Abstract
Carbon microporous adsorbents obtained on the basis of polymers are promising adsorbents for the tasks of adsorption storage of natural gas due to the possibility of creating a precise porous structure, as well as optimal mechanical characteristics. A study of the adsorption of methane in a carbon adsorbent based on a composite polymer of furfural and epoxy resin in the temperature range from 178 to 360 K and pressures up to 25 MPa has been carried out. The thermodynamic functions of the adsorption system—the differential molar isosteric and integral heats of adsorption, as well as the isosteric entropy, enthalpy, and heat capacity of the system are calculated. The obtained thermodynamic functions are of fundamental importance in the analysis of the properties of nanodispersed adsorbate in the micropores of the adsorbent, and can also be used as input data in modeling the thermodynamic states of experimental systems for methane storage and transportation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Adsorbed natural gas (ANG) technology is an effective alternative to known liquefied and compressed natural gas technologies for a number of technical reasons, such as high specific capacity, safety, and energy efficiency [1–3]. The most promising adsorbents for use in ANG technology include active carbons [4, 5] and Metal-organic frameworks (MOF) [6–8], as well as composites [9, 10], which have a high adsorption capacity for methane, which is the main component of natural gas. In the case of MOF application, it is currently very difficult to resolve issues related to the operation of adsorption gas storage systems—increasing mechanical, cyclic stability and bulk density [11, 12]. Therefore, the possibility of wide use of these materials is not being discussed. Thus, active carbons with a special porous structure remain the best for application in ANG systems in the foreseeable future. In turn, polymer-based activated carbons are of particular interest as a class of promising adsorbents for the adsorption of methane due to the possibility of varying their structural and adsorption properties in wide ranges [1, 13, 14]. In addition, activated carbons obtained on the basis of furfural polymers and epoxy resin [15–17] possess advanced characteristics of mechanical strength, abrasion, and resistance to vibration loads, which is important for use in onboard methane storage systems.
When choosing a carbon adsorbent for the ANG system, its key indicator, which is considered as the main one in most works, is the adsorption activity towards methane [18–20]. However, it is known that the processes of adsorption and desorption of gases are accompanied, respectively, by the release and absorption of heat, which has a significant effect on the characteristics of the adsorption storage system [21–24]. Thus, the study of the thermodynamic characteristics of “carbon adsorbent–methane” adsorption systems is an important and urgent scientific problem.
The purpose of this work is to study the adsorption and thermodynamic characteristics of systems for the adsorption accumulation of methane based on a carbon adsorbent obtained from a thermosetting copolymer of furfural and epoxy resin.
2 OBJECTS OF STUDY
2.1 Adsorbent
The work investigated the ACFE (Active Carbon from Furfural and Epoxy resin) adsorbent from polymer raw materials obtained by liquid molding of a thermosetting copolymer of furfural GFS 98 and epoxy resin in the presence of sulfuric acid as a catalyst [15–17]. The resulting granules have a spherical shape with a smooth outer surface. After obtaining the granules, the polymer material undergoes two-stage carbonization in furnaces in the temperature range of 450–500 and 800–850°C, which is then sent to the activation section. The activation is carried out by a physical method in an environment of water vapor and carbon dioxide at a temperature of about 850°C for 5–6 h [15–17].
2.2 Adsorptive
In experimental studies on the adsorption methane with a main component content of 99.999 vol % was used. The physicochemical properties of methane: molecular weight μ = 16.0426 g/mol, boiling temperature T0 = 111.66 K, critical temperature Tcr = 190.77 K, and critical pressure Pcr = 4.641 MPa [25].
3 EXPERIMENTAL
3.1 Structural and Energy Characteristics of Adsorbent
The structural and energy characteristics of the ACFE adsorbent, such as specific volume of micropores W0, standard characteristic energy of adsorption E0, and effective half-width of micropores x0, were calculated according to the Dubinin–Radushkevich equation of the theory of volumetric filling of micropores on the basis of experimental data on the adsorption of standard benzene vapor at a temperature of 293 K [26]. Specific surface area SBET was calculated using the well-known BET equation using adsorption data for nitrogen vapor at 77 K [27]. The bulk density of the adsorbent was determined in accordance with [28].
Table 1 shows the structural and energy characteristics of a microporous carbon adsorbent, from which it follows that the adsorbent has a micro-mesoporous structure.
3.2 Measurement of Methane Adsorption
To experimentally measure the adsorption of methane in the pressure range from 5 Pa to 25 MPa and temperatures from 178 to 360 K, we used the original adsorption-vacuum, volumetric, and volumetric–weight installations, which are described in detail in [29–31].
Adsorption of methane a was defined as the total content of the adsorbed substance in the micropores of the adsorbent:
Here, N is the total amount of gas in the system, g; V is the total volume of the system, cm3; Va is the volume of adsorbent with micropores, cm3; ρg is the density of the gas phase at a given pressure P and temperature T, g/cm3; m0 is the mass of the outgassed adsorbent, g; and μ is the molecular weight of methane, g/mmol.
Within the framework of this work, to approximate the experimental adsorption isotherms, we used Bakaev’s transcendental adsorption equation obtained by the method of statistical thermodynamics [32]:
where k0, k1, k2, and k3 are the numerically selected coefficients of the equation and P is the equilibrium pressure, Pa.
According to the results of the approximation, the maximum regression error was no more than 3%, which made it possible to calculate with high accuracy adsorption and thermodynamic characteristics of the system.
4 THERMODYNAMIC FUNCTIONS OF THE ADSORPTION SYSTEM
4.1 Differential Molar Isosteric Heat of Adsorption
Differential molar isosteric heat of adsorption qst is an important thermodynamic parameter characterizing the heat effects of adsorption processes [33–35]. By definition [33], isosteric heat of adsorption qst is the difference between enthalpy of the equilibrium gas phase hg and differential molar enthalpy of the adsorption system h1 (3):
In [34–36], when considering issues related to the determination of the thermodynamic functions of adsorption processes, it was shown that the most complete equation for calculating the differential molar isosteric heat of adsorption from the point of view of taking into account the properties of nonidealized systems is the equation obtained by Bakaev
where Z is the compressibility factor of the equilibrium gas phase at pressure P, Pa, and temperature T, K; vg is the specific volume of the gas phase, m3/kg; R is the universal gas constant, J/(mol K); Va = V0/m0 is the specific reduced volume of the adsorbent–adsorbate adsorption system, cm3/g; V0 is the volume and m0 is the mass of the regenerated adsorbent.
Equation (4) most fully takes into account the physical factors affecting the value of the differential molar isosteric heat of adsorption: adsorption isothermal strain (∂Va/∂a)T, thermal isosteric strain (∂Va/∂T)a, the slope of the adsorption isotherm (∂P/∂a)T and isoster [∂lnP/∂(1/T)]a, and non-ideality gas phase Z.
Nevertheless, it was shown in [35] that the contribution from the strain corrections for carbon adsorbents under the conditions under consideration is minimal and they can be disregarded when calculating qst. Therefore, within the framework of this study, to calculate qst, we used an equation that does not take into account the corrections for adsorption-induced and thermal deformation, but takes into account the non-ideality of the gas phase and the slope of the adsorption isotherms:
When calculating the differential molar isosteric heat of adsorption, it is important to determine its initial value \(q_{{{\text{st}}}}^{0}.\) The value of initial differential molar isosteric heat of adsorption \(q_{{{\text{st}}}}^{0}\) is calculated by the formula
Bakaev’s equation for the approximation of adsorption isotherms (1) also makes it possible to estimate the values of the initial heat of adsorption \(q_{{{\text{st}}}}^{0}.\) When the pressure in (1) tends to zero, it is possible to calculate Henry constant KΓ, presenting the equation in the form
The processes of sorption and desorption of methane are accompanied by the release and absorption of a large amount of heat. To assess the heat effect, the values of the integral heat of adsorption are calculated:
4.2 Differential Molar Isosteric Entropy of the System
The entropy of the adsorbent–adsorbate adsorption system is the most important thermodynamic function of the state, which makes it possible to obtain information about the state of the adsorbate in the pores of the adsorbent and the nature of their interaction. The equation for calculating the differential molar entropy of the system has the following form [34, 35]:
Enthalpy is a thermodynamic function of the state of an adsorption system, which determines the amount of energy in the system that can potentially be converted into heat—in other words, its heat content:
4.3 Differential Molar Isosteric Heat Capacity of the Adsorption System
From the point of view of thermodynamic description of the adsorption of molecules in micropores, the value of the differential molar isosteric heat capacity of the system is indicative. To calculate the differential molar isosteric heat capacity, the Kirchhoff equation is used, which, after differentiating Eq. (9) with respect to temperature, has the following form [34, 35]:
5 DISCUSSION
5.1 Adsorption of Methane in Wide Ranges of Temperatures and Pressures
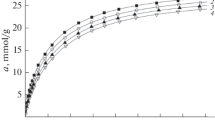
Experimental isotherms of methane adsorption on ACFE adsorbent at pressures up to 25 MPa and temperatures from 178 to 360 K are shown in Fig. 1.
Table 2 shows the numerical values of the constants of Bakaev adsorption equation (2) for the approximation of adsorption isotherms.
The shape of adsorption isotherms in Fig. 1 is typical for physical adsorption on microporous adsorbents. The isotherms are reversible; with increasing temperature, the adsorption value decreases and the maximum adsorption at 178 K reaches a value of about 11.5 mmol/g.
The isosteres of methane adsorption on ACFE shown in Fig. 2 are satisfactorily approximated by straight lines and do not undergo qualitative changes when passing through Ps and critical temperature limit Pcr, which corresponds to the similar conclusions of works [37, 38]. With an increase in the degree of adsorption filling, the slope of the isosteres becomes flatter and the angle of inclination to the abscissa decreases.
Table 3 shows the calculated data on specific volumetric capacity of the adsorption accumulation system VANG based on the ACFE, taking into account bulk density d = 500 kg/m3 (Table 1) and compressed gas systems without adsorbent VCNG calculated by the formulas from [1] for pressures of 5 (216–273 K) and 7 MPa (300–360 K).
From Table 3, it follows that, in the entire considered temperature range, VANG is almost double the value of VCNG, which may indicate the prospects of using this material in gas storage systems.
5.2 Differential Molar Isosteric Heat of Adsorption
Initial heat of adsorption of 22 kJ/mol (Fig. 3) indicates a high heterogeneity of the adsorbent surface with the presence of high-energy adsorption sites. With an increase of adsorption from 0 to 2 mmol/g, a decrease in heat is observed due to the depletion of adsorption sites, after which the decrease slows down in the range from 2 to 6 mmol/g, where the volumetric filling of the micropores with methane molecules occurs. When approaching the region of maximal fillings, above 8 mmol/g, the curves of the differential heat of adsorption demonstrate sharp drop down to zero values due to the limiting saturation of the pores with adsorbate.
As follows from Fig. 3, in the initial region of adsorption equilibria, the character of dependences qst(a) is practically the same; however, with increasing of adsorption amount, the curves start to disperse. This fact is observed due to the non-ideality of the adsorptive gas phase, which is taken into account in Eq. (5).
Table 4 shows the calculated values of the integral heat of adsorption of methane in a carbon adsorbent at various temperatures within the limits of integration from 0 to amax.
From the data in the table, it follows that, with an increase in temperature, in the studied ranges of pressure and temperature, there is a decrease in the value of the integral heat of adsorption in the range from 154 kJ/kg at 178 K to 107 kJ/kg at 360 K. Obtained data Q can be used to calculate the thermal characteristics of adsorption systems for accumulating methane on an adsorbent ACFE.
5.3 Differential Molar Isosteric Entropy of Methane Adsorption
Figure 4 shows the dependences of the differential molar entropy of the adsorption system of adsorption.
In the region of initial fillings (less than 0.5 mmol/g), a sharp drop in entropy occurs due to the adsorption of molecules on vacant high-energy adsorption sites. In the range of average fillings of 1–7 mmol/g, the entropy decrease slows down due to the further filling of micropores with methane molecules and the formation of associates [39, 40]. In the region of high fillings, there is a sharp rise in the curves s1(a), which is probably associated with a change in the properties of associates and the formation of a denser adsorbate structure in the pores [41]. A further increase in adsorption, as was shown in [38, 42], leads to a small local maximum and a sharp drop of entropy in the region of high fillings of micropores [38].
It also follows from the figure that s1(a) depends on temperature; as the temperature increases, the entropy increases and the curves shift upward. The greatest convergence of entropy isotherms is characteristic for the region of small values of adsorption, while the greatest divergence is for high values.
5.4 Differential Molar Isosteric Enthalpy of the Adsorption System
Calculated differnetial molar isosteric enthalpies of adsorption system are shown in Fig. 5. As follows from Fig. 5, the enthalpy of the adsorption system has a strong temperature dependence, which is minimum in the region of initial fillings and maximum in the region of high fillings, which is connected with the temperature dependence of the bulk gas phase of methane.
The most intense increase in the enthalpy value is observed at fillings from 0 to 1 mmol/g for all temperatures and from 7 to 10 mmol/g at temperatures above the critical one. Negative values of enthalpy along the ordinate axis are due to the choice of the standard state of enthalpy of the gas phase for calculating h1 according to Eq. (10).
5.5 Differential Molar Isosteric Heat Capacity
Figure 6 shows that, at low fillings, the enthalpy of the system increases almost linearly, which is associated with the temperature invariance of the heat of adsorption, qst ≠ f(T), but hg is linearly dependent on temperature. However, with growth of the fillings, dependences h1(T) become nonlinear: with increasing temperature, the slope of derivative dh1/dT increases.
Differentiation of h1 by temperature at constant adsorption allows one to obtain the calculated dependences of the heat capacity of the system on temperature Ca(T). Figure 7 shows the dependences of the differential isosteric heat capacity of the system, as well as the isobaric heat capacity of the gas phase of methane, on temperature Cp(T) selected for a qualitative comparison.
Dependences of differential molar isosteric heat capacities of the system Ca (numbering without dash) and gas phase Cp (numbered with a stroke) in the initial region of fillings on temperature at adsorption values, mmol/g, (1) 0.1, (2) 0.3, (3) 1, (4) 2, (5) 5, (6) 6.8, (7) 7.8, (8) 8.5, and (9) 9. Bars of relative error (maximum deviation) ± 30%.
From Fig. 7, it follows that, in the region of low temperatures and the range of initial fillings, the heat capacity of the system is identical in magnitude to the isobaric heat capacity of the gas phase (curves 1, 3, and 4 and 1', 2', and 4'). However, as the temperature rises above 220 K, the differences become more significant and the heat capacity of the adsorption system begins to exceed the values of Cp. Thereafter, with an increase in adsorption and an increase in temperature, the heat capacity also increases, and the higher the temperature, the more Ca(T) dependences differ.
According to [38], where the thermodynamic functions of a large number of adsorption systems were observed, in most cases dependence Ca(T) has a local maximum, which, with an increase in the adsorption filling, shifts towards a decrease in temperature. A similar situation, obviously, will be observed in our case for the ACFE–methane system in the case of an expansion of the temperature range.
It is important to note that this behavior of the Ca(T) curves cannot be connected with the influence of the gas phase, since the dependences diverge significantly (see curves 7 and 7 ' in Fig. 7). It is also unlikely that the properties of the adsorbent itself will overlap for ACFE, as an integral part of the entire adsorption system, since there is practically no change in its structure.
In this case, the only logical explanation for this effect is the change in the state of the substance in the micropores of the adsorbent—the formation of adsorption associates in the micropores of the adsorbent.
6 CONCLUSIONS
This paper has investigated the adsorption characteristics of a carbon microporous adsorbent obtained from furfural, as well as the thermodynamic functions of the ACFE–methane adsorption system in the temperature range from 178 to 360 K and pressures up to 25 MPa. The highest methane adsorption at temperatures of 178–360 K reach values from 9 to 11.5 mmol/g, which may indicate that this material has prospects for use in gas storage systems. A sharp drop in the value of the differential molar isosteric heat of adsorption of methane from 22 to 16 kJ/mol in the range of fillings from 0 to 1 mmol/g indicates a high heterogeneity of the adsorbent surface. This property is confirmed by the thermodynamic characteristic of the entropy of the adsorption system, where it drops sharply in the region of initial fillings. In this case, in the region of high fillings, a sharp increase in the entropy of the adsorption system is observed, which may be associated with the rearrangement of the adsorbate structure inside the pores of the adsorbent—the formation of adsorption associates in the micropores of the adsorbent. Calculation of the heat capacity of the adsorption system showed that, in the low-temperature range and in the range of initial fillings, the heat capacity of the system is identical in magnitude to the isobaric heat capacity of the gas phase. However, as the temperature rises above 220 K, the differences become more significant and the heat capacity of the adsorption system begins to exceed the values of Cp. The obtained data can be used to calculate the thermal characteristics of methane accumulation systems and to solve problems of optimizing adsorption processes.
REFERENCES
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., et al., Russ. Chem. Rev., 2018, vol. 87, no. 10, pp. 950–983.
Tsivadze, A.Yu., Aksyutin, O.E., Ishkov, A.G., et al., Russ. Chem. Rev., 2019, vol. 88, no. 9, pp. 925–978.
Kumar, K.V., Preuss, K., Titirici, M.M., and Rodríguez-Reinoso, F., Chem. Rev., 2017, vol. 117, no. 3, pp. 1796–1825. https://doi.org/10.1021/acs.chemrev.6b00505
Men’shchikov, I.E., Shiryaev, A.A., Shkolin, A.V., Vysotskii, V.V., Khozina, E.V., and Fomkin, A.A., Korean J. Chem. Eng., 2021, vol. 38, pp. 276–291. https://doi.org/10.1007/s11814-020-0683-2
Men’shchikov, I.E., Fomkin, A.A., Shkolin, A.V., et al., Russ. Chem. Bull., 2018, vol. 67, no. 10, pp. 1814–1822. https://doi.org/10.1007/s11172-018-2294-1
Knyazeva, M.K., Solovtsova, O.V., Tsivadze, A.Yu., et al., Russ. J. Inorg. Chem., 2019, vol. 64, pp. 1507–1512. https://doi.org/10.1134/S0036023619120064
Knyazeva, M.K., Tsivadze, A.Yu., Solovtsova, O.V., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, pp. 9–14. https://doi.org/10.1134/S2070205119010064
Makal, T.A., Li, J.-R., Lu, W., and Zhou, H.-C., Chem. Soc. Rev., 2012, vol. 41, pp. 7761–7779.
Solovtsova, O.V., Shkolin, A.V., Men’shchikov, I.E., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 6, pp. 1080–1084. https://doi.org/10.1134/S2070205119060303
Solovtsova, O.V., Shkolin, A.V., Men’shchikov, I.E., et al., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 5, pp. 826–832. https://doi.org/10.1134/S207020511905023X
Rubio-Martinez, M., Avci-Camur, C., Thornton, A.W., Imaz, I., Maspoch, D., and Hill, M.R., Chem. Soc. Rev., 2017, vol. 46, pp. 3453–3480.
Valizadeh, B., Nguyen, T.N., and Stylianou, K.C., Polyhedron, 2018, vol. 145, pp. 1–15.
Fomkin, A.A., Pribylov, A.A., Tkachev, A.G., et al., Prot. Met. Phys. Chem. Surf., 2020, vol. 56, no. 1, pp. 3–7.
Men’shchikov, I.E., Fomkin, A.A., Tsivadze, A.Y., et al., Adsorption, 2017, vol. 23, no. 2–3, pp. 327–339.
Gur’yanov, V.V., Mukhin, V.M., and Kurilkin, A.A., Catal. Ind., 2013, vol. 5, pp. 156–163.
Mukhin, V.M., Tarasov, A.V., and Klushin, V.N., Aktivnye ugli Rossii (Active Carbons of Russia), Moscow: Metallurgiya, 2000.
Mukhin, V.M., et al., Sorbtsionnye Khromatogr. Protsessy, 2009, vol. 9, no. 2, pp. 191–195.
Casco, M.E., Martínez-Escandell, M., and Gadea-Ramos, E., Chem. Mater., 2015, vol. 27, no. 3, pp. 959–964.
Wang, Y., Ercan, C., Khawajah, A., and Othman, R., AIChE J., 2012, vol. 58, no. 3, pp. 782–788.
Kockrick, E., Schrage, C., Borchardt, L., Klein, N., Rose, M., Senkovska, I., and Kaskel, S., Carbon, 2010, vol. 48, no. 6, pp. 1707–1717.
Men’shchikov, I.E., Shkolin, A.V., Strizhenov, E.M., et al., Nanomaterials, 2020, vol. 10, no. 11, p. 2243. https://doi.org/10.3390/nano10112243
Men’shchikov, I., Shkolin, A., Khozina, E., and Fomkin, A., Nanomaterials, 2020, vol. 10, no. 7, p. 1379. https://doi.org/10.3390/nano10071379
Ridha, F.N., Yunus, R.M., Rashid, M., and Ismail, A.F., Exp. Therm. Fluid Sci., 2007, vol. 32, pp. 14–22.
Feroldi, M., Neves, A.C., Borba, C.E., and Alves, H.J., J. Cleaner Prod., 2018, vol. 172, pp. 921–926.
Sychev, V.V., Vasserman, A.A., Zagoruchenko, V.A., et al., Termodinamicheskie svoistva metana (Thermodynamic Properties of Methane), Moscow: Izd. Standartov, 1979.
Dubinin, M.M., Prog. Surf. Membr. Sci., 1975, vol. 9, pp. 1–70.
Brunauer, S., Emmett, P.H., and Teller, E., J. Am. Chem. Soc., 1938, vol. 60, no. 2, pp. 309–319.
GOST (State Standard) no. R 55959-2014: Activated Carbon. Standard Test Method for Bulk Density, Moscow: Standartinform, 2014.
Shkolin, A.V. and Fomkin, A.A., Russ. Chem. Bull., 2008, vol. 57, pp. 1799–1805.
Pribylov, A.A., Serpinskii, V.V., and Kalashnikov, S.M., Zeolites, 1991, vol. 11, pp. 846–849.
Fomkin, A.A., Shkolin, A.V., Men’shchikov, I.E., et al., Meas. Tech., 2016, vol. 58, no. 12, pp. 1387–1391. https://doi.org/10.1007/s11018-016-0904-6
Bakaev, V.A., Dokl. Akad. Nauk SSSR, 1966, vol. 167, pp. 369–372.
Hill, T.L., in Advances in Catalysis and Related Subjects, Frankerburg, Y.I., , Eds., New York: Academic Press, 1952, vol. 4, pp. 211–258.
Fomkin, A.A., Adsorption, 2005, vol. 11, no. 3, pp. 425–436.
Shkolin, A.V., Fomkin, A.A., and Potapov, S.V., Russ. Chem. Bull., 2017, vol. 66, no. 4, pp. 607–613.
Bakaev, V.A., Doctoral Sci. (Phys.-Math.) Dissertation, Moscow: Moscow State Univ., 1989.
Fomkin, A.A., Men’shchikov, I.E., Pribylov, A.A., et al., Colloid J., 2017, vol. 79, no. 1, pp. 144–151. https://doi.org/10.1134/S1061933X16060053
Fomkin, A.A., Doctoral Sci. (Phys.-Math.) Dissertation, Moscow, 1993.
Anuchin, K.M., Fomkin, A.A., Korotych, A.P., and Tolmachev, A.M., Prot. Met. Phys. Chem. Surf., 2014, vol. 50, no. 2, pp. 173–177.
Shkolin, A.V., Fomkin, A.A., Tsivadze, A.Yu., et al., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, no. 6, pp. 955–963.
Tovbin, Yu.K., in Adsorbtsiya, adsorbenty i adsorbtsionnye protsessy v nanoporistykh materialakh (Adsorption, Adsorbents, and Adsorptive Processes in Nano-Porous Materials), Moscow: Granitsa, 2011.
Fomkin, A.A., Serpinskii, V.V., and Fidler, K., Izv. Akad. Nauk SSSR, Ser. Khim.,1982, no. 6, p. 1207.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Men’shchikov, I.E., Fomkin, A.A. & Shkolin, A.V. Thermodynamics of Methane Adsorption in a Microporous Carbon Adsorbent Prepared From Polymer Composition. Prot Met Phys Chem Surf 57, 883–889 (2021). https://doi.org/10.1134/S2070205121050191
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121050191