Abstract
Algal lipids provide essential fatty acids for higher trophic levels in the marine food web, and understanding the fatty acid composition in phytoplankton is critical for evaluating its value as a diet. Nineteen microalgal species, mainly originating from the Baltic Sea, covering major algal classes were grown in different growth conditions. Samples were taken during both the exponential and stationary growth phases and analysed regarding their fatty acid methyl esters and free fatty acids. Our results show that across all screened species, total fatty acids increased significantly from exponential to stationary growth phase. Furthermore, it was observed that warm-water species contained more lipids and differed in their lipid profile as compared with the cold-water species. Brackish water species also showed a slightly higher lipid content than the marine species, but their lipid profile was not significantly different. Plotting changes in lipids against changes in cell nitrogen revealed a significant dependency between decrease in cell nitrogen and increase in lipids across all tested species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the aquatic food web, phytoplankton lipids represent an important metabolic fuel for higher trophic levels (Lee et al. 1971; Budge and Parrish 1998). Algal lipids can comprise unusual structures exhibiting peculiar bioactivities and contain essential fatty acids that organisms at other trophic levels have limited abilities to synthesize (Guschina and Harwood 2006). Omega-3 fatty acids, in particular eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are of vital importance to aquatic consumers, and there are numerous studies on their importance in the food web (e.g. Müller-Navarra et al. 2000). Unicellular algae can accumulate very high concentrations of lipids, i.e. >30 % of their biomass (Sheehan et al. 1998), and the cultivation of phytoplankton has received attention as a potential feed for aquaculture, and also recently as a potential raw material for biofuel production. Very few studies have examined the lipid profiles of different species in different growth stages (Kattner et al. 1983; Reitan et al. 1994; Lv et al. 2010). The mechanisms regulating their lipid content and composition are not fully resolved (Hu et al. 2008). Such knowledge is required both for understanding energy flows and organism dynamics in aquatic food webs, and for developing applications where lipids derived from phytoplankton can be commercially produced.
Several environmental parameters, such as temperature, light and nutrition, affect the lipid content and lipid profile of phytoplankton. Different types of nutrient limitation have been shown to increase the overall lipid content per cell in algal species where lipids represent the primary storage product. In particular, nitrogen starvation has often been reported as a trigger for increasing the lipid content (Hu et al. 2008; Williams and Laurens 2010). Under optimal growth conditions, phytoplankton use photosynthetically fixed carbon for growth, and the lipids present in the cell are primary structural components in membranes. During the transition from optimal to limited growth conditions, due to, e.g., nutrient starvation, cell division ceases and excess carbon can be stored as an energy reserve. This could be in the form of either starch or lipids.
We investigated the hypothesis that a growth-phase-dependent change in fatty acid pattern and lipid quality is a general phenomenon that can be observed in a broad diversity of microalgal species. In contrast to many previous studies, we investigated species from all major algal groups and determined lipid profiles in both exponential growth phase (EX) and stationary growth phase (ST). Our experiments included algae adapted to different salinities and temperatures to test whether a dependency exists between growth-phase-dependent shifts in lipid characteristics and growth conditions.
Materials and methods
Cultures
Nineteen phytoplankton strains were cultivated and screened for lipid contents in EX and ST. Brackish water strains were isolated from the Baltic Sea, except Isochrysis species (CCAP 927/12), which was isolated from brackish water in England. Strains labelled TV were obtained from the culture collection of Tvärminne Zoological Station (Hällfors and Hällfors 1992), strain HEM was obtained from the working group of K. Sivonen (Sivonen et al. 1989), and K. Spilling isolated the TVK strains. Warm-water marine strains (CCAP strains) were obtained from the Culture Collection of Algae and Protozoa (Table 1).
Taxonomic determination
In order to confirm the taxonomic classification of Scenedesmus obliquus and Chaetoceros muelleri, 16S ribosomal DNA (rDNA) analysis was carried out. Genomic DNA was extracted with a modified cetyltrimethylammonium bromide method (Doyle and Doyle 1987), and a fragment of the 16S rDNA was amplified by PCR using the Eubac27F and 1492R primers (Lane 1991). The resulting DNA fragments were sequenced and compared to known sequences in the database of the National Centre for Biotechnology Information. The sequence of the S. obliquus sample was 100 % consistent with that of S. obliquus strain UTEX 393 (DQ396875). The 16S rDNA fragment of C. muelleri analysed was 100 % identical with C. muelleri isolate C12 (FJ002219.1).
Growth conditions
The algae were grown in f/2 medium (Guillard and Ryther 1962; Guillard 1975), with the only difference that the macronutrients were adjusted to the molar ratios N/P = 8 and N/Si = 1, so that cells would be nitrogen limited in the ST. Dissolved silicate (Na2SiO3·9H2O) was only added for the diatoms. The medium was prepared using deionized water with added salt, which was autoclaved, and the nutrients were added though a sterile 0.2-μm filter. Based on their environmental requirements, the strains were divided into three cultivation groups: (1) cold-water brackish strains, (2) warm-water brackish strains and (3) warm-water marine strains (Table 1). Groups 1 and 2, the brackish water strains, were cultivated in artificial sea water with 6 g L−1 salt (Instant Ocean, Aquarium Systems Mentor, OH, USA). Strains were kept either at 4 °C (group 1) or at 18 °C (group 2). For the marine strains, salinity was adjusted to 35 g L−1 salt, and they were kept at 18 °C. Experimental cultures were grown in 8-L polycarbonate bottles (cold-water strains) or in 5-L Erlenmeyer flasks using artificial sea water as a medium. Cultures were illuminated with 100 μmol photons m−2 s−1 in a 16-h light/8-h dark cycle. Cultures were continuously aerated with sterile air (pumped through 0.2-μm filters, Sartorius, Göttingen, Germany).
Sampling
For the individual culture strains, no replicas were used, but for the diatom Phaeodactylum tricornutum, we used two different strains isolated from both brackish and marine water. Samples for analyses were collected during EX and ST. At the time of sampling, algal biomass (dry weight) varied on average from 74 (±53) mg L−1 in EX to 207 (±97) mg L−1 in ST. Samples of 1–4 L were concentrated using a centrifuge (3,000–3,500 rpm) or by filtering through a 10-μm mesh so that the final volume was typically 25 or 50 mL. For biomass analysis (chlorophyll a, particulate nutrients and dry weight), an aliquot was then withdrawn. The remaining cells were harvested by centrifugation (10,000 rpm), and the pellets were frozen and stored at −80 °C prior to the lipid analyses. Gymnodinium species had a very low biomass yield and therefore was sampled only in ST. The cyanobacteria, i.e. Synechococcus species and Nodularia spumigena, were sampled only in EX.
Monitoring growth
For defining the different growth phases, growth was monitored 3–5 times per week using chlorophyll a (Chl a) in vivo fluorescence and one to two times per week by extracted Chl a. Chl a in vivo fluorescence was measured using a Cary Eclipse Fluorescence Spectrophotometer (Varian NMR Instruments Palo Alto, California, USA). Samples were dark-acclimated for a few minutes, then 350 μL of subsamples was placed into 96-well plates, and Chl a fluorescence was measured using excitation at 440 nm and emission at 682 nm (10-nm slits). For samples exhibiting high fluorescence, cells were diluted with fresh culture medium in order to measure in the linear range of the method.
To extract Chl a, 0.5–10 mL of samples was filtered onto 13-mm acid-washed glass fibre filters (made from 47-mm filters, Whatman GF/F, Whatman, ME, USA). One millilitre of 96 % ethanol was added, and the samples were extracted for 24 h at room temperature. The samples were then either analysed after 24 h or stored at −20 °C. Chl a was analysed with a Cary Varian Eclipse spectrofluorometer calibrated with pure Chl a (Sigma Inc., St. Louis, MO, USA) using excitation at 430 nm and emission at 670 nm (5-nm slits).
Samples for dry weight (DW) were filtered onto combusted (4 h at 450 °C), acid-washed, preweighed Whatman GF/F filters. Samples were dried (12 h at 80 °C) and weighed. Samples for particulate organic nitrogen (N) and carbon (C) were filtered onto acid-washed and precombusted Whatman GF/F filters and analysed with a Roboprep/Tracermass mass spectrometer (Europa Scientific, UK) according to literature (Spilling et al. 2010).
Lipid extraction and determination
For cell disruption, 500 μL chilled (−20 °C) methanol with 0.1 % butylated hydroxytoluene was added to 5 mg DW algae sample and incubated at −20 °C for 10 min in a reaction vial. In prechilled racks, algae samples were disrupted with two 4-mm stainless steel balls in each vial in a mixer mill (3 min, 25 Hz, MM 301, Retsch, Haan, Germany). Lipids were extracted using a modification of the method of Bligh and Dyer (1959). Transmethylation and gas chromatography (GC) were performed as in the previous studies (Spilling et al. 2013).
Statistical analysis
All statistical analyses were performed with SigmaPlot 11.0 (Systat Software, Inc., CA, USA) and R 2.15.2 (R Core Team 2012). To analyse the influence of cultivation conditions and growth phase on phytoplankton total lipid content, one- and two-way analyses of variance (ANOVA) were used. To inspect how these factors influence the complete 30-dimensional lipid profile, a permutation-based nonparametric multivariate ANOVA (PERMANOVA) was computed through the vegan R package (ver. 2.0–5) (Anderson 2001). The 2D principal component analysis (PCA) projections of the lipid data were computed with R prcomp function. Linear regression was used to compare the dependency between changes in cell nitrogen and cell lipids, and the differences in slopes were examined (GT2 method; Sokal and Rohlf 1980). T tests were performed to test the pooled differences between unsaturated fatty acids (UFA), saturated fatty acids (SAFA) and eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) contents in EX and ST, respectively.
Results
Influence of the growth phase, temperature and salinity on lipid quantity
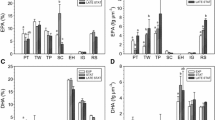
The lipid content of algal species increased from EX to ST (Table 1; Fig. 1). Within the brackish cold-water strains, the total fatty acid content in EX varied between 2.8 and 7.3 % of DW and increased to between 10.9 and 26.5 % of DW in ST. In comparison, the brackish warm-water strains showed similar lower limits of lipid content, 3.5 % of DW in EX and 21.5 % of DW in ST, but some species had a lipid content up to 23.0 % of DW in EX and 40.6 % of DW in ST. The difference between lipid content in EX and ST was less within the screened marine species (5.3–13.3 % of DW in EX, 7.1–32.1 % of DW in ST) compared to both other cultivation groups. A two-way ANOVA confirmed the significant influence of the growth phase on the lipid content of the screened microalgal species (Table 2); lipid quantity was significantly higher in the ST (Fig. 1), whereas high temperature and low salinity had also a minor increasing effect on total lipids (Table 2).
Influence of the growth phase, temperature and salinity on the lipid profiles
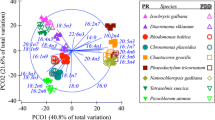
The influences of growth stage, salinity and temperature were first inspected with PCA by using scaled and centred data (Fig. 2). The clustering of lipid profiles for the cold- and warm-water species can be visually observed by inspecting the first principal component (PC1). This result indicates that the cultivation temperature leads to structural differences in lipid profiles of both cultivation groups. In contrast, salinity does not seem to significantly affect the lipid structure. The second principal component (PC2) mainly illustrates the magnitude of change in the overall lipid structure during the transition from EX to ST. If scaling to unit variance is not used, the large overall increase in lipid concentration from EX to ST will dominate the image (not shown). These results were further confirmed with a permutational multivariate ANOVA (PERMANOVA) test: the results in Table 3 show that temperature and growth phase affect the lipid profiles, but salinity does not have such a role in this data set.
A two-dimensional projection of the 30-dimensional lipid data (computed with PCA by using centred and scaled data). Differences in lipid profiles due to different cultivation temperature are seen on the horizontal axis (PC1); differences in lipid profiles due to the growth stage can be observed from the vertical axis (PC2). The extent of change in a lipid profile between both growth stages is shown by the length of the arrow. Small and large circles indicate the salinity (6 g L−1 and 35 g L−1, respectively), and the colour indicates the temperature (blue = 4 °C, red = 18 °C). (Color figure online)
Changes in lipid profiles were specified by calculating changes in percentages of SAFA, UFA, EPA and DHA between EX and ST. SAFA content decreased significantly from 45.4 % of total fatty acids in EX to 35.7 % in ST, while UFA increased in the same range (p = 0.031, T 32 = ±2.263). The decrease in EPA from 6.5 to 4.9 % of total fatty acid content was not significant (p = 0.477, T 37 = 0.719). Also, DHA did not show a significant shift between the two growth phases (mean EX: 5.0 % of total fatty acids, mean ST: 5.6 % of total fatty acids; p = 0.841, T 35 = −0.203).
Linkage between changes in cell nitrogen and lipid content
We wanted to test whether the general differences in lipid content observed between growth phases were linked to nitrogen limitation (indicated by shifts in cellular C/N ratios) during stationary growth. A linear regression revealed a significant dependency of lipid content on nitrogen limitation (Fig. 3). Taking only the species within their respective cultivation group (cold-water brackish, warm-water brackish, warm-water marine) into consideration, the slopes were within similar ranges (0.0397, 0.0427 and 0.0592, respectively). Differences in slope were tested with the GT2 method (Sokal and Rohlf 1980), and no significant differences were observed, which means that all species behave similar. The different offsets of the three cultivation groups indicate that species behave characteristic within their respective cultivation group (Table 4).
Increase in algal lipid concentrations from the exponential (EX) to the stationary (ST) growth phase as a function of changes in cell stoichiometry (C/N ratio). Blue dots indicate the cold-water brackish species, red dots the warm-water brackish species and green dots the warm-water marine species. Coloured lines show the respective linear regression of values of the three cultivation groups, and the black solid line shows the linear regression of all species pooled. The dotted line indicates the 95 % confidence interval of the pooled values. (Color figure online)
Discussion
One aim of our study was to investigate whether dependency of lipid content on growth phase is a general phenomenon in microalgal populations. Evidence for a dependency comes from different sources (Kattner et al. 1983; Borowitzka and Borowitzka 1988; Takagi et al. 2006). However, there has been no consistent testing of this hypothesis across a broad range of algal groups or species that were grown under controlled conditions, perhaps with the exception of Shifrin and Chisholm (1981) screening 30 marine and freshwater species, mainly green algae and diatoms. In contrast to Shifrin and Chrisholm, we focused in the present study on Baltic species that were also grown under different culture conditions (batch culture, light–dark cycle, two different cultivation temperatures). Additionally, we analysed the fatty acid composition and drew conclusions about the influence of environmental conditions on lipid quantity and quality. Nevertheless, our results of twofold to threefold higher lipid contents in the stationary growth phase are in line with Shifrin and Chrisholm’s results. Some previous studies indicate that lipid production is affected by salinity and temperature (Tomaselli et al. 1988; Thompson et al. 1992; De Oliveira et al. 1999; Renaud et al. 2002). Hence, we investigated species from major algal groups previously grown under different salinity and temperature conditions to check whether the hypothetical relationship between growth phase and lipid content is a general phenomenon independent of taxonomic classification and cultivation conditions. As we did not chose an experimental design testing systematically all species in all culture conditions, one has to take into consideration that detected differences in lipid quality and quantity of single algae species are not necessarily only due to culture conditions but also due to differences between the algal species.
The results of our study strongly support the hypothesis that lipid content depends on growth phase. Our experiments showed that the lipid content increases on average 2.6 times from EX to ST. This is consistent with the results from a meta-analysis comparing the lipid contents of several microalgal species between EX and ST (Hu et al. 2008; Williams and Laurens 2010). Hu and co-workers explain lipid accumulation in most microalgae as a response to nitrogen deficiency, a phenomenon first described in the 1950s (Collyer and Fogg 1954). Nevertheless, data to support this hypothesis are not presented in Hu’s review. Furthermore, also deficiency of other nutrients such as phosphorus can result in lipid accumulation and changes in lipid profile (Reitan et al. 1994). Our results support the assumption of a dependency between available nitrogen and cell lipids. We found an overall significant relationship between nitrogen limitation and lipid content across a wide range of microalgae from different taxonomical backgrounds and grown under different cultivation conditions. The positive correlation between the increase in the C/N ratio and the lipid concentration for the various algal groups suggests that this is a general phenomenon. Under nitrogen-deficient conditions, synthesis of N-rich proteins stops, whereas photosynthetic C fixation may continue. The sugars produced during photosynthesis affect the osmotic potential, and the cells need to store carbon in another form to avoid problems with osmoregulation. The most common storage products are lipids and starch, and the preferred storage product is species specific (Rai 1995). Carbon in these forms can be stored in much higher concentrations than as sugars, increasing the C/N ratio. It is assumed that a nitrogen deficiency leads to a decrease in the growth rate. Large amounts of adenosine triphosphate and nicotinamide adenine dinucleotide phosphate, which are normally used for cell growth, are then channelled into fatty acid biosynthesis and lipid accumulation (Harwood and Jones 1989; Thompson 1996). Most studies limited their tested species to a small selected group of microalgae grown under the same conditions (Suen et al. 1987; Larson and Rees 1996; Zhila et al. 2005; Li et al. 2008), originating from the same territory (Harrison et al. 1990; Viso and Marty 1993; Renaud et al. 1999) or which are close relatives (Mansour et al. 1999; Yeesang and Cheirsilp 2011). With the present study, a relationship between a decrease in cell nitrogen and an increase in lipids was demonstrated for a wide range of microalgal species grown under different growth conditions.
Furthermore, we investigated the putative dependency between salinity and lipids. Our results indicate that an increase in the salinity might lead to a decrease in lipid content. Several studies have shown that each algal species has an optimal salinity level for growth and lipid production (Chaffin et al. 2012; Fuentes-Grünewald et al. 2012). Depending on the physiological state, this level might be different for different microalgal species. It was shown that three genes of the fatty acid biosynthesis which are activated among others by a change in salinity in Haematococcus pluvialis have a significant influence on the lipid production (Lei et al. 2012). The algal species we investigated were mainly from Baltic Sea and, therefore, adapted to low salinities. Therefore, it is not surprisingly that our calculations indicate less optimal conditions for algae growing in higher-salinity medium. This result might not be a general phenomenon but probably applies for many Baltic species.
To investigate whether, next to the lipid quantity, also lipid composition shows a significant change, we performed PCA. Results indicate a significant influence of temperature on the lipid profiles. In accordance with literature, all screened species revealed a significant increase of about 10 % in UFA upon reaching ST (Pal et al. 2011; Lei et al. 2012). Generally, low temperatures diminish enzymatic processes and synthetic pathways and result in decreased membrane fluidity (Gurr et al. 2002; Peterson et al. 2007). Most organisms living in a cold environment have a high proportion of UFAs in order to maintain their membrane fluidity (Harwood 1994). Studies on macroalgae showed that algae from cold-water regions are generally richer in polyunsaturated fatty acids (PUFA) than those that are adapted to warm water (Colombo et al. 2006). Also in microalgae, it was found that acclimation to colder conditions increases the proportion of UFAs (Lynch and Thompson 1982, 1984). More recent studies on Dunaliella salina investigated the mechanism protecting photosystem II in cold-acclimated D. salina against freezing damages (Zchut et al. 2003). The results indicated that the level of unsaturated plasma membrane lipids increased in cells acclimated to cold temperatures. Also, cyanobacteria decrease the FA saturation level in cold temperatures (Murata and Wada 1995; Sakamoto et al. 1998). Two very important PUFA are the fatty acids EPA and DHA which do not exhibit a significant shift in quantities between the two growth phases in our study. EPA is a highly important lipid marker for estimating the energy transfer between primary producers and consumers in freshwater food webs (Müller-Navarra et al. 2000). A low EPA content in microalgal communities is related to low zooplankton growth and egg production and, therefore, to low energy transfer efficiency between different trophic levels. Due to different zooplanktons in marine food webs, DHA is maybe even more important (Evjemo et al. 2008). As an essential component of phospholipids in membranes of the central nerve system, DHA was shown to be important for survival, eye development and pigmentation in halibut larvae (Shields et al. 1999).
Our results indicate a clear relationship between the growth stage and lipid content of the investigated algae. It is well established that the lipid content and fatty acid composition are critical elements affecting the food quality of the phytoplankton for higher trophic levels. During a blooming phase, e.g. during the spring bloom in temperate areas, it could be expected that the food quality of the phytoplankton community would improve at the end of the bloom, but factors such as light environment will also affect how much lipids would be accumulated. In natural waters, increased allocation of fixed carbon to lipids, instead of proteins and polysaccharides, has been observed during nutrient-depleted conditions, phytoplankton bloom peak and abundance of certain species (diatoms/cryptophytes) (Lindqvist and Lignell 1997; Rai et al. 1997; Smith et al. 1997; Parrish et al. 2005). It is still an open question to what extent the lipid composition changes as a result of growth stage in a natural environment.
As microalgae are essential as a potential feed for aquaculture and raw material for biofuel production, it is highly important first to understand the factors that influence lipid production. This knowledge is essential to optimize cultivation of commercially interesting microalgae and, hence, to grow them in a cost-effective or even profitable way. Besides the commercial usage, microalgae are also an important metabolic fuel for higher trophic levels in food webs. By understanding the factors influencing microalgal lipids, the occurrence of marker fatty acids in food webs can be explained. These markers can be used to infer environmental factors such as average temperature, illumination and water quality. This study is a first step towards such an understanding; further studies with more factors and algal species have to be performed.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust J Ecol 26:32–46
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA, Borowitzka LJ (1988) Micro-algal biotechnology. University Press, Cambridge
Budge SM, Parrish CC (1998) Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay. Newfoundland. II. Fatty acids. Org Geochem 29(5–7):1547–1559
Chaffin JD, Mishra S, Kuhaneck RM, Heckathorn SA, Bridgeman TB (2012) Environmental controls on growth and lipid content for the freshwater diatom, Fragilaria capucina: a candidate for biofuel production. J Appl Phycol 24(5):1045–1051
Collyer D, Fogg GE (1954) Studies on fat accumulation by algae. J Exp Bot 6(17):256–275
Colombo ML, Rise P, Giavarini F, De Angelis L, Galli C, Bolis CL (2006) Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods Hum Nutr 61(2):67–72
De Oliveira M, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac Int 7(4):261–275
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Evjemo JO, Tokle N, Vadstein O, Olsen Y (2008) Effect of essential dietary fatty acids on egg production and hatching success of the marine copepod Temora longicornis. J Exp Mar Biol Ecol 365:31–37
Fuentes-Grünewald C, Garcés E, Alacid E, Sampedro N, Rossi S, Camp J (2012) Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J Ind Microbiol Biotechnol 39(1):207–216
Guillard R (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 26–60
Guillard R, Ryther J (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Gurr MI, Harwood JL, Frayn KN (2002) Lipid Biochemistry. Lipid biochemistry- An Introduction 5th Edition, Wiley-Blackwell, Oxford
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45(2):160–186
Hällfors G, Hällfors S (1992) The Tvärminne collection of algal cultures. In: Tvärminne Studies, vol 5. University of Helsinki, pp 15–17
Harrison P, Thompson P, Calderwood G (1990) Effects of nutrient and light limitation on the biochemical composition of phytoplankton. J Appl Phycol 2(1):45–56
Harwood JL (1994) Environmental factors which can alter lipid metabolism. Prog Lipid Res 33(1–2):193–202
Harwood JL, Jones AL (1989) Lipid metabolism in algae. Adv Bot Res 16:1–53
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Kattner G, Gercken G, Eberlein K (1983) Development of lipids during a spring plankton bloom in the northern North Sea: I. Particulate fatty acids. Mar Chem 14(2):149–162
Lane DJ (1991) Nucleic acid techniques in bacterial systematics. Wiley, New York
Larson TR, Rees TAV (1996) Changes in cell composition and lipid metabolism mediated by sodium and nitrogen availability in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 32(3):388–393
Lee RF, Nevenzel JC, Paffenhöfer GA (1971) Importance of wax esters and other lipids in the marine food chain: phytoplankton and copepods. Mar Biol 9(2):99–108
Lei A, Chen H, Shen G, Hu Z, Chen L, Wang J (2012) Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol Biofuels 5(1):18
Li Y, Horsman M, Wang B, Wu N, Lan C (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81(4):629–636
Lindqvist K, Lignell R (1997) Intracellular partitioning of 14CO2 in phytoplankton during a growth season in the northern Baltic. Mar Ecol Prog Ser 152:41–50
Lv X, Zou L, Sun B, Wang J, Sun M-Y (2010) Variations in lipid yields and compositions of marine microalgae during cell growth and respiration, and within intracellular structures. J Exp Mar Biol Ecol 391(1–2):73–83
Lynch DV, Thompson GA Jr (1982) Low temperature-induced alterations in the chloroplast and microsomal membranes of Dunaliella salina. Plant Physiol 69(6):1369–1375
Lynch DV, Thompson GA Jr (1984) Chloroplast phospholipid molecular species alterations during low temperature acclimation in Dunaliella. Plant Physiol 74(2):198–203
Mansour MP, Volkman JK, Jackson AE, Blackburn SI (1999) The fatty acid and sterol composition of five marine dinoflagellates. J Phycol 35(4):710–720
Müller-Navarra DC, Brett MT, Liston AM, Goldman CR (2000) A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403(6765):74–77
Murata N, Wada H (1995) Acryl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 308:1–8
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90(4):1429–1441
Parrish CC, Thompson RJ, Deibel D (2005) Lipid classes and fatty acids in plankton and settling matter during the spring bloom in a cold ocean coastal environment. Mar Ecol Prog Ser 286:57–68
Peterson ME, Daniel RM, Danson MJ, Eisenthal R (2007) The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem J 402:331–337
R Core Team (2012) A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/
Rai H (1995) The influence of photon flux density (PFD) on short term 14C incorporation into proteins, carbohydrates and lipids in freshwater algae. Hydrobiologia 308:51–59
Rai H, Arts MT, Wainman BC, Dockal N, Krambeck HJ (1997) Lipid production in natural phytoplankton communities in a small freshwater Baltic lake, Lake Schöhsee, Germany. Freshw Biol 38:581–590
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrition limitation on fatty acid and lipid content of marine microalgae. J Phycol 30(6):972–979
Renaud SM, Thinh L-V, Parry DL (1999) The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 170(2):147–159
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211(1–4):195–214
Sakamoto T, Shen GZ, Higashi S, Murata N, Bryant DA (1998) Alteration of low-temperature susceptibility of the cyanobacterium Synechococcus sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch Microbiol 169(1):20–28
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae. National Renewable Energy Laboratory
Shields RJ, Bell JG, Luizi FS, Gara B, Bromage NR, Sargent JR (1999) Natural copepods are superior to enriched artemia nauplii as feed for halibut larvae (Hippoglossus hippoglossus) in terms of survival, pigmentation and retinal morphology: relation to dietary essential fatty acids. J Nutr 129(6):1186–1194
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: Interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Sivonen K, Himberg K, Luukkainen R, Niemela SI, Poon GK, Codd GA (1989) Preliminary characterization of neurotoxic cyanobacteria blooms and strains from Finland. Toxic Assess 4(3):339–352
Smith REH, Gosselin M, Kattner G, Legendre L, Pesant S (1997) Biosynthesis of macromolecular and lipid classes by phytoplankton in the Northeast Water Polynya. Mar Ecol Prog Ser 147:231–242
Sokal RR, Rohlf FJ (1980) Biometry. W. E. Freeman and Company, New York
Spilling K, Tamminen T, Andersen T, Kremp A (2010) Nutrient kinetics modeled from time series of substrate depletion and growth: dissolved silicate uptake of Baltic Sea spring diatoms. Mar Biol 157:427–436
Spilling K, Brynjólfsdóttir Á, Enss D, Rischer H, Svavarsson HG (2013) The effect of high pH on structural lipids in diatoms. J Appl Phycol. doi:10.1007/s10811-012-9971-5
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J Phycol 23:289–296
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302(1):17–45
Thompson PA, Guo MX, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature: II. On the fatty acid composition of eight species of marine phytoplankton. J Phycol 28(4):488–497
Tomaselli L, Giovannetti L, Sacchi A, Bochi F (1988) Effects of temperature on growth and biochemical composition in Spirulina platensis strain M2. In: Stadler T, Mellion J, Verdus MC, Karamanos Y, Morvan H, Christian D (eds) Algal Biotechnology. Elsevier Applied Science, London, pp 303–314
Viso A-C, Marty J-C (1993) Fatty acids from 28 marine microalgae. Phytochemistry 34(6):1521–1533
Williams PJL, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3(5):554–590
Yeesang C, Cheirsilp B (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102(3):3034–3040
Zchut S, Nir Keren, Ohad I, Uri Pick (2003) Cold-acclimation protects photosystem II against freezing damage in the halotolerant alga Dunaliella salina. J Plant Physiol 160(2):185–192
Zhila NO, Kalacheva GS, Volova TG (2005) Effect of nitrogen limitation on the growth and lipid composition of the green alga Botryococcus braunii Kutz IPPAS H-252. Russ J Plant Physiol 52(3):311–319
Acknowledgments
This study was funded by the Academy of Finland Research Programme “Sustainable energy—SusEn” and the Nordic Energy Research Programme “N-INNER” and MAREX-Exploring Marine Resources for Bioactive Compounds: From Discovery to Sustainable Production and Industrial Applications (Call FP7-KBBE-2009-3, Project nr. 245137). We would like to thank Anke Kremp, Kaarina Sivonen and Seija and Guy Hällfors for providing cultures and Tuulikki Seppänen-Laakso for the development of the lipid extraction and analysing method and helpful discussion about it. The excellent technical assistance of Airi Hyrkäs and Jaana Rikkinen and by the staff of the Tvärminne Zoological Station is gratefully acknowledged. Furthermore, we acknowledge the reviewers for very helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schwenk, D., Seppälä, J., Spilling, K. et al. Lipid content in 19 brackish and marine microalgae: influence of growth phase, salinity and temperature. Aquat Ecol 47, 415–424 (2013). https://doi.org/10.1007/s10452-013-9454-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-013-9454-z