Abstract
Phthalate esters (PAEs) are mainly used in the polymer industry as external plasticizers in PVC, and tend to migrate slowly out of the plastic, either into the air by volatilization or into water or other solvents by dissolution. Di-n-butyl phthalate (DBP), butyl benzyl phthalate (BBP) and di-2-ethylhexyl phthalate (DEHP) are three members of PAEs, identified as priority controlled hazardous substances by the United States Environmental Protection Agency, and have been shown to have potential for endocrine disrupting effects on vertebrates and humans. The effects of DBP, BBP and DEHP on survival and reproduction of the freshwater rotifer Brachionus calyciflorus were studied using life-table demographic methods. The results showed that all the life-table demographic parameters of B. calyciflorus were markedly affected by DBP and BBP, but not by DEHP. The net reproductive rate representing the output of reproduction was more affected than all the other parameters representing population growth, development or survival of the rotifers. Compared to the solvent control, DBP and BBP, both at 500 μg l−1, significantly increased the net reproductive rate, and prolonged the generation time and the life expectancy at hatching of the rotifers. DBP at 50 μg l−1 markedly decreased the intrinsic rate of population increase of the rotifers, but the reverse was true for BBP at 50 and 500 μg l−1. Among all the parameters, the intrinsic rate of population increase was the most sensitive to DBP and BBP. The levels of PAEs in water from all the studied rivers and lakes in the world did not affect the population growth of rotifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs) are a family of industrial chemicals used as softeners, adhesives or solvents by a variety of industries. They are mainly used in the polymer industry as plasticizers in PVC and to a lesser extent in the non-polymer industry for different consumer products (sealants, paints, printing inks, cosmetics, coatings of different products such as cars, coils, cables or fabrics, etc.) (OSPAR Commission 2006). Because phthalates are external plasticizers which soften the resins without reacting chemically with them, they tend to migrate slowly out of the plastic, either into the air by volatilization or into water or other solvents by dissolution (Laughlin et al. 1978). In China, the highest level of PAEs in rivers and lakes was 263.8 μg l−1, which was almost the same as those from the other rivers and lakes in the world (Tian et al. 2003; Sha et al. 2007).
Di-n-butyl phthalate (DBP), butyl benzyl phthalate (BBP) and di-2-ethylhexyl phthalate (DEHP) are three members of PAEs, and identified as priority controlled hazardous substances by the United States Environmental Protection Agency (USEPA) (Jin 1990). In recent years, they have been shown to have potential for endocrine disrupting effects on vertebrates and humans (OSPAR Commission 2006). Although some information on the acute and chronic toxicities of these three PAEs to aquatic microorganisms, algae, invertebrates and fish is available (Stamples et al. 1997; Huang et al. 1998, 1999; Ministry of Environment, Government of Japan 2002, 2004; Bradlee and Thomas 2003; Norman et al. 2007), relatively little is known about their chronic toxicities on rotifers (Zhao et al. 2007).
Being a natural food link between the primary producers (algae) and zooplanktivorous fish, rotifers are important to maintain an ecological balance in aquatic ecosystems (Nogrady et al. 1993). So, there is an increasing trend in the use of rotifers as bioassay organisms for aquatic ecotoxicological studies in recent 20 years (Snell and Janssen 1995; Snell and Joaquim-Justo 2007). Among all the reported studies, analysis of the survival and reproduction, integrated by life-table calculations, can provide information on the intrinsic rate of population increase, net reproductive rate, generation time and life expectancy at hatching under a given set of environmental conditions, and be an important theoretical basis to measure the effects of chronic exposure of populations to toxicants (Ferrando et al. 1993).
The main purpose of the present study is to assess the effects of three phthalate esters, DBP, BBP and DEHP, on survival and reproduction of the freshwater rotifer B. calyciflorus using life-table demographic techniques, and to screen out sensitive endpoints which could be used to monitor the ecological effects of sublethal concentrations of the three phthalate esters.
Materials and methods
The rotifer B. calyciflorus used in this experiment was obtained by hatching resting eggs collected from sediments of Lake Jinghu and then clonally culturing under controlled laboratory conditions. Stock rotifer cultures were kept at 25 ± 1°C in natural light and daily fed on 2.0 × 106 cells ml−1 of Scenedesmus obliquus. For mass cultures as well as for experiments, reconstituted hardwater (EPA medium) (USEPA 1985) was used as the medium. Algae were grown in a semi-continuous culture using HB-4 medium (Li et al. 1959) renewed daily at 20%. Algae in exponential growth were centrifuged and then resuspended in EPA medium.
DBP, BBP and DEHP (standard grade, ≥97%, Sigma-Aldrich, Germany) were used as the toxicants. Stock solutions were prepared by dissolving DBP, BBP and DEHP in 100% acetone, then diluted to the desired concentrations using EPA medium, respectively.
Based on the concentrations of PAEs in water from all the studied rivers and lakes in China (Tian et al. 2003; Sha et al. 2007) and the results of preliminary tests, seven toxicant concentrations (0.005, 0.05, 0.5, 5, 50, 500, 5,000 μg l−1), a blank control (EPA medium) and a solvent control [containing 0.5% (v/v) acetone which was the same as that in the toxicant at 5,000 μg l−1] were selected for the present study, each consisting of four replicates of 10 rotifers. Before experiment commenced, rotifers with amictic eggs were randomly removed from the stock cultures and placed into a glass dish containing 10 ml of EPA medium with 2.0 × 106 cells ml−1 of S. obliquus which is optimal for survival and reproduction of the rotifers (Xi et al. 2001). After 3 h, ten neonates for each replicate were collected and transferred into a 6-ml glass cup containing 5 ml of test solution with 2.0 × 106 cells ml−1 of S. obliquus. Thereafter, the number of neonates produced and the number of original test individuals alive were recorded and then neonates were removed every 8 h (Xi and Hu 2003; Huang et al. 2007; Zha et al. 2007). The original rotifers alive were transferred into freshly prepared test solution every 24 h. The life-table experiments were conducted in darkness (expect when the rotifers were observed under a light microscope) (Janssen et al. 1994; Marcial et al. 2005) at 25 ± 1°C until each individual of every cohort died.
Based on the data collected, age-specific survivorship (l x ) and age-specific fecundity (m x ) were constructed for each cohort (replicate) using conventional life-table techniques (Poole 1974). Intrinsic rate of population increase (r m ), net reproductive rate (R 0), generation time (T) and life expectancy at hatching (e 0) were calculated according to Krebs (1985) and Lotka (1913).
One-way analysis of variance (ANOVA), with the concentration of DBP, BBP or DEHP as the independent variable, and each of the life-table demographic parameters as the dependent variable, followed by Dunnett’s test was conducted for pair-wise comparisons of each concentration of test chemicals to the solvent control (Zar 1999).
Results
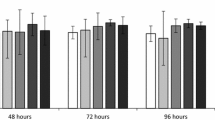
DBP and BBP both had a lethal effect at 5,000 μg l−1 before the rotifers reached sexual maturity. When compared to the solvent control, DBP at 500 μg l−1 increased significantly the age-specific fecundity of rotifers, but none of the three phthalate esters at the other concentrations significantly affected the age-specific fecundity. The age-specific survival of the rotifers exposed to all the three phthalates was similar to the solvent control (Fig. 1).
Compared to the blank control, the solvent (acetone at 0.5%) did not significantly affect any of the life-table demographic parameters of the rotifers.
DBP significantly influenced all the life-table demographic parameters of B. calyciflorus (Table 2). Compared to the solvent control, DBP at 500 μg l−1 significantly increased the net reproductive rate, and prolonged the generation time and the life expectancy at hatching of the rotifers, but DBP at 50 μg l−1 markedly decreased the intrinsic rate of population increase of the rotifers (Table 1).
BBP also significantly affected all the life-table demographic parameters of B. calyciflorus (Table 2). Compared to the solvent control, BBP at 500 μg l−1 markedly increased the net reproductive rate, and prolonged the generation time and the life expectancy at hatching of rotifers; BBP at 50 and 500 μg l−1 significantly increased the intrinsic rate of population increase of the rotifers (Table 1).
DEHP did not markedly affect the net reproductive rate, the intrinsic rate of population increase, the generation time nor the life expectancy at hatching of B. calyciflorus (Tables 1, 2).
Discussion
As a consequence of chronic toxicant stress, a reduction in net reproductive rate was observed in B. patulus, B. calyciflorus, B. plicatilis and D. magna (Rao and Sarma 1986; Fernandez-Casalderrey et al. 1991, 1993; Janssen et al. 1994; Huang et al. 1999; Xi and Hu 2003; Chu et al. 2005; Xu et al. 2005). However, sublethal concentrations of aldrin, chlordecone and DBP increased the net reproductive rates of B. calyciflorus and D. magna (Huang et al. 1999, 2007; Zha et al. 2007). Similarly, in the present study, DBP and BBP both at 500 μg l−1 increased the net reproductive rate of B. calyciflorus. The above stated results indicated that sublethal concentrations of pollutants with endocrine disrupting activity for vertebrates might have an intriguing effect on the reproduction of the rotifers, but higher concentrations of them might have a toxic effect. It is worthy of further research as to how sublethal concentrations of DBP, BBP and DEHP stimulate the reproduction of the rotifers.
The population growth of rotifers decreased under the stress of all the tested pollutants with estrogenic activity for vertebrates (Rao and Sarma 1986, 1990; Fernandez-Casalderrey et al. 1991, 1993; Ferrando et al. 1993; Janssen et al. 1994; Snell and Carmona 1995; Gallardo et al. 1997; Preston et al. 2000; Preston and Snell 2001; Radix et al. 2002; Mariraz-Perez et al. 2004; Xi and Feng 2004; Marcial et al. 2005; Chu et al. 2005), except micromolar concentrations of gamma-aminobutyric acid, growth hormone, human chorionic gonadotropin and 5-hydroxytryptamine, which increased the population growth of B. plicatilis, aldrin and DBP, which increased the intrinsic rate of population increase of B. calyciflorus and D. magna (Gallardo et al. 1997; Huang et al. 1999, 2007), and juvenile hormone, 20-hydroxyecdysone and tri-iodothyronine, which had no effect on population growth (Gallardo et al. 1997). In the present study, BBP at 50 and 500 μg l−1 increased the intrinsic rate of population increase of the rotifers, but the reverse was also true for DBP at 50 μg l−1. DEHP at 0.005–5,000 μg l−1 did not affect the intrinsic rate of population increase.
Sublethal concentrations of aldrin increased the life expectancy at hatching of B. calyciflorus (Huang et al. 2007), and glyphosate, aldrin and chlordecone prolonged the mean lifespan of B. calyciflorus (Chu et al. 2005; Huang et al. 2007; Zha et al. 2007). Identical to the effects of those pollutants, in the present study DBP and BBP both at 500 μg l−1 prolonged the life expectancy at hatching of the rotifers, but DEHP at 0.005–5,000 μg l−1 did not affect it. The above stated results indicate that sublethal concentrations of pollutants with endocrine disrupting activity might be beneficial to not only the reproduction but also the survival of rotifers.
Several studies have found that sublethal concentrations of aldrin and chlordecone prolonged the generation time of rotifers, but Cu, lindane, thiophanate-methyl and deltamethrin shortened the generation time of rotifers (Janssen et al. 1994; Xi and Hu 2003; Xu et al. 2005; Huang et al. 2007; Zha et al. 2007). In the present study, DBP and BBP, both at 500 μg l−1, prolonged the generation time of B. calyciflorus, but DEHP at 0.005–5,000 μg l−1 did not affect it.
Similar to the non-significant dose–response relationship between the concentration of aldrin as well as chlordecone and each of all the life-table demographic parameters of B. calyciflorus (Huang et al. 2007; Zha et al. 2007), any significant dose–response relationship between the concentration of DBP, BBP as well as DEHP and each of the life-table demographic parameters of B. calyciflorus was not observed in the present study, which might be attributed to 10-fold pollutant concentration interval.
The lifespan and the net reproductive rate of B. calyciflorus differed with the rotifer clones. At 25°C and under toxicant free conditions, the B. calyciflorus clone which has been used in our laboratory for ecotoxicologial studies had a lifespan of 89–148 h, a life expectancy at hatching of 88–126 h and a net reproductive rate of 11–22 (Huang et al. 2007; Zha et al. 2007), and that studied by Araujo and McNair (2007) had a lifespan of 12–14 days and a net reproductive rate of 17–19. Considering the significantly intriguing effect of BBP at 500 μg l−1 on the population growth of B. calyciflorus (Zhao et al. 2007), another rotifer clone with a shorter lifespan and a lower net reproductive rate was used in the present study to detect the possibly intriguing effects of the three PAEs on its survival and reproduction. The present results showed that, although the B. calyciflorus clone used in the present study had a shorter life expectancy at hatching of 64 h and a lower net reproductive rate of 3.19 (Table 1), it could be used to sensitively detect the intriguing effects of DBP and BBP on survival and reproduction of rotifers.
Compared with the conventional test animals such as D. magna and Pimephales promelas (fathead minnow), rotifers had comparable sensitivities to most compounds, but no single species was consistently the most sensitive to all compounds (Snell et al. 1991a, b; Persoone et al. 1993, Persoone and Janssen 1993). As far as the intriguing effect of DBP on the reproduction of zooplankton was concerned, B. calyciflorus had the same sensitivity to DBP as D. magna, because DBP at 0.5 mg l−1 stimulated the net reproductive rate of both B. calyciflorus and D. magna (Huang et al. 1998, 1999; the present study).
The 48-h LC50 values of DBP and BBP were 3.0 and 1.0–4.7 mg l−1 for D. magna, respectively (Mayer and Ellersieck 1986; Barera and Adams 1981). The 10-day LC50 values of DBP and BBP were 0.63 and 0.46 mg l−1 for Hyalella azteca; and 2.48 and 1.23 mg l−1 for Lumbriculus variegates, respectively (Call et al. 2001). DBP and DEHP were less toxic to a crustacean, a green alga, and a bacterium than BBP (Jonsson and Baun 2003). Similarly, the comparison among the effects of the three phthalate esters on the survivorships of B. calyciflorus obtained in the present study showed that the toxicity of BBP was the highest, and that of DEHP was the lowest.
Conclusion
All the life-table demographic parameters of B. calyciflorus were markedly affected by DBP and BBP, but not affected by DEHP. The net reproductive rate which represents the output of reproduction was more affected than all the other parameters which represent population growth, development or survival of the rotifers. Among all the parameters, the intrinsic rate of population increase was the most sensitive to DBP and BBP. Sublethal concentrations of DBP and BBP had intriguing effects on survival and reproduction of the rotifers, but the levels of PAEs in water from all the studied rivers and lakes in the world did not affect the population growth of the rotifers.
References
Araujo A, McNair NJ (2007) Individual- and population-level effects of antibiotics on the rotifers, Brachionus calyciflorus and B. plicatilis. Hydrobiologia 593:185–199
Barera Y, Adams WJ (1981) Resolving some practical questions about Daphnia magna acute toxicity tests. In: Bishop et al (eds) Aquatic toxicology and hazard assessments: the sixth symposium ASTP STP 802. ASTM Philadelphia
Bradlee AC, Thomas P (2003) Aquatic toxicity of phthalate esters. The handbook of environmental chemistry, vol 3. Part Q, 263–298
Call DJ, Markee TP, Geiger DL, Brooke LT, VandeVenter FA, Cox DA, Genisot KI, Robillard KA, Gorsuch JW, Parkerton TF, Reiley MC, Ankley GT, Mount DR (2001) An assessment of the toxicity of phthalate esters to freshwater benthos. I. Aqueous exposures. Environ Toxicol Chem 20:1798–1804
Chu ZX, Xi YL, Xu XP (2005) Effect of glyphosate on the life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Chin J Appl Ecol 16:1142–1145 (in Chinese)
Fernandez-Casalderrey A, Ferrando MD, Andreu-Moliner E (1991) Demographic parameters of Brachionus calyciflorus Pallas (Rotifers) exposed to sublethal endosulfan concentrations. Hydrobiologia 226:103–110
Ferrandez-Casalderrey A, Ferrando MD, Andreu-Moliner E (1993) Chronic toxicity of methylparathion to the rotifer Brachionus calyciflorus fed on Nannochloris oculata and Chlorella pyrenoidosa. Hydrobiologia 255/256:41–49
Ferrando MD, Janssen CR, Andreu E, Persoone G (1993) Ecotoxicological studies with the freshwater rotifer Brachionus calyciflorus. II. An assessment of the chronic toxicity of lindane and 3, 4-dichloraniline using life tables. Hydrobiologia 255/256:33–40
Gallardo WG, Hagiwara A, Tomita Y, Soyano K, Snell TW (1997) Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Müller. Hydrobiologia 358:113–120
Huang GL, Sun HW, Gao J, Chen YS (1998) Study on toxic effects of dibutyl phthalate on Daphnia magna. Environ Chem 17:428–433 (in Chinese)
Huang GL, Sun HG, Song ZH (1999) Interactions between dibutyl phthalate and aquatic organisms. Bull Environ Contam Toxicol 63:759–765
Huang L, Xi YL, Zha CW, Zhao LL (2007) Effect of aldrin on life history characteristics of rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 79:524–528
Janssen CR, Persoone G, Snell TW (1994) Cyst-based toxicity tests.VIII. Short chronic test with the freshwater rotifer Brachionus calyciflorus. Aquatic Toxicol 28:243–258
Jin XC (1990) The organic compound pollution chemistry. Qinghua, Beijing (in Chinese)
Jonsson S, Baun A (2003) Toxicity of mono- and diesters of o-phthalic esters to a crustacean, a green alga, and a bacterium. Environ Toxicol Chem 22:3037–3043
Krebs CJ (1985) Ecology: the experimental analysis of distribution and abundance. Harper & Row, New York
Laughlin BR, Neff JM (1978) The effects of three phthalate esters on the larval development of the grass shrimp Palaemonetes pugio (holthuis). Water, Air, Soil Pollut 9:323–336
Li S, Zhu H, Xia Y, Yu M, Liu K, Ye Z, Chen Y (1959) The mass culture of unicellular green algae. Acta Hydrobiol Sinica 4:462–472 (in Chinese)
Lotka AJ (1913) A natural population norm. J Wash Acad Sci 3:241–248
Marcial SH, Hagiwara A, Snell TW (2005) Effect of some pesticides on reproduction of rotifer Brachionus plicatilis Müller. Hydrobiologia 546:569–575
Mariraz-Perez T, Sarma SSS, Nandini S (2004) Effects of mercy on the life table demography of the rotifer Brachionus calyciflorus Pallas (Rotifera). Ecotoxicology 13:535–544
Mayer P, Ellersieck MR (1986) Manual of acute toxicity: interpretation and data Base for 410 chemicals and 66 species of freshwater animals, resource Pub. 160. U.S. Department of the Interior, Fish and Wildlife Service, Washington, DC
Ministry of Environment, Government of Japan (2002) Environmental risk initial assessment 29: di (2-ethylhexyl) phthalate (in Japanese)
Ministry of Environment, Government of Japan (2004) Synopsis of the results of ecological effects tests. Available at http://www.env.go.jp/chemi/sesaku/seitai.html (in Japanese)
Nogrady T, Wallace RL, Snell TW (1993) Rotifera. vol 1: biology, ecology and systematics. SPB Academic, The Hague
Norman A, Borjeson H, David F, Tienpont B, Norrgren L (2007) Studies of putake, elimination, and late effects in Atlantic Salmon (Salmo salar) dietary exposed to di-2 ethylhexyl pathalate (DEHP) during early life. Arch Environ Contam Toxicol 52:235–242
OSPAR Commission (2006) OSPAR background document on phthalates. Available at http://www.ospar.org/documents/dbase/publications
Persoone G, Janssen CR (1993) Freshwater invertebrate toxicity tests. In: Calow P (eds) Handbook of ecotoxicology. Blackwell, UK
Persoone G, Goyvaerts M, Janssen CR, Coen W, Vangheluwe M (1993) Cost effective acute hazard monitoring of polluted waters and waste dumps with the aid of Toxkits. Final Report EEC, Contract ACE 89/BE 2D3. Commission of the European Communities, Brussels
Poole RW (1974) An introduction to quantitative ecology. McGraw-Hill, New York
Preston BL, Snell TW (2001) Full life cycle toxicity assessment using rotifer resting egg production: implications for ecological risk assessment. Environ Pollut 114:399–406
Preston BL, Snell TW, Roberston TL, Dingmann BJ (2000) Use of freshwater rotifer Brachionus calyciflorus in screening assay for potential endocrine disruptors. Environ Toxicol Chem 19:2923–2928
Radix P, Severin G, Schamm KW, Kettrup A (2002) Reproduction disturbances of Brachionus calyciflorus (rotifer) for the screening of environmental endocrine disruptors. Chemosphere 47:1097–1101
Rao TR, Sarma SSS (1986) Demographic parameters of Brachionus patulus Müller (Rotifera) exposed to sublethal DDT concentrations at low and high food levels. Hydrobiologia 139:193–200
Rao TR, Sarma SSS (1990) Interaction of Chlorella density and DDT concentration on the population dynamics of the rotifer Branchionus patulus (Rotifera). Indian J Environ Health APR 32:57–60
Sha Y, Xia X, Yang Y (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River, China. Environ Monit Assess 124:277–287
Snell TW, Carmona MJ (1995) Comparative toxicity sensitivity of sexual and asexual reproduction in the rotifer Brachionus calyciflrorus. Environ Toxicol Chem 14(3):415–420
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313/314:231–247
Snell TW, Joaquim-Justo C (2007) Workshop on rotifers in ecotoxicology. Hydrobiologia 593:227–232
Snell TW, Moffat BD, Janssen CR, Persoone G (1991a) Acute toxicity tests using rotifers. III. Effects of temperature, strain and exposure time on the sensitivity of Brachionus plicatilis. Ecotoxicol Toxicol Wat Qual 6:63–75
Snell TW, Moffat BD, Janssen CR, Persoone G (1991b) Acute toxicity tests using rotifers. IV. Effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicol Environ Saf 21:308–317
Stamples CA, Adams WJ, Parkerton TF, Gorsuch JW (1997) Aquatic toxicity of eighteen phthalate esters. Environ Toxicol Chem 16:875–891
Tian HJ, Shu WQ, Zhang XK, Wang YM, Cao J (2003) Organic pollutions in source water in Jialing River and Yangtze River (Chongqing section). Resour Environ Yangtze Basin 12:118–123 (In Chinese)
USEPA (1985) Methods for measuring the acute toxicity of effluents to freshwater and marine organisms. Peltier WH, Weber CI (eds) EPA/600/4 - 85/013. US Environ Protec Agency, Washington DC
Xi YL, Hu HY (2003) Effect of thiophanate-methyl on the reproduction and survival of the refreshwater rotifer Brachionus calyciflorus. Bull Environ Contam Toxicol 71:722–728
Xi YL, Feng LK (2004) Effect of thiophanate-methyl and glyphosate on asexual and sexual reproduction in the rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 73:644–651
Xi YL, Huang X-F, Jin H-J, Liu J-K (2001) The effect of food concentration on the life history of three types of Brachionus calyciflorus females. Internat Rev Hydrobiol 86:209–215
Xu XP, Xi YL, Chu ZX, Chen F (2005) The effect of deltamethin on experimental population dynamics of freshwater rotifer Brachionus calyciflorus. Acta Zool Sin 51:251–256 (In Chinese)
Zar JH (1999) Biostatistical analysis, 4. Prentice Hall, NJ
Zha CW, Xi YL, Huang L, Zhao LL (2007) Effect of sublethal exposure to chlordecone on life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Bull Environ Contam Toxicol 81:71–75
Zhao LL, Xi YL, Huang L, Zha CW (2007) Effects of phthalate acid esters on population growth and sexual reproduction of rotifer Brachionus calyciflorus. Acta Zool Sin 53:250–256 (In Chinese)
Acknowledgements
This work was supported by Natural Scientific Foundation of China (30470323), Natural Scientific Foundation of Educational Ministry of China (051286), Excellent Youth Foundation in Anhui Province (04043050), and Natural Scientific Foundation of Educational Committee of Anhui Province, China (2003kj032zd).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, LL., Xi, YL., Huang, L. et al. Effects of three phthalate esters on the life-table demography of freshwater rotifer Brachionus calyciflorus Pallas. Aquat Ecol 43, 395–402 (2009). https://doi.org/10.1007/s10452-008-9179-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-008-9179-6