Abstract
Four nanoporous carbons obtained from different polymers: polypyrrole, polyvinylidene fluoride, sulfonated styrene–divinylbenzene resin, and phenol–formaldehyde resin, were investigated as potential adsorbents for carbon dioxide. CO2 adsorption isotherms measured at eight temperatures between 0 and 60 °C were used to study adsorption properties of these polymer-derived carbons, especially CO2 uptakes at ambient pressure and different temperatures, working capacity, and isosteric heat of adsorption. The specific surface areas and the volumes of micropores and ultramicropores estimated for these materials by using the density functional theory-based software for pore size analysis ranged from 840 to 1990 m2 g−1, from 0.22 to 1.47 cm3 g−1, and from 0.18 to 0.64 cm3 g−1, respectively. The observed differences in the nanoporosity of these carbons had a pronounced effect on the CO2 adsorption properties. The highest CO2 uptakes, 6.92 mmol g−1 (0 °C, 1 atm) and 1.89 mmol g−1 (60 °C, 1 atm), were obtained for the polypyrrole-derived activated carbon prepared through a single carbonization-KOH activation step. The working capacity for this adsorbent was estimated to be 3.70 mmol g−1. Depending on the adsorbent, the CO2 isosteric heats of adsorption varied from 32.9 to 16.3 kJ mol−1 in 0–2.5 mmol g−1 range. Overall, the carbons studied showed well-developed microporosity and exceptional CO2 adsorption, which make them viable candidates for CO2 capture, and for other adsorption and environmental-related applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The newest report of “The International Panel on Climate Change” clearly indicates the ongoing and deepening climate change (IPCC, Climate Change 2013). The report states the anthropogenic greenhouse gas emissions contribute mostly to the climate change, and the resulting changes already have a negative impact on human health, agriculture, water resources, and ecosystems on land and ocean. The main source of greenhouse gases—primarily CO2, water vapor, CH4 and N2O (Piacentini and Mujumdar 2009)—is burning of fossil fuels, in industrial processes or otherwise. The IPCC stressed the importance of reduction of greenhouse gas emissions, particularly the anthropogenic CO2 emissions, and proposed carbon capture and storage (CCS) as a viable solution. Adsorption on nanoporous solids is one of the possible implementations of CCS.
So far various materials for CO2 adsorption and CCS were explored such as nanoporous carbons (Ludwinowicz and Jaroniec 2015a; Seredych et al. 2014), modified porous silica (Heydari-Gorji et al. 2011) and organosilica (De Canck et al. 2013), zeolites (Su and Lu 2012), porous polymers (Patel et al. 2012), and organometallic networks (Liu et al. 2012). Among these materials, activated carbons commonly have high surface areas, large pore volumes, and proper pore sizes; all beneficial for CO2 adsorption. In addition, carbons can be prepared in variety of morphologies, tailorable surface chemistry, and high chemical (water, acids and bases) and thermal stability (Liu et al. 2015). Activated carbons are commonly prepared from natural precursors, such as coal, lignite, peat, wood, fruit pits, coal tar, and various biomasses (Jankowska et al. 1991; Marsh and Rodriguez-Reinoso 2006).
Alternatively, synthetic wastes can be used as carbon precursors as well. For instance, polymers or polymeric wastes used as carbon precursors include: polyethylene (PE), polypropylene (PP), polystyrene (PS), poly(vinyl chloride) (PVC), poly(ethylene terephtalate) (PET), poly(methyl metacrylate) (PMMA), polyacrylonitrile (PAN), and more (Mishra et al. 2003; Arienillas et al. 2005; Migahead et al. 2010; Gupta et al. 2014; Choma et al. 2014a). These, and other polymers, are used every day for production of common objects, e.g., building components, household appliances, textiles, CDs and DVDs, and computer equipment. Carbons prepared by carbonization of polymers typically require subsequent activation to develop their surface area and porosity to be viable adsorbents. Activation is typically classified as either physical or chemical, with the first including activation with CO2 or water vapor (Górka and Jaroniec 2011), and the second, activation with KOH (de Souza et al. 2013) or ZnCl2 (Ludwinowicz and Jaroniec 2015b). Wickramaratne and Jaroniec (2013) found that the CO2 activation of carbon spheres prepared by Stöber method resulted in the development of microporosity and high CO2 uptake of 8.05 mmol g−1 (0 °C, 1 atm). Activated carbons are effective CO2 adsorbents mainly due to the large amount of ultramicropores (micropores with size <1 nm), which were shown to enhance CO2 adsorption (Lee and Park 2013; Liu et al. 2015).
In this article, we examine four carbon adsorbents prepared by carbonizing and activating different polymers, study their structure and CO2 adsorption properties at different temperatures ranging from 0 to 60 °C. This study aims to explore the opportunities in the development of microporosity in the polymer-derived carbons for enhancing CO2 uptake at ambient conditions.
2 Experimental
2.1 Materials
2.1.1 Activated carbon from polypyrrole (AC-PPy)
One of the samples studied, namely polypyrrole-derived activated carbon (AC-PPy), was reported by Choma et al. (2015). This carbon (AC-PPy) was obtained by a single-step carbonization-KOH activation of a self-made polypyrrole (PPy). First, polypyrrole was prepared by polymerization of pyrrole (3.8 g) in the presence of FeCl3 (200 mL of 0.5 M FeCl3 aqueous solution) under stirring for 2 h at room temperature; afterwards, the product was filtered, washed with distilled water, and dried at 80 °C for 12 h. The resulting polymer was mixed with KOH (1:4 C:KOH weight ratio) and heated at 700 °C for 2 h using 3 °C min−1 temperature ramp in nitrogen flow (40 dm3 h−1). The resulting activated carbon was washed with a 1 M HCl aqueous solution and distilled water, and dried at 110 °C for 12 h.
2.1.2 Activated carbon from polyvinylidene fluoride (AC-PVDF)
Another activated carbon studied, AC-PVDF, was reported by Choma et al. (2015). In this case, polyvinylidene fluoride granules were carbonized at 450 °C for 30 min in flowing nitrogen (heating rate = 5 °C min−1 and nitrogen flow = 100 dm3 h−1). Then, the resulting carbon was mixed with KOH (1:5 C:KOH weight ratio) and activated at 700 °C for 1 h using the same heating rate and nitrogen flow as in the case of carbonization. Finally, the activated carbon was washed with a 35 wt% HCl aqueous solution and distilled water, and dried at 100 °C for 24 h.
2.1.3 Activated carbon from sulfonated styrene–divinylbenzene resin (AC-SDVB)
The third activated carbon studied, AC-SVDB, was prepared according to the recipe of Choma et al. (2014b) using a commercially available sulfonated styrene–divinylbenzene resin Amberjet 1200H (Rohm and Haas, Philadelphia, USA). The resin beads (d ≈ 5 mm; 120 g) were dried at 100 °C for 3 h, immersed in 300 cm3 of 50 wt% orthophosphoric acid, and evaporated in a rotary evaporator at 180 °C. Next, the cooled down sample was carbonized at 350 °C for 90 min under static conditions (i.e., no gas flow), followed by activation with KOH (1:4 C:KOH weight ratio) at 700 °C, washing and drying as reported by Choma et al. (2014b).
2.1.4 Ordered mesoporous carbon from phenol–formaldehyde resin (OMC-PF)
OMC-PF ordered mesoporous carbon was prepared by soft-templating procedure reported by Choma et al. (2012). The XRD patterns and TEM images for phenolic resin-based OMCs, confirming their mesostructural ordering, are already available in literature (de Souza et al. 2013; Wang et al. 2008). The synthesis was carried out under acidic (HCl) conditions using resorcinol and formaldehyde as the carbon precursors, and Pluronic F127 triblock copolymer (polyethylene oxide-polypropylene oxide-polyethylene oxide) as the structure-directing agent. First, 2.5 g of resorcinol and 2.5 g of Pluronic F127 block copolymer were dissolved in a mixture of 11.9 mL of ethanol and 6.6 mL of distilled water. After complete dissolution, 2.2 mL of HCl (35–38 wt% aqueous solution) was added and the mixture was stirred for 30 min. Then, 2.5 mL of formaldehyde (37 wt% aqueous solution) was added and the stirring was continued until the solution separated into two layers. The bottom organic layer was collected, transferred to a Petri dish, and dried at 100 °C for 24 h. The resulting organic–organic composite was carbonized in nitrogen flow (20 dm3 h−1) using the following temperature program: first, temperature was ramped to 180 °C using 2 °C min−1 and dwelled for 5 h, then, temperature was ramped to 400 °C using 2 °C min−1, and immediately (no dwell), temperature was ramped to 850 °C using 5 °C min−1 and dwelled for 2 h. The procedure yielded ≈1 g of the carbon product.
2.2 Measurements
Nitrogen adsorption–desorption isotherms at −196 °C and carbon dioxide adsorption isotherms at 0, 10, 20, 25, 30, 40, 50, and 60 °C were measured on an ASAP 2020 surface area and porosity analyzer manufactured by Micromeritics Instrument Corp., Norcross, GA, USA. Each sample was degassed at 200 °C for 2 h in vacuum before every measurement.
2.3 Calculations
The apparent specific surface area (SBET) was determined based on the low-temperature nitrogen adsorption by the Brunauer–Emmett–Teller method (Brunauer et al. 1938) in the relative pressure range 0.05–0.20 using the nitrogen cross-section area of 0.162 nm2. Total pore volume (Vt) was calculated by converting the volume of nitrogen adsorbed at the relative pressure ≈0.99 to the volume of liquid nitrogen at the experiment’s conditions. Pore size distributions (PSD) and specific surface areas (SDFT) were calculated based on the low-temperature nitrogen adsorption using the non-local density functional theory method (NLDFT) for carbon slit-shaped pores with energetic heterogeneity and geometrical corrugation developed by Jagiello and Olivier (2013a, b) and implemented in SAIEUS software by Jagiello. Volumes of micropores and small mesopores (V2) were calculated by integration of the PSD curves under the first and second peak in the range from zero to the endpoint of the second peak. Volumes of small micropores (V1) were calculated by integration of the PSD curves under the first peak in the range from zero to the PSD minimum after the first peak. The sizes of small (w1) and large (w2) micropores were estimated at the maxima of the first and second PSD peaks, respectively. Microporosity was calculated as the ratio of V2 to Vt and expressed in %. Isosteric heats of CO2 adsorption (qst) were calculated based on the CO2 adsorption isotherms at 0, 10, 20, 25, 30, 40, 50, and 60 °C using the Clausius–Clapeyron equation:
where R is the universal gas constant, T is the adsorption temperature, p is the equilibrium pressure, and a is the amount adsorbed (Myers 2002).
3 Results and discussion
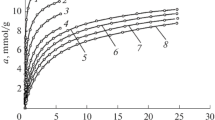
The CO2 adsorption properties of four selected carbon adsorbents derived from different polymers were investigated to explore the opportunities in the development of microporosity in the polymer-derived carbons for achieving high CO2 uptakes at ambient pressure and different temperatures between 0 and 60 °C. A series of eight CO2 adsorption isotherms was measured for each of the four carbon studied. The corresponding nitrogen adsorption isotherms and PSDs are presented in Fig. 1. The isotherm obtained for AC-PPy shows type I(a) according to the new IUPAC classification (Thommes et al. 2015), indicating the presence of small micropores. On the other hand, the isotherm recorded for AC-PVDF is type I(b), which also indicates microporous structure, but one with broad pore size distribution and possibly small mesopores. In contrast, AC-SDVB and OMC-PF both have isotherms being a combination of type I and IV (Kruk and Jaroniec 2001). The PSD curves support these observations: AC-PPy has exclusively pores <1 nm, AC-PVDF has a bimodal PSD spanning from small micropores up to ≈ 4 nm, and AC-SDVB and OMC-PF have both micropores and mesopores present, although of different sizes and quantities.
Nitrogen adsorption–desorption isotherms at −196 °C (left panel) and the corresponding differential pore size distributions calculated using 2D-NLDFT method (right panel; inset shows the mesopore range of PSD for AC-SDVB) for all carbons studied. AC-PPy, AC-PVDF, AC-SDVB and OMC-PF denote carbons prepared from polypyrrole, polyvinylidene fluoride, sulfonated styrene–divinylbenzene resin, and phenol–formaldehyde resin, respectively. Nitrogen adsorption isotherms for AC-PVDF, AC-PPy and AC-SDVB were reported by Choma et al. (2015)
Table 1 lists the structural parameters calculated based on nitrogen adsorption isotherms for all carbons studied. These carbons feature very large specific surface areas, SDFT, reaching 2000 m2 g−1 in all instances and the apparent SBET surface areas from 1810 to 2920 m2 g−1, except OMC-PF, which had a smaller surface area, SDFT = 840 m2 g−1 and SBET = 660 m2 g−1. Similarly, the total pore volumes are large for these carbons in the range of 0.87–1.64 cm3 g−1, and slightly smaller for OMC-PF, Vt = 0.65 cm3 g−1. All carbons possess ultramicropores (pores < 1 nm, labeled as V1) ranging from 0.18 to 0.64 cm3 g−1 depending on the polymer used. The average size of these pores (w1) varies from 0.6 to 0.9 nm, as indicated by the position of the first PSD peak for each carbon sample. The carbons possess larger micropores as well, some with broad PSDs extending up to small mesopores. The peaks’ maxima in this range (w2) vary from 1.2 to 2.1 nm. The volume of all micropores and small mesopores (if present, labeled as V2) range from 0.22 to 1.47 cm3 g−1. In addition, OMC-PF and AC-SDVB also possess some amounts of mesopores, as apparent form their PSDs.
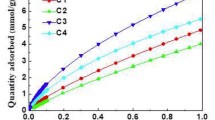
Extensive CO2 adsorption studies were performed for all carbons studied. CO2 adsorption measurements were done at multiple temperatures from 0 to 60 °C and the results were used to calculate the temperature dependence of CO2 uptake, working capacity of each adsorbent, and isosteric heat of CO2 adsorption. Figure 2 shows all CO2 adsorption isotherms and Table 2 lists the CO2 uptakes for all materials at each temperature. The CO2 uptakes recorded at 0 °C and 1 atm are in the range of 3.5–6.9 mmol g−1, and those at 60 °C are in the range of 1.0–1.9 mmol g−1. AC-PPy consistently scored the highest uptake at each temperature, while the order of the other sorbents changed depending on the temperature. To start with, the uptakes at 0 °C follow the order: AC-PPy > AC-SDVB > AC-PVDF > OMC-PF, but at higher temperatures, OMC-PF slowly moves from the fourth to the third place at 10 °C, and then to the second position at 50 °C. The final uptake order, at 60 °C, ends up being AC-PPy > OMC-PF > AC-SDVB > AC-PVDF. These interesting changes are attributed to the presence of extremely small micropores in the OMC-PF carbon that retain CO2 much stronger and result in much slower decay of the CO2 adsorption with temperature. This is supported by much higher isosteric heat of CO2 adsorption for OMC-PF at low values of adsorption as compared to the values obtained for other carbons (see Fig. 4).
CO2 adsorption isotherms measured at 0–60 °C for a AC-PPy, b AC-PVDF, c AC-SDVB, and d OMC-PF. Notation of the samples as in Fig. 1
Figure 3 shows the CO2 uptakes at 0.0013 atm (1 mmHg) and 1 atm (760 mmHg) as functions of temperature for all carbons studied. The uptakes show almost perfect exponential dependence on temperature in the 0–60 °C interval (note the ordinate axis is in logarithmic scale). For all straight lines in Fig. 3 the correlation coefficient exceeds 0.99. The exponential dependence has been previously reported by Ludwinowicz and Jaroniec (2015a) and used to estimate the working capacity in a CO2 pressure-swing adsorption process (PSA). Aaron and Tsouris (2005) described PSA where adsorption occurs at 30 °C and 2 atm and desorption is carried out at 60 °C and 0.0013 atm. Here, we calculate the working capacity based on this information, but instead of adsorption at 2, 1 atm is used (the working capacity at 2 atm would be even higher). Table 3 lists the adsorption and desorption uptakes and the final working capacity.
CO2 uptakes at 0.0013 atm (hollow symbols) and 1 atm (full symbols) as functions of temperature for all carbons studied. The ordinate axis is in logarithmic scale. Notation of the samples as in Fig. 1
As the result of very small uptake under desorption conditions, the working capacities are virtually equal to the CO2 uptakes at the adsorption conditions. Consequently, AC-PPy scored the highest working capacity of 3.70 mmol g−1. The other materials achieved values in the range 1.95–2.65 mmol g−1, which are comparatively high as well (Samanta et al. 2012).
To complement the CO2 adsorption studies, the isosteric heats of adsorption were calculated based on the CO2 adsorption and the Clausius–Clapeyron equation. Figure 4 shows the calculated heats of adsorption as functions of the CO2 uptake for all carbons studied. The noticeable small steps in the plots at higher values of the CO2 uptake are due to the decreasing number of the CO2 isotherms used to calculate qst. These steps represent a sudden decrease in the accuracy not the actual changes. As expected, the heats of adsorption decrease with the amount of adsorbed CO2, but the values and their decline are quite different among the carbons studied. For instance, the highest initial heat of adsorption (32.9 kJ mol−1 at CO2 uptake of 0.1 mmol) is observed for OMC-PF, followed by those for AC-PPy (29.2 kJ mol−1), AC-SDVB (25.5 kJ mol−1), and AC-PVDF (24.9 kJ mol−1). However, the fastest decreases in qst, 35 and 29 %, in the range of 0–2.5 mmol are observed for AC-PVDF and OMC-PR, respectively, while the corresponding decreases for AC-PPy and AC-SDVB are about 14 %.
4 Conclusions
Four carbon adsorbents prepared from different polymers: polypyrrole (AC-PPy), polyvinylidene fluoride (AC-PVDF), sulfonated styrene–divinylbenzene resin (AC-SDVB), and phenol–formaldehyde resin (OMP-PF) were examined as potential adsorbents for CO2 capture. CO2 adsorption isotherms measured at different temperatures between 0 and 60 °C range were used to evaluate the adsorption properties such as CO2 uptakes at different temperatures, working capacity, and isosteric heat of adsorption in relation to the polymer used as carbon precursor.
All carbons studied showed the well developed porous structures, either exclusively microporous (AC-PPy and AC-PVDF) or micro-mesoporous (AC-SDVB and OMC-PF). The highest CO2 uptake at 0 °C, 6.92 mmol g−1, was obtained for AC-PPy followed by those for AC-SDVB, AC-PVDF, and OMC-PF. At higher temperatures, AC-PPy consistently scored best CO2 uptakes among carbons studied, while the order of the remaining samples changed with temperature. The temperature dependence of CO2 uptake in 0–60 °C range followed an exponential trend, as previously reported by Ludwinowicz and Jaroniec (2015a). Based on these data, the working capacities for all adsorbents were calculated assuming a pressure-swing CO2 adsorption process with adsorption at 1 atm and 30 °C and desorption at 0.0013 atm and 60 °C. The resulting values range from 1.95 to 3.70 mmol g−1, with the best result for AC-PPy. Overall, the carbons studied were highly microporous, which resulted in high CO2 uptakes at ambient conditions, making them viable candidates for carbon capture and other adsorption and environmental-related applications.
References
Aaron, D., Tsouris, C.: Separation of CO2 from flue gas: a review. Sep. Sci. Technol. 40, 321–348 (2005)
Arienillas, A., Rubiera, F., Parra, J.B., Ania, C.O., Pils, J.J.: Surface modification of low cost carbons for their application in the environmental protection. Appl. Surf. Sci. 252, 619–624 (2005)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Choma, J., Kalinowska, A., Jedynak, K., Jaroniec, M.: Reproducibility of the synthesis and adsorption properties of ordered mesoporous carbons obtained by the soft-templating method (in Polish). Ochr. Srodowiska 34(3), 3–10 (2012)
Choma, J., Osuchowski, L., Jaroniec, M.: Properties and applications of activated carbons obtained from polymeric materials: a review (in Polish). Ochr. Srodowiska 36(2), 3–16 (2014a)
Choma, J., Osuchowski, L., Marszewski, M., Jaroniec, M.: Highly microporous polymer-based carbons from CO2 and H2 adsorption. RSC Advances 4, 14795–14802 (2014b)
Choma, J., Stachurska, K., Osuchowski, L., Dziura, A., Jaroniec, M.: Carbon dioxide adsorption on activated carbons obtained from polymeric precursors (in Polish). Ochr. Srodowiska 37(4), 3–8 (2015)
De Canck, E., Ascoop, I., Sayari, A., Van Der Voort, P.: Periodic mesoporous organosilicas functionalized with a wide variety of amines for CO2 adsorption. Phys. Chem. Chem. Phys. 15, 9792–9799 (2013)
De Souza, L.K.C., Wickramaratne, N.P., Ello, A.S., Costa, M.J.F., da Costa, C.E.F., Jaroniec, M.: Enhancement of CO2 adsorption on phenolic resin-based mesoporous carbons by KOH activation. Carbon 65, 334–340 (2013)
Górka, J., Jaroniec, M.: Hierarchically porous phenolic resin-based carbons obtained by block copolymer-colloidal silica templating and post-synthesis activation with carbon dioxide and water vapor. Carbon 49, 154–160 (2011)
Gupta, V.K., Nayak, A., Agarwal, S., Tyagi, I.: Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J. Colloid Interface Sci. 417, 420–430 (2014)
Heydari-Gorji, A., Belmabkhout, Y., Sayari, A.: Polyethyleneimine-impregneted mesoporous silica: effect of amine loading and surface alkyl chains on CO2 adsorption. Langmuir 27, 12411–12416 (2011)
ICPP, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, 2013
Jagiello, J., Olivier, J.P.: 2D-NLDFT Adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 55, 70–80 (2013a)
Jagiello, J., Olivier, J.P.: Carbon slit pore model incorporating surface energetical heterogeneity and geometrical corrugation. Adsorption 19, 777–783 (2013b)
Jankowska, H., Swiatkowski, A., Choma, J.: Active Carbon. Ellis Horwood Ltd., Chichester (1991)
Kruk, M., Jaroniec, M.: Gas adsorption characterization of ordered organic–inorganic nanocomposite materials. Chem. Mater. 13, 3169–3183 (2001)
Lee, S., Park, S.: Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 389, 230–235 (2013)
Liu, J., Thallapally, P.K., McGrail, B.P., Brown, D.R., Liu, J.: Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 41, 2308–2322 (2012)
Liu, J., Wickramaratne, N.P., Qiao, S.Z., Jaroniec, M.: Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 14, 763–774 (2015)
Ludwinowicz, J., Jaroniec, M.: Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 82, 297–303 (2015a)
Ludwinowicz, J., Jaroniec, M.: Effect of activating agents on the development of microporosity in polymeric-based carbon for CO2 adsorption. Carbon 94, 673–679 (2015b)
Marsh, H., Rodriguez-Reinoso, F.: Activated Carbon. Elsevier, Amsterdam (2006)
Migahead, M.A., Abdul-Raheim, A.M., Atta, A.M., Brostow, W.: Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly(ethylene terephthalate). Mater. Chem. Phys. 121, 208–214 (2010)
Mishra, S., Goje, A.S., Zope, V.S.: Chemical recycling, kinetics, and thermodynamics of poly(ethylene terephthalate) (PET) waste powder by nitric acid hydrolysis. Polym. React. Eng. 11, 79–99 (2003)
Myers, A.L.: Thermodynamics of adsorption in porous materials. AIChE J. 48, 145–160 (2002)
Patel, H.A., Karadas, F., Canlier, A., Park, J., Deniz, E., Jung, Y., Atilhan, M., Yavuz, C.T.: High capacity carbon dioxide adsorption by inexpensive covalent organic polymers. J. Mater. Chem. 22, 8431–8437 (2012)
Piacentini, R.D., Mujumdar, A.S.: Climate change and drying of agricultural products. Drying Technol. 27, 629–635 (2009)
Samanta, A., Zhao, A., Shimizu, G.K.H., Sarkar, P., Gupta, R.: Post-combustion CO2 capture using solid sorbents: a review. Ind. Eng. Chem. Res. 51, 1438–1463 (2012)
Seredych, M., Jagiello, J., Bandosz, T.J.: Complexity of CO2 adsorption on nanoporous sulfur-doped carbons—is surface chemistry an important factor? Carbon 74, 207–217 (2014)
Su, F., Lu, C.: CO2 capture from gas stream by zeolite 13x using a dual-column temperature/vacuum swing adsorption. Energy Environ. Sci. 5, 9021–9027 (2012)
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. (2015). doi:10.1515/pac-2014-1117
Wang, X., Liang, C.D., Dai, S.: Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol–formaldehyde and block copolymers under highly acidic conditions. Langmiur 24, 7500–7505 (2008)
Wickramaratne, N.P., Jaroniec, M.: Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 5, 1849–1855 (2013)
Acknowledgments
J.C. acknowledges the National Science Centre (Poland) for support of this research under grant 2013/09/B/ST5/00076.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choma, J., Stachurska, K., Marszewski, M. et al. Equilibrium isotherms and isosteric heat for CO2 adsorption on nanoporous carbons from polymers. Adsorption 22, 581–588 (2016). https://doi.org/10.1007/s10450-015-9734-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9734-0