Abstract
In the Sanjiang Plain (North East China), narrowleaf small reed (Deyeuxia angustifolia) usually distributes widely in typical meadow or marsh, while reed (Phragmites australis), the concomitant species, is distributed sparsely in the D. angustifolia communities or relative open sites. To date, the mechanisms responsible for their different distribution patterns are far from clear. Both water level and light are important factors determining plant distribution in wetland ecosystems and therefore, the aim of this paper was to identify the role of these two factors and their potential interaction on plant distribution in this plain. Growth responses and biomass allocation of the two macrophytes were investigated by growing them in three irradiances (300, 100, 20 μmol m−2 s−1) and two water levels (0 and 5 cm) under greenhouse conditions. Biomass accumulation, mean relative growth rate (RGR), height and mean relative elongation rate (RER) of both species significantly decreased with the reduction of light availability. Biomass accumulation, RGR, height and RER of P. australis were significantly inhibited by higher water level. However, water level had no effect on the growth of D. angustifolia. Stem mass fraction was higher at 0-cm water level in D. angustifolia, and was not affected by water level in P. australis. These data suggest that D. angustifolia has a higher adaptive ability to acclimate to flooding and shade stresses than does P. australis, which might be an important reason for their different distribution patterns.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Sanjiang Plain (45°01′ ~ 48°28′N, 130°13′ ~ 135°05′E) is the largest freshwater marsh in China, about 108, 900 km2 (Luo and Xie 2009). In the plain, one of the dominant species Deyeuxia angustifolia usually distributes widely in typical meadow or marsh and occupies an area of about 1.24 × 104 km2, while Phragmites australis, the concomitant species, is distributed sparsely in the D. angustifolia communities or relative open sites with an area of about 8.87 × 102 km2 (Yi et al. 1985; Li et al. 2009). To date, little is known about the mechanisms controlling their distributional patterns.
Flooding is the predominant factor determining plant growth and distribution in wetland ecosystems mainly due to the reduced oxygen availability and redox potential (Eh) in the soil (Van Eck et al. 2006; Iwanaga and Yamamoto 2008; Luo and Xie 2009). Tolerance to flooding varies among species, which can be reflected by the distribution of species along water-level gradients. For flood-tolerant plants, adaptive characteristics include the changes of shoot: root ratios, elongation of stem, development of intercellular spaces in lenticels, formation of aerenchyma and adventitious roots, as well as shallow root systems (Iwanaga and Yamamoto 2008; Xie et al. 2008; Luo and Xie 2009). These morphological changes allow plants to acclimate to anaerobic conditions by increasing oxygen transport to roots or by reducing radial oxygen loss to the soil (Blom and Voesenek 1996; Luo and Xie. 2009).
Light is another limiting factor influencing plant survival and growth in wetlands, especially for seedlings (Krauss and Allen 2003; Going et al. 2008). For example, in D. angustifolia communities of Sanjiang Plain, thick litter layer (up to 20–30 cm) could decrease the radiation amount and light quality significantly, consequently inhibit seed germination and seedling establishment (Li et al. 2009). To survive in low light conditions, seedling usually adjusts their morphology, such as decreasing leaf thickness and increasing leaf area, shoot: root ratio and stem height, to increase their opportunities to exposure to ambient light (Kennedy et al. 2007; Going et al. 2008). Therefore, the different response of morphological plasticity among species might reflect their abilities to occupy habitats in the nature (Ryser and Eek 2000).
Moreover, plant growth is usually affected by flooding and shading simultaneously, which makes it difficult to predict plant distribution patterns on the basis of single factor (Chapin et al. 1987; Lenssen et al. 2003). Higher water level might facilitate (Mommer et al. 2005) or inhibit (Geiger and Servaites 1991; Lenssen et al. 2003) plant to acclimate to shade, then leading to the interactive effect differently with the expected influence on the basis of both separate effects. Furthermore, Lenssen et al. (2003) found that interactive effects existed in flood-sensitive species rather than flood-tolerant plants. Although many studies have been conducted on the effect of water level and light on plant performance, respectively (Xu et al. 2005; Van Eck et al. 2006; Iwanaga and Yamamoto 2008), the combined effects and their potential interactions in determining plant distributions are poorly understood (Mommer et al. 2005; Battaglia and Sharitz 2006).
Here we want to identify the role of water level, light and their potential interactions on the different distributions between D. angustifolia and P. australis in the Sanjiang Plain. To this end, seedlings of D. angustifolia and P. australis were treated with three irradiances and two water levels under greenhouse conditions and their growth performance and biomass allocation pattern were investigated to test the following hypothesis: (1) both high water level and shade have a more profoundly negative effect on the growth of P. australis than that of D. angustifolia, since the later as a dominant species should be more tolerant to stressful environments; (2) the adaptive strategies (such as biomass allocation, stem elongation) to acclimate to higher water level and shade are more effective in D. angustifolia than in P. australis, since the former as a dominant species should be a successful competitor to occupy habitat at higher water level and lower irradiance.
Materials and methods
Plant material
The seeds of both species were collected in August, 2006 from the Sanjiang Mire Wetland Experimental Station (47°35′N, 133°31′E), the Chinese Academy of Sciences, in the eastern part of Heilongjiang Province, China. The station is permanently inundated and referred to as eutrophic freshwater marsh. The average elevation is 56 m above sea level. The mean annual precipitation is 600 mm and the mean temperature was 1.9°C. The water and soil in marshes are completely frozen from October to April and begin to melt from late April to July (Ding et al. 2002). Prior to the experiment, the seeds were rinsed with distilled water, and then sterilized with potassium permanganate solution (0.1%) for 10 min, followed by a second rinse with distilled water, and finally soaked in distilled water for 24 h. Seeds were germinated in November 19, 2006 in 150 plastic bottles (1.5 L, 16 cm in height) for each species. Prior to sowing, each bottle was filled with 6-cm soil collected from the Sanjiang Freshwater Marsh Field Observation Station (31 g kg−1 organic matter, 155 mg kg−1 exchangeable N and 13.4 mg kg−1 exchangeable P). After that, 10 seeds of D. angustifolia were planted in each bottle, but 30 seeds of P. australis were planted per bottle due to low germination rates (Li et al. 2009). The temperature was kept at 25 ± 2°C during the day and 15 ± 2°C at night, and the light was provided by 400 watt sodium lamps at a photon flux density of 300 μmol m−2 s−1 (PAR) in a 14-h photoperiod. Tap water (containing 51.1 μg l−1 NH4 +-N, 176 μg l−1 NO3 −-N and 52.7 μg l−1 PO4 3+-P, pH = 7.2) was supplied daily according to the plant growth. After 14 days, only one seedling (size 7–8 cm in height) was left in each bottle.
Experimental design and harvest
A two-way factorial design was used in the experiment, which consisted of three irradiances (300, 100, 20 umol m−2 s−1) and two water levels (0 and 5 cm, relative to soil surface). The lowest light irradiance was chosen based on another experiment, which found that both the seedlings of P. australis and D. angustifolia can survive under 20 umol m−2 s−1 (Li et al. 2009). The water depth was chosen based on the work of Ding et al. (2002) who found that the standing water depth ranged from 0 to 5 cm in D. angustifolia marshes in this area. Prior to the experiment, growth parameters (biomass, height) of five seedlings for each species were measured. A total of 120 seedlings per species were chosen for the experiment. Therefore, 120 bottles (one seedling per bottle) were used for each treatment of each species. Light reduction was obtained by different layers of nylon-nets. Tap water was supplied daily to maintain the water level.
Plants were harvested at 7, 14, 21 and 28 days after the start of the experiment, respectively. For each harvest, five seedlings of each treatment were dug out, and care was taken to collect all the roots. Roots were cleaned with tap water. Plant height was measured, then the plants were divided into leaves, roots and stems, dried at 80°C for 48 h and weighed for biomass evaluation.
Calculation and data analysis
Plant biomass was the sum of each tissue mass. In order to perform functional growth analysis on biomass allocation to correct for size differences among plants, adjusted allometric analysis (biomass fractions in allocation) suggested by Poorter and Nagel (2000) was applied. Leaf mass fraction, stem mass fraction and root mass fraction were calculated as the ratio of leaf mass, stem mass and root mass to the plant biomass. Relative growth rate (RGR) and relative elongation rate (RER) were determined as: RGR = (lnw 2 − lnw 1)/(t 2 − t 1), RER = (lnh 2 − lnh 1)/(t 2 − t 1), where w 1 was the initial biomass, w 2 the biomass at harvest time t 2, h 1 the initial plant height, h 2 the plant height at harvest time t 2 and (t 2 − t 1) the experimental time.
Repeated-measures ANOVAs, using water level and light irradiance as dependent factors and harvest time as repeated-measures factor, were used to evaluate treatment effects on biomass, RGR, height, RER, leaf mass fraction, stem mass fraction and root mass fraction of the two species. Prior to analysis, sphericity was tested using Mauchly’s test. Data were log10-transformed, if necessary, to reduce heterogeneity of variances and normality and homogeneity were tested using Liljefor’s test and Levene’s test, respectively. Multiple comparisons of means were performed by Duncan’s test at 0.05 significance level.
Results
Biomass accumulation and mean relative growth rate (RGR)
Biomass accumulation of D. angustifolia was significantly reduced by the reduction of light availability alone (P < 0.001; Table 1). At the end of the experiment, biomass accumulation of D. angustifolia at high irradiance (149.12–154.70 mg per plant; Table 2) was 24.2–27.0 times higher than at low irradiance (6.12–5.72 mg per plant; Table 2). Biomass accumulation of P. australis decreased significantly in accord with the reduction of light availability (P < 0.001; Table 1) and enhancement of water level (P < 0.001; Tables 1, 2). Moreover, light and water level had a combined negative effect on biomass accumulation of P. australis at medium and high irradiance (P < 0.001; Table 1).
RGRs were similar to biomass accumulation, and decreased significantly with the reduction of light availability in both P. australis (P < 0.001; Table 1) and D. angustifolia (P < 0.001; Table 1). At the end of experiment, RGR of D. angustifolia at high irradiance (0.120–0.126 g g−1 day−1; Fig. 1) was 14.2–15.0 times higher than that at low irradiance (0.008–0.009 g g−1 day−1; Fig. 1). At 5-cm water level, RGR of P. australis was 15.2 times higher at high irradiance (0.128 g g−1 day−1; Fig. 1) than at low irradiance (0.008 g g−1 day−1; Fig. 1), while at 0-cm water level, RGR of P. australis at high irradiance was only 5 times higher than that at low irradiance. Higher water level led to a lower RGR in P. australis (P < 0.001; Table 1; Fig. 1), but had insignificant effect in D. angustifolia (P > 0.05; Table 1; Fig. 1).
Plant height and mean relative elongation rate (RER)
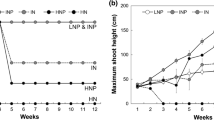
The height of P. australis was significantly affected by both light (P < 0.001; Table 1) and water level (P < 0.001; Table 1), with significant interactions (P < 0.001; Table 1). Plants were highest at high irradiance + 0 cm water level (53.2 cm per plant, 28 days; Table 3) and shortest at low irradiance + 5 cm water level (10.5 cm per plant, 28 days; Table 3). Moreover, significant effect of water level was only found at medium and high irradiance (Table 3). The height of D. angustifolia was only affected by light (P < 0.001; Table 1), rather than by water level (P > 0.05; Table 1). At the end of the experiment, the height of D. angustifolia at high irradiance (40.3–45.0 cm per plant; Table 3) was about 1.95 times higher than that at low irradiance (20.8–22.8 cm per plant; Table 3).
RER decreased significantly with the reduction of light availability in both D. angustifolia (P < 0.001; Table 1) and P. australis (P < 0.001; Table 1). From high to low irradiance, RER decreased 57.6–75.6% in D. angustifolia but 78.0–80.8% in the seedlings of P. australis (Fig. 2). RER of D. angustifolia seedlings at low irradiance (0.016–0.023 cm cm−1 day−1; Fig. 2) was higher than that of P. australis (0.014–0.018 cm cm−1 day−1; Fig. 2). However, RER of D. angustifolia at high irradiance (0.054–0.064 cm cm−1 day−1; Fig. 2) was lower than that of P. australis (0.065–0.091 cm cm−1 day−1; Fig. 2). RER of P. australis was also significantly affected by water level (P < 0.05; Table 1). At medium and high irradiance, RER at 0-cm water level (0.053–0.091 cm cm−1 day−1; Fig. 2) was about 1.4 times higher than that at 5-cm water level (0.037–0.065 cm cm−1 day−1; Fig. 2). However, water level had no effect on the RER of D. angustifolia (P > 0.05; Table 1).
Biomass allocation
Biomass allocation of both species were significantly affected by light availability (P < 0.001; Table 1; Fig. 3), except for the leaf mass fraction of D. angustifolia (P > 0.05; Table 1; Fig. 3). The root mass fraction of D. angustifolia at 5-cm water level significantly decreased with the reduction of light availability. However, opposite result was found in P. australis seedlings. Seedlings of D. angustifolia had a higher stem mass fraction at low and medium irradiance than that at high irradiance, while the stem mass fraction of P. australis was highest at high irradiance. Water level had a significant influence on the biomass allocation of both species (P < 0.001; Table 1; Fig. 3), except for the root mass fraction of D. angustifolia (P > 0.05; Table 1; Fig. 3) and the stem mass fraction of P. australis (P > 0.05; Table 1; Fig. 3). At low irradiance, the root mass fraction of P. australis at 5-cm water level was higher than that at 0-cm water level. The stem mass fraction of D. angustifolia was higher at 0-cm than at 5-cm water level.
Discussion
Seedling phase is the most crucial and sensitive stage in the plant life history (Peterson and Baldwin 2004). Many environmental factors, such as light, flooding, competition and nutrients may act as an environmental sieve influencing the recruitment of new seedlings, and determining plant distribution to a certain degree. Our study confirmed that light significantly decreases biomass, RGR, height and RER of both D. angustifolia and P. australis, which suggests that light plays an important role in determining plant distributions in the Sanjiang Plain. Furthermore, biomass allocation of both species were also influenced by light limitation except for the leaf mass fraction of D. angustifolia. Higher stem mass fraction in D. angustifolia at low irradiance indicated that more energy was allocated to the stem to facilitate its elongation, which is a useful strategy for plant to grow out of the shade and to grow more emergent leaves for photosynthesis (Wang et al. 2008). However, the stem mass fraction of P. australis decreased significantly with the reduction of irradiance, suggesting that it could not resist shade stress by stem elongation. Moreover, higher RER of D. angustifolia at low irradiance suggested that D. angustifolia had a higher ability to escape from shade stresses than that of P. australis. Our result is also supported by another study (Li et al. 2009), which showed that the survival rate of D. angustifolia at low irradiance was higher than that of P. australis due to higher leaf morphological plasticity.
The growth responses to water level were species-specific. Enhancement of stem mass fraction and RER are important adaptive strategies for plant to acclimate to flooding stress (Laan and Blom 1990). In this experiment, biomass accumulation, RGR, height and RER of P. australis were significantly inhibited by higher water level. However, water level had no effects on the growth of D. angustifolia. These results indicated that D. angustifolia could grow better than P. australis at 5-cm water level. For P. australis, the negative effects of flooding on plant growth were also found in other studies (Blanch et al. 1999; Edwards et al. 2003), due to poor soil aeration and depletion of oxygen in the rhizosphere of plants (Xie et al. 2008). Moreover, Deegan et al. (2007) also confirmed that the below-ground parts of P. australis usually had low aeration efficiency. However, the stem mass fraction of P. australis was not affected by water level and RER was lower at 5-cm water level. This result is contrary to the study of Vretare et al. (2001), which showed that P. australis could allocate more energy to the above-ground part to increase the stem height when treated with 70–75 cm water depth. The response of plants to the same environmental stress might be different with life stage or stress intensities (Battaglia et al. 2000). Stem mass fraction of D. angustifolia at 5-cm water level was lower than that at 0-cm water level, indicating that enhancement of stem mass fraction was not the effective strategy for D. angustifolia to acclimate to flooding. Study by Xie et al. (2008) confirmed that D. angustifolia could resist flooding stress mainly by the positive adjustment of root growth, such as shallow root systems and increased root porosity.
When plants were stressed with flooding and shade simultaneously, their growths were not only influenced by these two factors, respectively, but also by their interactions. Generally, three modes of interactions could be occurred: (1) amplified effect, which means that the adjustment of plant to acclimate to shade would be prevent by higher water level, then lead to the total effect on flooded, shaded plants is stronger than expected on the basis of both separate effects (Lenssen et al. 2003); (2) independently effect, no interactions could be found in this case; (3) reduced effect, which could be occurred in the case that one stress is so strong that additional limitations hardly depress plant growth any further (Chapin et al. 1987; Urbas and Zoble 2000; Lenssen et al. 2003). In our experiment, interactions were found in P. australis, for the influence of water level changed greatly under different light conditions. At low irradiance, similar growth performance between two water levels indicated that the negative effect of shade was so strong that making water level hardly inhibit P. australis growth any more. Reduced effect was also found in some other studies, but in contrast to our study, these studies confirmed that higher water level was the stronger limitation on plant growth than shade (Dale and Causton 1992; Lenssen et al. 2003). One possible reason for the difference is that the water level in our study was so shallow (only 5 cm) that resulting its negative effect was smaller than shading. However, interactions had insignificant effect on the growth of D. angustifolia, mainly because the growth was not affected by water level and these differences confirmed that D. angustifolia had a higher adaptive abilities than did P. australis to acclimate to the simultaneous stresses of flooding and shade, which might be an important reason accounting for their different distribution patterns in the Sanjiang Plain.
References
Battaglia LL, Sharitz RR (2006) Responses of floodplain forest species to spatially condensed gradients: a test of the flood-shade tolerance trade off hypothesis. Oecologia 147:108–118
Battaglia LL, Foré SA, Sharitz RR (2000) Seedling emergence, survival and size in relation to light and water availability in two bottomland hardwood species. J Ecol 88:1041–1050
Blanch SJ, Ganf GG, Walker KF (1999) Growth and resource allocation in response to flooding in the emergent sedge Bolboscoenus medianus. Aquat Bot 63:145–160
Blom CWPM, Voesenek LACJ (1996) Flooding: the survival strategies of plants. Trends Ecol Evol 11:290–295
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Dale MP, Causton DR (1992) The ecophysiology of Veronica cbamaedrys, V. montana and V. officinalis. П. The interaction of irradiance and water regime. J Ecol 80:493–504
Deegan BM, White SD, Ganf GG (2007) The influence of water level fluctuations on the growth of four emergent macrophyte species. Aquat Bot 86:309–315
Ding WX, Cai ZC, Tsuruta H, Li XP (2002) Effect of standing water depth on methane emissions from freashwater marshes in Northeast China. Atomos Environ 36:5149–5157
Edwards AL, David WL, Jennifer HR (2003) Responses to a fluctuating environment: effects of water depth on growth and biomass allocation in Eleocharis cellulosa Torr. (Cyperaceae). Can J Bot 8:964–975
Geiger DR, Servaites JC (1991) Carbon allocation and response to stress. In: Mooney HA, Winner WE, Pell EJ (eds) Response of plants to multiple stress. Academic press, San Diego, pp 104–127
Going B, Simpson J, Even T (2008) The influence of light on the growth of watercress (Nasturtium officinale R. Br.). Hydrobiologia 607:75–85
Iwanaga F, Yamamoto F (2008) Effects of flooding depth on growth, morphology and photosynthesis in Alnus japonica species. New Forest 35:1–14
Kennedy S, Black K, CO’ Reilly, Dhubháin AN (2007) The impact of shade on morphology, growth and biomass allocation in Picea sitchensis, Larix × eurolepis and Thuja plicata. New Forest 33:139–153
Krauss KW, Allen JA (2003) Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat Bot 77:311–324
Laan P, Blom CWPM (1990) Growth and survival responses of Rumex species to flooded and submerged conditions: the importance of shoot elongation, underwater photosynthesis and reserve carbohydrates. J Exp Bot 41:775–783
Lenssen IPM, Menting FBJ, Van der Putten WH (2003) Plant responses to simultaneous stress of waterlogging and shade: amplified or hierarchical effects? New Phytol 157:281–290
Li YZ, Zhang CM, Xie YH, Huang JS, Yang G (2009) Growth responses of Deyeuxia angustifolia and Phragmites communis seedlings to low-light stress in the Sanjiang Plain. Chin J Appl Environ Biol 15(1):53–58 (in Chinese)
Luo WB, Xie YH (2009) Growth and morphological responses to water level and nutrient supply in three emergent macrophyte species. Hydrobiologia 624:151–160
Mommer L, Kroon HD, Pierik R, Böögemann GM, Visser EJW (2005) A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytol 167:197–206
Peterson JE, Baldwin AH (2004) Seedling emergence from seed banks of tidal freshwater wetlands: response to inundation and sedimentation. Aquat Bot 78:243–254
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Ryser P, Eek L (2000) Consequences of phenotypic plasticity vs. interspecific differences in leaf and root traits for acquisition of aboveground and belowground resources. Am J Bot 87:402–411
Urbas P, Zoble K (2000) Adaptive and inevitable morphological plastisity of three herbaceous species in a multi-species community: field experiment with manipulated nutrients and light. Acta Oecologica 21:139–147
Van Eck WHJM, Lenssen JPM, Van de Steeg HM, Blom CWPM, de Kroon H (2006) Seasonal dependent effects of flooding on plant species survival and zonation: a comparative study of 10 terrestrial grassland species. Hydrobiologia 565:59–69
Vretare V, Weisner SEB, Strand JA, Granéli W (2001) Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat Bot 69:127–145
Wang HY, Yu D, Xiao KY (2008) The interactive effects of irradiance and photoperiod on chara vulgaris L: concerted responses in morphology, physiology, and reproduction. Hydrobiologia 610:33–41
Xie YH, Luo WB, Wang KL, Ren B (2008) Root growth dynamics of Deyeuxia angustifolia seedlings in response to water level. Aquat Bot 89:292–296
Xu K, Ye W, Li G, Li J (2005) Phenotypic plasticity in response to light intensity in the invasive species alternanthera philoxeroides. J Wuhan Bot Res 23(6):560–563 (in Chinese)
Yi FK, Li CH, Zhao KY, Ding SQ (1985) Study on vegetation type in the Sanjiang Plain. In Huang XT (ed) Study on Marsh in China. Science Press, Beijing, pp 162–171 (in Chinese)
Acknowledgments
The authors greatly appreciate Dr. Kayla Duke and Dr. Caiyan Chen for language improvement and Prof. Liyun Chen for providing experimental conditions in Hunan Agriculture University. We also thank Dr. Wenbo Luo for the date analysis. This research was supported by the Directional Program of the Chinese Academy of Science (KZCX2-YW-435-02;KZCX1-YW-08-01-02), the National Basic Research Program of China (2009CB421103) and the National Natural Science Foundation of China (30770362).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
The authors Feng Li and Youzhi Li co-first author.
Rights and permissions
About this article
Cite this article
Li, F., Li, Y., Qin, H. et al. Plant distribution can be reflected by the different growth and morphological responses to water level and shade in two emergent macrophyte seedlings in the Sanjiang Plain. Aquat Ecol 45, 89–97 (2011). https://doi.org/10.1007/s10452-010-9334-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-010-9334-8