0. Post hoc tests were examined using Tukey’s test for two-way ANOVA and Dunnett’s test for multivariate ANOVA. The assumptions of homogeneity of variance and normality were assessed by scatter plots of residuals and normal curves of residuals, respectively. The significance level was set at 0.05 for all tests unless otherwise stated.
Results
Effects of fresh U. linza thalli on G. lemaneiformis
In the fresh thalli co-culture experiment, the presence of U. linza significantly suppressed the growth (Fig. 1a) (two-way ANOVA, p < 0.001) and photosynthesis (Fig. 1b) (two-way ANOVA, p < 0.001) of G. lemaneiformis along with incubation time of 96 h. At the end of the experiment, the biomass of the monocultured G. lemaneiformis (1G) increased by 11.7 % and Y(II) increased by 12.1 %. In contrast, G. lemaneiformis biomass was reduced by 9.4, 13.3, and 30.6 % in the three co-culture systems (1G1U, 1G3U, and 1G5U), respectively. Y(II) of G. lemaneiformis co-cultured with the three U. linza treatments followed a concentration-dependent trend and decreased by 27.2, 39.2, and 84.7 %, respectively. By linear regression analysis, growth rate and Y(II) of G. lemaneiformis were positively correlated (R 2 = 0.916) in both the monocultured and co-cultured systems (Fig. 1c). The photographs in Fig. 2 show the morphological changes of G. lemaneiformis when cultured with different amounts of U. linza at 48 h. In the co-culture experiment, the tinct of G. Lemaneiformis faded and intenerated with the passage of time, the more of the U. linza biomass, the heavier the color fading and decomposition (Fig. 2a, b, c, d).
Morphological changes of G. lemaneiformis after 48 h in the fresh thalli batch co-culture experiment. a the control without U. linza addition; b G. lemaneiformis (1.25 g wet weight L−1) co-cultured with 1.25 g wet weight L−1 of U. linza; c G. lemaneiformis (1.25 g wet weight L−1) co-cultured with 3.75 g wet weight L−1 of U. linza; d G. lemaneiformis (1.25 g wet weight L−1) co-cultured with 6.25 g wet weight L−1 of U. linza
Effects of fresh G. lemaneiformis thalli on U. linza
Ulva linza grew well both in monoculture and in co-cultures with G. lemaneiformis. Two-way ANOVA analysis indicated that different quantities of G. lemaneiformis had no significant effects on the growth of U. linza compared to the control (two-way ANOVA, p = 0.799). By the end of the experiment, the biomass of monocultured U. linza (1U) increased by 40.3 % and that in the three co-culture treatments (1U1G, 1U3G, and 1U5G) increased by 39.0, 38.4, and 30.9 %, respectively. Although the presence of higher quantities of G. lemaneiformis resulted in lower values of Y(II) for U. linza, the difference was not significant (two-way ANOVA, p = 0.308) (Fig. 3a). Y(II) of U. linza first increased and then decreased in both monoculture and co-culture. After 96 h, Y(II) of U. linza had increased by 12.5 % in monoculture (1U) and 10.5, 9.3, and 1.2 % in the three co-culture treatments (1U1G, 1U3G, and 1U5G), respectively (Fig. 3b). Via linear regression analysis, a positive correlation (R 2 = 0.713) between growth rate and photosynthetic efficiency Y(II) of U. linza was obtained in both the monoculture and co-culture systems (Fig. 3c).
Nutrient changes in fresh thalli co-culture
Figure 4 shows the changes in nutrient concentration over culture time in the co-culture experiments. Two-way ANOVA analysis demonstrates that both NO3–N and PO4–P concentrations significantly declined with time in both fresh thalli co-culture systems (two-way ANOVA, p < 0.001). In the G. lemaneiformis monoculture, the concentrations of NO3–N (Fig. 4a) and PO4–P (Fig. 4b) decreased by about 36.9 and 30.5 %, from an initial 240 to 151.36 μmol L−1 and from an initial 30 to 20.86 μmol L−1 after incubation for 96 h, respectively. NO3–N (Fig. 4c) and PO4–P concentrations (Fig. 4d) in monocultured U. linza decreased to much lower levels, from an initial 240 to 0 μmol L−1 and from an initial 30 to 0.8 μmol L−1 after 96 h of incubation, respectively. In the G. lemaneiformis co-cultured with three concentrations of U. linza, NO3–N concentration decreased from 240 to 107.07, 62.36, and 27.29 μmol L−1 (Fig. 4a), and for U. linza co-cultured with three concentrations of G. lemaneiformis, NO3–N concentration decreased from 240 to 107.17, 89.48, and 27.18 μmol L−1 after 96 h of incubation (Fig. 4c). The decrease of NO3–N was faster in the monoculture control of U. linza than that in the co-cultures of U. linza and G. lemaneiformis. The concentrations of PO4–P, however, displayed a different pattern compared to that of NO3–N when the thalli addition exceeded 1.25 g wet weight L−1. In the 1G3U and 1G5U co-cultured systems, the PO4–P concentration declined significantly to 2.98 and 0.87 μmol L−1 after 12 h of incubation, respectively, and the concentration reached zero after 24 h of incubation (Fig. 4b). However, in the 1U3G and 1U5G co-cultured systems, PO4–P concentration decreased gently to 1.78 and 1.41 μmol L−1 after 96 h of incubation, respectively (Fig. 4d).
a Changes in nitrate concentrations and b phosphorus concentrations with culture time for G. lemaneiformis co-cultured with various amounts of U. linza; c changes in nitrate concentrations and d phosphorus concentrations with culture time for U. linza co-cultured with various amounts of G. lemaneiformis
pH changes in fresh thalli co-culture
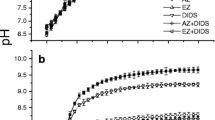
The pH of the culture medium used in the monoculture and co-culture systems was initially 7.97. Over time, the pH values in all treatments increased (Table 1). A clear biomass-dependent relationship was observed between the initial concentration of fresh thalli (either U. linza or G. lemaneiformis) and pH values measured after 96 h of incubation (Table 1). The pH values increased from 7.97 to 9.98, 10.13, 10.18, 10.02 and 10.06 in 1G1U, 1G3U, 1G5U, 1U3G, and 1U5G co-culture systems after 96 h cultivation, respectively.
Effects of macroalgae culture filtrate
Figures 5 and 6 show the results of the experiments in which U. linza or G. lemaneiformis was cultured with the macroalgae culture filtrate of G. lemaneiformis or U. linza, respectively. The U. linza culture filtrate dramatically inhibited growth (two-way ANOVA, p < 0.001) and Y(II) (two-way ANOVA, p < 0.001) of G. lemaneiformis in comparison to the control. After 96 h of incubation, the biomass (Fig. 5a) and Y(II) (Fig. 5b) of G. lemaneiformis decreased by 11.4 and 50.1 %, whereas that in the control increased by 11.7 and 12.2 %, respectively. In contrast, the G. lemaneiformis culture filtrate had no significant effect on the growth (two-way ANOVA, p = 0.831), but significant influence on Y(II) (two-way ANOVA, p < 0.001) of U. linza over the course of the experiment compared to the control (Fig. 6a) and Y(II) (Fig. 6b). After 96 h of incubation, the biomass of U. linza in this treatment increased by 53.5 and 4.3 %, respectively.
Discussions
Although algal blooms, including those considered disastrous, can be natural phenomena, the global problem of “green tides” has increased over the last three decades both in extent and in how it is perceived by the public (Ye et al. 2011). They are great threats to fisheries, public health, and economies around the world (Tang and Gobler 2011). Whether or not algal blooms cause net increases in local or regional species biodiversity or exclude native algal assemblages is a controversial issue (White and Shurin 2011). However, some reports demonstrated that algal blooms can bring negative impacts on the neighbors. Ulva cf. lactuca, which is one of the causative agents of “green tides”, reportedly can produce toxic substances that negatively affect other species in the community and lead to reductions in primary productivity and biodiversity (Friedlander et al. 1996).
Multiple factors, such as resource competition (Huo et al. 2011), environmental factors (Wang et al. 2009), potential effects of materials density (White and Shurin 2011), and negative allelopathy (Tang and Gobler 2011), may account for negative interactions among species. Huo et al. (2011) indicated that the growth of Karenia mikimotoi was suppressed by Gracilaria verrucosa mainly through competition for nutrients, especially nitrogen. The results of the present study demonstrated that the “green tide” macroalga U. linza was able to restrict the growth and photosynthesis of G. lemaneiformis. In contrast, G. lemaneiformis had little effect on the growth of U. linza.
In the present study, high-level nutrient assimilation clearly occurred and U. linza exhibited higher nutrient uptake than G. lemaneiformis. In the culture filtrate of U. linza in which nutrients were added every 24 h, both growth and Y(II) of G. lemaneiformis were significantly inhibited by 11.4 and 50.1 % in comparison to the control, which suggests that allelochemicals released by U. linza may contribute to these negative effects. Meanwhile, the increasing rate of biomass and photosynthesis of target species decreased gradually with increasing biomass of U. linza or G. lemaneiformis added in the fresh thalli co-culture system. Moreover, the inhibition extent of the two parameters of G. lemaneiformis from the culture filtrate was slighter than those from the high biomass fresh thalli co-culture systems (1G3U and 1G5U). Thus, potential effects of materials density should not be neglected in haste in our experiments, and it might come from the increasing overall algal density and the interspecific competition for nutrient. After all, more biomass in culture system leads to more serious nutrient limitation and more fierce competition. White and Shurin (2011) manipulated Sargassum muticum in the low intertidal zone to test its effects via both light and space competition at different densities over two consecutive growing seasons and found non-linear, density-dependent effects of S. muticum on native macroalgae richness.
Previous studies have also shown that macroalgae can change the pH of the culture medium and make it unsuitable for microalgal growth (Schmidt and Hansen 2001; Lundholm et al. 2005). In the present study, the pH levels in G. lemaneiformis co-cultured with various quantities of U. linza increased significantly (range 7.97–10.19) compared to that in monocultured G. lemaneiformis (range 7.97–9.06) (Table 1). U. linza displayed higher values of photosynthesis than G. lemaneiformis. Two previous studies showed that high rates of photosynthesis may draw down CO2 and increase pH levels, and high pH has been invoked to account for the allelopathic affects of some autotrophs (Schmidt and Hansen 2001; Lundholm et al. 2005). Thus, it seemed that high photosynthesis levels of U. linza leading to pH increase might account for the negative effects on G. lemaneiformis. However, the existence of allelopathic effects of U. linza on G. lemaneiformis cannot be concluded based only on this result. In the culture filtrate experiments (Fig. 5), in which pH was adjusted to 8.0 ± 0.01 every 24 h, both growth and Y(II) of G. lemaneiformis were also dramatically inhibited. These data show that the high pH increase was not responsible for the observed negative effects on G. lemaneiformis.
Light limitation was another factor that may play a role in the interactions. For example, Sargassum muticum at high cover excluded native species and reduced richness through light competition by shading smaller, understory macroalgae (White and Shurin 2011). However, the light limitation could be eliminated in the present study. Firstly, the fresh algae were incubated in 500-mL flasks containing 400 ml of culture medium, so the space was large enough for the samples to grow normally. Secondly, the experiment was conducted in an illuminated incubator at 100 μmol photons m−2 s−1 provided from all directions. And thirdly, all the flasks were shaken manually twice every day at fixed times, and the materials could change their positions to receive enough illumination in the culture medium.
Our results show that allelochemicals released by U. linza and nutrient competition may be the two key causes for the negative effects on growth and Y(II) of G. lemaneiformis co-cultured with U. linza. Inhibition of phytoplankton by exudates from macrophytes has been observed in many studies (Mulderij et al. 2005; Wang et al. 2009; Tang and Gobler 2011). The extent of the allelopathic inhibition of G. lemaneiformis growth by U. linza in our study (9–31 %) is similar to inhibitory effects reported for other macrophytes. For instance, the presence of the aquatic macrophyte Stratiotes aloides led to 14–80 % growth inhibition of the green alga Scenedesmus obliquu (Mulderij et al. 2005). Tang and Gobler (2011) reported that the addition of live U. lactuca thalli in bottle and mesocosm experiments consistently led to >50 % reduction in cell densities of Aureococcus anophagefferens in 48 h.
“Green tides” are great threats to fisheries, public health, and economies around the world (Ye et al. 2011). In order to understand and control free-floating macroalgae blooms, many studies have focused on identifying the mechanism that triggers them. One hypothesis about the possible triggering mechanisms of “green tides” is the presence of elevated nutrient levels in coastal waters, or coastal eutrophication. Many biotic and abiotic factors produce excess nutrients, thereby elevating nitrogen and phosphorus content and causing eutrophication. This in turn triggers blooms of green macroalgae such as U. prolifera and U. linza (Worm et al. 2001; Charlier et al. 2008). Apart from excess nutrient loadings, growing greenhouse gas emissions have raised the ocean temperature, and global warming may strengthen the rapid expansion of “green tides”. The human removal of native species may be another causative mechanism for “green tide” blooms (Behrenfeld 2011). Valentine and Johnson (2003) found that removal of the native canopy resulted in a significant increase of the introduced kelp Undaria pinnatifida on the substratum. The last and the most important reason for the increase in “green tides” may lie in the features that give them a competitive edge for rapid and successful colonization in eutrophic conditions: copious production of reproductive spore bodies, high and rapid inorganic nitrogen uptake and storage, and a wide tolerance for adverse environmental conditions (e.g., temperature, light intensity, salinity, and anoxia) (Fu et al. 2008; Ma et al. 2009; Gao et al. 2010; Zhang et al. 2010). Furthermore, results of the present study indicate that the allelopathic effects of “green tide” algae represent another important reason for large-scale blooms. The consequence is that biodiversity reduction and reconstruction of the community caused by “green tides” may increase the scale and frequency of blooms.
The results of the present study help to reduce the large gap in the existing knowledge about allelopathic interactions between “green tide” macroalgae and other macroalgae species. However, our conclusions are elementary and based on laboratory experiments. Moreover, “green tides” are no longer related only to specific species in specific estuaries or coastal areas. To gain more insight into the role of “green tides” and to understand the ecological importance of allelopathic interactions, it is necessary to conduct field or enclosure experiments that focus on the effects of “green tide” macroalgae on the whole phytoplankton community in different seasons. Furthermore, identifying metabolites secreted by “green tide” macroalgae is essential to understanding their strong inhibitory effects.
Conclusions
The green tide-forming macroalga U. linza was found to be able to significantly restrict growth and photosynthesis of G. lemaneiformis when they were co-cultured in the same environment. The causative mechanism for the reduced growth and photosynthesis observed in G. lemaneiformis from U. linza was not light limitation and change of pH, but combination of allelochemicals released by U. linza and nutrient competition. The results from this study provide evidence for the mechanisms of “green tide” formation by U. linza: potent allelopathic effects on and faster nutrients uptake than its competitors such as G. lemaneiformis.
References
Behrenfeld M (2011) Uncertain future for ocean algae. Nat Clim Change 1:33–34
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Brawley S, Fei XG (1988) Ecological studies of Gracilaria asiatica and Gracilaria lemaneiformis in Zhanshan Bay, Qingdao. Chin J Oceanol Limnol 6:22–34
Charlier RH, Morand P, Finkl CW, Thys A (2007) Green tides on the Brittany coasts. Environ Res Eng Manag 41:52–59
Charlier RH, Morand P, Finkl CW, Thys AC (2008) Dealing with green tides on Brittany and Florida coasts. In: Proceedings of the international symposium on environmental science and technology. vol 1, p 1435–1441
Friedlander M, Gonen Y, Kashman Y, Beer S (1996) Gracilaria conferta and its epiphytes: 3. Allelopathic inhibition of the red seaweed by Ulva cf. lactuca. J Appl Phycol 8:21–25
Fu G, Yao JT, Liu FL, Liu JD, Wang XL, Fu WD, Li DP, Zhou MJ, Sun S, Duan DL (2008) Effect of temperature and irradiance on the growth and reproduction of Enteromorpha prolifera J. Ag. (Chlorophycophyta, Chlorophyceae). Chin J Oceanol Limnol 26:357–362
Gao S, Chen XY, Yi QQ, Wang GC, Pan GH, Lin AP, Peng G (2010) A strategy for the proliferation of Ulva prolifera, main causative species of green tides, with formation of sporangia by fragmentation. PLoS ONE 5:1–7
Gao ZQ, Meng CX, Zhang XW, Xu D, Miao XX, Wang YT, Lv HX, Yang LM, Chen LL, Ye NH (2012) Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS ONE 7:1–7
Gao ZQ, Li DM, Meng C, Xu D, Zhang XW, Ye NH (2013) Survival and proliferation characteristics of the microalga Chlamydomonas sp. ICE-L after hypergravitational stress pretreatment. Icarus 226:971–979
Garcia-Jimenez P, Marian FD, Rodrigo M, Robaina RR (1999) Sporulation and sterilization method for axenic culture of Gelidium canariensis. J Biotech 70:227–229
Gross E (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
Huo YZ, Zhang JH, Xu SN, Tian QT, Zhang YJ, He PM (2011) Effects of seaweed Gracilaria verrucosa on the growth of microalgae: a case study in the laboratory and in an enclosed sea of Hangzhou Bay, China. Harmful Algae 10:411–418
Jeong JH, Jin HJ, Sohn CH, Suh KH, Hong YK (2000) Algicidal activity of the seaweed Corallina pilulifera against red tide microalgae. J Appl Phycol 12:37–43
Jin Q, Dong SL, Wang CY (2005) Allelopathic growth inhibition of Prorocentrum micans (Dinophyta) by Ulva pertusa and Ulva linza (Chlorophyta) in laboratory cultures. Eur J Phycol 40:31–37
Krause G, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol 42:313–349
Lundholm N, Hansen PJ, Kotaki Y (2005) Lack of allelopathic effects of the domoic acid-producing marine diatom Pseudo-nitzschia multiseries. Mar Ecol Prog Ser 288:21–33
Ma JH, Ji JM, Xu R, He PM, Zhang TF, Wang XK, Li YH, Ren S, Xu P, Lu QQ (2009) Preliminary study on life history of Ulva linza Linnaeus [Enteromorpha linza (L.) J. Ag.]. J Fish Chin 33:45–52
McLachlan J (1979) Growth media-marine. In: Stein JR (ed) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, Cambridge, pp 25–51
Molish H (1937) Der einfluss einer pflanze auf die andere: allelopathie. von Gustav Fischer, Jena
Mu XW, Lu QQ, Hu CM, Chen SY, Zhang T, Zhang XF, Xu P (2010) Preliminary report on the green algae investigation in mariculture ponds along the coastline of Jiangsu Province. In: Wang QY (ed) Marine aquaculture industry based on ecologic system. China Ocean, Beijing, pp 28–37
Mulderij G, Mooij WM, Smolders AJP, Van Donk E (2005) Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides. Aqua Bot 82:284–296
Nan CR, Zhang HZ, Lin SZ, Zhao GQ, Liu XY (2008) Allelopathic effects of Ulva lactuca on selected species of harmful bloom-forming microalgae in laboratory cultures. Aqua Bot 89:9–15
Raffaelli DG, Raven JA, Poole LJ (1998) Ecological impact of green macroalgal blooms. Oceanogr Mar Biol 36:97–125
Scebba F, Canaccini F, Castagna A, Bender J, Weigel HJ, Ranieri A (2006) Physiological and biochemical stress responses in grassland species are influenced by both early-season ozone exposure and interspecific competition. Environ Pollut 142:540–548
Schmidt LE, Hansen PJ (2001) Allelopathy in the prymnesiophyte Chrysochromulina polylepis: effect of cell concentration, growth phase and pH. Mar Ecol Prog Ser 216:67–81
Svirski E, Beer S, Friedlander M (1993) Gracilaria conferta and its epiphytes. 2. Interrelationship between the red seaweed and Ulva cf. lactuca. Hydrobiology 260/261:391–396
Tang YZ, Gobler CJ (2011) The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 1:480–488
Valentine JP, Johnson CR (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295:63–90
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
Vermeij MJA, Dailer ML, Smith CM (2011) Crustose coralline algae can suppress macroalgal growth and recruitment on Hawaiian coral reefs. Mar Ecol Prog Ser 422:1–7
Wang Y, Yu ZM, Song XX, Tang XX, Zhang SD (2007) Effects of macroalgae Ulva pertusa (Chlorophyta) and Gracilaria lemaneiformis (Rhodophyta) on growth of four species of bloom-forming dinoflagellates. Aqua Bot 86:139–147
Wang Y, Zhou B, Tang XX (2009) Effects of two species of macroalgae Ulva pertusa and Gracilaria lemaneiformis on growth of Heterosigma akashiwo (Raphidophyceae). J Appl Phycol 21:375–385
White LF, Shurin JB (2011) Density dependent effects of an exotic marine macroalga on native community diversity. J Exp Mar Biol Ecol 405:111–119
Worm B, Heike K, Sommer U (2001) Algal propagules banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia 128:281–293
Ye NH, Zhang XW, Mao YZ, Liang CW, Xu D, Zou J, Zhuang ZM, Wang QY (2011) “Green tides” are overwhelming the coastline of our blue planet: taking the world’s largest example. Ecol Res 29:541–546
Zhang XW, Wang HX, Mao YZ, Liang CW, Zhuang ZM, Wang QY, Ye NH (2010) Somatic cells serve as a potential propagule bank of Enteromorpha prolifera forming a green tide in the Yellow Sea, China. J Appl Phycol 22:173–180
Zhou Y, Yang HS, Hu HY, Liu Y, Mao YZ, Zhou H, Xu XL, Zhang FS (2006) Bioremediation potential of the macroalga Gracilaria lemaneiformis (Rhodophyta) integrated into fed fish culture in coastal waters of north China. Aquaculture 252:264–276
Acknowledgments
The present study was supported by a project from Science and Technology Commission of Qingdao Shinan District (2012-5-008-SW), National Natural Science Foundation of China (41176153, 41106124, 31170279), Natural Science Foundation of Shandong Province (2009ZRA02075, ZR2011DM006, ZR2011CQ010), Shandong Science and Technology plan project (2011GHY11528), the Hi-Tech Research and Development Program (863) of China (2012AA052103), the Specialized Fund for the Basic Research Operating expenses Program (20603022012004, 2010-ts-03), Qingdao Municipal Science and Technology plan project (11-3-1-5-hy), National Marine Public Welfare Research Project (200805069), and the National Science and Technology Pillar Program, (2008BAD95B11, 2010BAC68B03) and the Supporting Project for Young Teachers in Shandong University of Technology (4072-110045).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhengquan Gao and Dong Xu have contributed equally to this work.
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
Gao, Z., Xu, D., Meng, C. et al. The green tide-forming macroalga Ulva linza outcompetes the red macroalga Gracilaria lemaneiformis via allelopathy and fast nutrients uptake. Aquat Ecol 48, 53–62 (2014). https://doi.org/10.1007/s10452-013-9465-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-013-9465-9