Abstract
A series of soft-templated ordered mesoporous carbons (OMCs) was synthesized by using resorcinol and formaldehyde as carbon precursors, triblock copolymer Pluronic F127 as a soft-template, and an organic acid (acetic, benzoic, citric, oxalic, or succinic) as a polymerization reaction catalyst. The aforementioned organic acids were strong enough to facilitate the formation of ordered mesophases by the block copolymer template used and to catalyze the polymerization reaction of resorcinol and formaldehyde in this template. The use of weak organic acids instead of strong inorganic acids such as HCl eliminated inorganic anions from the reaction environment and resulted in high surface area OMCs. Basically, the resulting carbons showed the surface areas and pore volumes comparable to those reported for the carbons prepared under similar conditions but in the presence of strong inorganic acids. Electron microscopy analysis proved the presence of ordered mesopores, whereas thermogravimetric analysis showed a good thermal stability of these carbons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Within the last decade, the research devoted to the development of ordered mesoporous carbons (OMCs) has been intensified. The reason for this growing interest is a high demand for applications of OMCs ranging from adsorption (Jun et al. 2000), catalysis (Chai et al. 2004), and separations (Liang et al. 2003) to energy storage and conversion (Kalbour et al. 2006). OMCs have been mainly prepared by hard and soft-templating. The former method makes the use of ordered mesoporous silica materials (Joo et al. 2001; Ryoo et al. 1999, 2001; Lee et al. 2004), colloidal silica (Han and Hyeon, 1999; Li and Jaroniec, 2001; Jaroniec et al., 2008), and colloidal crystals (Zakhidov et al. 1998; Yoon et al. 2005) as templates. The latter method employs triblock copolymers (Liang and Dai, 2006; Meng et al. 2006; Jaroniec et al. 2009; Gorka et al. 2009; Choma et al. 2010) as soft templates, phenol derivatives (such as phenol, resorcinol, or phloroglucinol) and formaldehyde as carbon precursors along with HCl or NaOH used to catalyze polymerization of carbon precursors. In the first stage of soft-templating, a mesostructured polymer–polymer composite is formed by the self-assembly of carbon precursors and block copolymer. Subsequent thermal treatment at elevated temperatures yields a mesoporous polymer, which subjected to carbonization yields a mesoporous carbon. Soft-templating is a simple and effective method for controlling the mesoporous structure of carbons; for instance, pH of the synthesis mixture influences the average pore size of the resulting carbons. The initial synthesis conditions, basic or acidic, affect the average pore size (which is about 4 or 8 nm, respectively) as well as the cross-linking and thickness of pore walls that determine chemical, mechanical, and thermal stability of the resulting carbons (Meng et al. 2006; Wang et al. 2008).
The synthesis of OMCs under basic conditions requires a precise pH control during the entire process. In addition, this recipe requires an additional step of pre-polymerization of phenol and formaldehyde to obtain resol, which is self-assembled with a block copolymer template. The base-catalyzed soft-templating was introduced for the first time by Zhao and co-workers (Meng et al. 2005, 2006; Zhang et al. 2005). Liang and Dai (2006), Wang et al. (2008) developed an alternative recipe under acidic conditions, which is less sensitive to pH changes. One of the disadvantages of the latter recipe is a high concentration of chloride ions introduced to the reaction environment along with HCl. The presence of these ions is not desirable in certain applications of OMCs. That is why more and more efforts have been made to develop the feasible and more environmentally friendly routes for the synthesis of OMCs. Lu et al. (2008) proposed the soft-templating synthesis of OMCs by using resorcinol and formaldehyde, triblock co-polymer Pluronic F127, and glutamic acid. The resulting mesoporous carbons possessed the specific surface area over 700 m2/g, total pore volume of ca. 0.60 cm3/g, and the thickness of pore walls of about 7 nm. In addition, Liu et al. (2011) synthesized soft-templated OMCs by using citric acid as a polymerization catalyst of resorcinol and formaldehyde. The resulting carbon materials were found to possess good thermal stability, hexagonally ordered mesoporous structure with the mean pore size of ca. 5.2 nm. The specific surface area varied in the range between 612 and 851 m2/g, and the total pore volume between 0.46 and 0.62 cm3/g. It was reported (Liu et al. 2011) that the aforementioned carbon materials can be useful for CO2 adsorption and/or sequestration.
In the current work, organic acid-assisted soft-templating synthesis of OMCs is studied. A series of carbon samples was prepared by using resorcinol and formaldehyde as carbon precursors, triblock copolymer Pluronic F127 as a soft-template, and either: acetic, benzoic, citric, oxalic, or succinic acid as a polymerization catalyst of the carbon precursors. It is shown that the resulting carbons possess the surface areas and pore volumes, which are comparable to those of the block copolymer-templated carbons prepared in the presence of strong inorganic acids.
2 Experimental
2.1 Chemicals
Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer (EO106PO70EO106, Pluronic F127) was generously provided by BASF Corp. (Germany). Resorcinol (C6H4(OH)2; 98 %), and formaldehyde (HCHO, 37 %), were acquired from Sigma–Aldrich (Germany). Citric, acetic, oxalic, succinic, and benzoic acid were purchased from POCh (Poland), while ethanol (96 %) from Chempur (Poland).
2.2 Synthesis of mesoporous carbons
Mesoporous carbons were prepared by extending the soft-templating recipe reported by Liu et al. (2011). Approximately 2.5 g of Pluronic F127 and 1.65 g of resorcinol were dissolved in 20 cm3 of ethanol and 20 cm3 of deionized water. After complete dissolution, the reaction mixture was supplied with 6.3 g of either: citric, acetic, oxalic, succinic or benzoic acids and stirred vigorously for 1 h. Then, 2.5 cm3 of formaldehyde was added to the solution and stirred for additional 80 min. The resulting solution was homogeneous and yellowish; it was transferred into a Teflon-lined autoclave and heated at 60 °C for 72 h. The polymeric monolith was collected by filtration, washed with water, and dried in an oven at 80 °C for 12 h; finally, an orange-red monolith was obtained. Thermal treatment and carbonization of this monolith were performed in a tube furnace under flowing nitrogen (20 dm3/h) using a heating rate of 1 °C/min up to 600 °C and keeping the sample at that temperature for 3 h. The procedure yielded 0.6–1.2 g of each mesoporous carbon obtained with different organic acid. The final products were denoted as ST-〈organic acid〉 where 〈organic acid〉 denotes the name of organic acid used during synthesis.
2.3 Characterization
Low-temperature nitrogen adsorption/desorption isotherms were measured at −196 °C on ASAP 2020 volumetric analyzer manufactured by Micromeritics Inc. (Norcross, GA, USA). Prior to measurements, all samples were degassed at 200 °C for at least 2 h.
High-resolution thermogravimetric (TG) analysis was conducted using Q500 thermogravimetric analyzer manufactured by TA Instruments (New Castle, DE, USA). TG and DTG curves were recorded from 30 to 800 °C in air with a heating rate of 5 °C/min.
Scanning electron microscopy images were taken using LEO1530 scanning electron microscope manufactured by Zeiss (Germany) operated at 2 kV accelerating voltage.
Transmission electron microscopy images were taken using 200 kV FEI Tecnai F20 TEM equipped with a field emission gun. The samples for TEM analysis were first suspended in ethanol, and then a droplet of the sample was put onto a carbon-coated copper TEM grid (400-mesh). TEM specimens were allowed to air-dry and kept in vacuum for a few hours to minimize the contamination during TEM observation.
2.4 Calculations
The BET (Brunauer–Emmett–Teller) specific surface area S BET was calculated from nitrogen adsorption isotherms in the range of relative pressures from 0.05 to 0.2 using cross-sectional area of 0.162 nm2 per nitrogen molecule (Brunauer et al. 1938). Single-point total pore volume V t (Gregg and Sing, 1982) was estimated from the amount adsorbed at a relative pressure of ~0.99. Micropore volume V mi was calculated by the α s plot method (Gregg and Sing, 1982; Jaroniec et al., 1989) in the range of α s from 0.8 to 1.2. Mesopore volume V me was obtained by subtraction of the micropore volume V mi from the total pore volume V t. Pore size distribution (PSD) curves were calculated based on the adsorption branch of nitrogen isotherm using the improved Kruk–Jaroniec–Sayari (KJS) method for cylindrical pores smaller than 12 nm (Kruk et al. 1997b). The statistical film thickness (t curve) calculated by fitting the low-temperature (−196 °C) nitrogen adsorption isotherm measured on the nonporous carbon Cabot BP280 (Kruk et al. 1997a) to the multilayer region of the t curve derived for ordered mesoporous silica materials MCM-41 (Choma et al. 2002), and the modified Kelvin equation, were used in the KJS method. Maxima of PSD curves were used to determine the micropore and mesopore widths, w mi and w me accordingly. Mesoporosity, expressed in %, was calculated as the ratio of the mesopore volume V me to the total pore volume V t.
3 Results and discussion
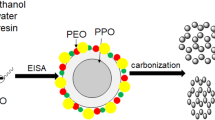
The main focus of the conducted studies was to investigate the feasibility of the organic acid-assisted soft-templating synthesis of OMCs. The secondary goal was to study the impact of the organic acid used on adsorption properties of the resulting carbon materials. Figure 1 depicts a general synthetic path of the soft-templating synthesis of mesoporous carbons carried on in the presence of an organic acid. The first step of this synthesis is to introduce triblock copolymer and organic acid into an aqueous–alcoholic solution.
Schematic illustration of the soft-templating synthesis of mesoporous carbons in the presence of organic acids; scheme analogous to that in Gorka dissertation (2010)
Resorcinol and formaldehyde, used as carbon precursors, were introduced subsequently into the reaction solution and underwent organic acid-catalyzed polymerization in hydrophilic domains of the ordered mesophase of block copolymer. Next, the resulting polymer–polymer mesostructured composite was subjected to the thermal treatment in flowing nitrogen. The initial treatment at elevated temperatures resulted in the cross-linking of phenolic resin and the creation of mesopores due to the decomposition of block copolymer template; further heating of the resulting mesoporous phenolic resin in nitrogen at higher temperatures (so-called carbonization) afforded OMC.
Experimental nitrogen adsorption–desorption isotherms for the carbon studied are shown in Fig. 2. All isotherms are Type IV according to IUPAC classification (Sing et al. 1985) with well-developed hysteresis loops classified as Type H1 (Sing et al. 1985). Microporosity of all samples was evaluated by the α s plot method from nitrogen adsorption isotherms (Gregg and Sing, 1982). Four samples, which were synthesized with citric, succinic, acetic, and benzoic acid, possess well-developed, and fairly similar microporosity, whereas microporosity of the carbon sample obtained with use of oxalic acid is less developed as compared to other samples. The mesopore volume of the samples was obtained by subtracting the micropore volume from the single-point pore volume; of course, its value is larger for the samples having higher step of capillary condensation. Thus, the carbon obtained in the presence of citric acid possessed the most developed mesoporous structure among all samples studied. A comparable mesoporosity was found for the carbon obtained in the presence of succinic acid. The least developed mesoporous structure was observed for the carbon prepared with the help of oxalic acid.
Basic structural parameters of the carbon materials studied were calculated based on experimental nitrogen adsorption isotherms shown in Fig. 2. The resulting parameters are shown in Table 1.
The BET specific surface area (S BET) values varied from 601 m2/g for the ST-Oxalic acid sample to 746 m2/g for the ST-Acetic acid sample, whereas the single-point pore volume (V t) values ranged from 0.40 cm3/g for the ST-Oxalic acid to 0.59 cm3/g for the ST-Citric acid. Since the target of this study was to obtain carbon materials with the well-developed mesoporosity, therefore the volumes of mesopores (V me) should be dominant. The micropore volumes do not exceed 0.1 cm3/g except those for the ST-Oxalic acid and ST-Benzoic acid samples, having micropore volumes of 0.14 and 0.13 cm3/g, respectively. The mesopore volumes recorded for the ST-Citric acid and ST-Succinic acid samples are 0.51 and 0.49 cm3/g, respectively.
The highest mesoporosity value of 86 % was observed for the ST-Citric acid sample, whereas the lowest value of 65 % was for the ST-Oxalic acid sample. The mesoporosity values reported in Table 1 show that in all carbon samples mesopores dominate over micropores.
Pore size distribution functions (PSDs) for the carbon studied were calculated using Kruk–Jaroniec–Sayari method (KJS) (Kruk et al. 1997b) and are shown in Fig. 3. All PSD curves exhibit two maxima. The first, occurring for pore sizes <2 nm, corresponds to the width of micropores, whereas the letter (about 6 nm), corresponds to the width of mesopores. As can be seen from Table 1, the micropore size, ca. 1.9 nm, is analogous for all the samples studied regardless of the organic acid used. On the other hand, the width of mesopores is changing slightly with the organic acid used during the synthesis. The smallest value, 5.88 nm, was obtained for the ST-Acetic acid sample, whereas the largest mesopores, 6.19 nm, were obtained when succinic acid was used (the ST-Succinic acid sample). Based on above it can be concluded that the organic acids used during soft-templating synthesis affect slightly the mesoporous structure of the resulting carbons; an exception is the sample obtained in the presence of oxalic acid.
It was mentioned in introduction that the initial synthesis conditions, basic or acidic, affect the average size of mesopores; smaller mesopores (~4 nm) are usually reported for the carbons prepared from resol (basic conditions; Meng et al. 2006) and larger mesopores (~8 nm) are reported for carbons obtained under acidic conditions (Wang et al. 2008). The current study confirms this case. The average mesopore size for the carbon prepared in the presence of citric acid is ~6 nm, while the value of about 8 nm was reported for the corresponding sample prepared in the presence of HCl (see a comparison of nitrogen adsorption isotherms and pore size distributions in Fig. 4). It seems that in the case of the block copolymer used, higher acidity favors the formation of larger micelles and consequently, larger mesopores; similar finding was reported by Grant and Jaroniec (2012) for the block copolymer-templated alumina samples.
A comparison of nitrogen adsorption isotherms (top panel) and pore size distributions (bottom panel) for the carbon samples prepared in the presence of citric acid and HCl; data for the latter sample are reported by Choma et al. (2012)
The TG profile was recorded for the ST-Citric acid sample. The data were measured in the temperature range from 30 to 800 °C in flowing air. The TG data indicate that the mesoporous carbons are thermally stable in air atmosphere up to 400 °C and start to decompose afterward with a rapid combustion at ~430 °C. A very small value of residue, i.e. 0.61 %, shows that the carbons studied contain negligible amount of inorganic impurities.
An important aspect is the morphology of the mesoporous carbons studied. Figures 5 and 6 show SEM images of the ST-Benzoic acid and ST-Succinic acid samples, respectively. These images show that the carbons studied display some kind layered organization at the macroscopic level. This structure can be well seen at the edge of the ST-Benzoic acid sample shown in Fig. 5. In addition, ordered nature of uniform mesopores can be noticed in Fig. 6 showing the SEM image of the ST-Succinic acid sample. The convincing evidence of mesostructural ordering is provided by TEM analysis, which is presented below.
As it was previously mentioned, the main focus of the current study was to obtain mesoporous carbon materials with ordered and uniform mesoporosity through an organic acid-assisted soft-templating synthesis. TEM studies were conducted to prove the ordered and uniform nature of mesopores in the carbon materials prepared in the presence of organic acids. Figure 7 shows the TEM image of the mesoporous carbon obtained using citric acid (the ST-Citric acid sample). The well-ordered mesopores of this carbon are clearly seen in Fig. 7 and their size is in a good agreement with adsorption-based pore size analysis.
4 Conclusions
The current work shows that the organic acid-assisted soft-templating represents a feasible route for the synthesis of well-ordered mesoporous carbon materials. Citric, acetic, oxalic, succinic, and benzoic acids were successfully employed in this soft-templating synthesis as catalysts for the polymerization of resorcinol and formaldehyde carbon precursors. The use of the aforementioned organic acids was sufficient for the preparation of mesoporous carbons with good structural properties, i.e., specific surface area reaching 700 m2/g, total pore volume of about 0.60 cm3/g, and the mesopore width of about 6 nm. It is noteworthy that the organic acid-assisted synthesis eliminated chloride ions from the reaction environment because HCl catalyst was not used. Acidity values of the organic acids used, expressed in terms of pKa (Table 1) show that pKa in the range of 3–4 is sufficiently strong to catalyze the polymerization reaction of resorcinol and formaldehyde during soft-templating synthesis of mesoporous carbons. In addition, it was shown that triblock copolymer Pluronic F127 works well as a soft template, in the presence of not only a strong acid such as HCl but also in the presence of weak organic acids such as citric, acetic, succinic, or benzoic acids. The resulting mesoporous carbon materials possess the well-developed mesoporous structures and relatively large volumes of mesopores.
References
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Chai, G.S., Yoon, S.B., Kim, J.H., Yu, J.-S.: Spherical carbon capsules with hollow macroporous core and mesoporous shell structures as a highly efficient catalyst support in the direct methanol fuel cell. Chem. Commun. 23, 2766–2767 (2004)
Choma, J., Jaroniec, M., Kloske, M.: Improved pore-size analysis of carbonaceous adsorbents. Adsorption Sci. Technol. 20, 307–315 (2002)
Choma, J., Jedynak, K., Jamioła, D., Jaroniec, M.: Influence of carbonization temperature on the adsorption and structural properties of mesoporous carbons obtained by soft templating (in Polish). Ochrona Srodowiska (Poland) 34–2, 3–8 (2012)
Choma, J., Zubrowska, A., Gorka, J., Jaroniec, M.: Adsorption properties of phenolic resin-based mesoporous carbons obtained by using mixed templates of Pluronic F127 and Brij 58 or Brij 78 polymers. Adsorption 16, 377–383 (2010)
Gorka, J. (Jaroniec, M—advisor): Polymer-based mesoporous carbons: soft-templating synthesis, adsorption and structural properties. Dissertation, Kent State University (2010)
Gorka, J., Fenning, C., Jaroniec, M.: Influence of temperature, carbon precursor/copolymer ratio and acid concentration on adsorption and structural properties of mesoporous carbons prepared by soft-templating. Coll. Surf. A 352, 113–117 (2009)
Grant, S.M., Jaroniec, M.: Effect of acid concentration on pore size in polymer-templated mesoporous alumina. J. Mater. Chem. 22, 86–92 (2012)
Gregg, S.J., Sing, K.S.W.: Adsorption, surface area and porosity, 2nd edn. Academic Press, London (1982)
Han, S., Hyeon, T.: Simple silica particle template synthesis of mesoporous carbons. Chem. Commun. 19, 1955–1956 (1999)
Joo, S.H., Choi, S.J., Oh, I., Kwak, J., Liu, Z., Terasaki, O., Ryoo, R.: Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412, 169–172 (2001)
Jaroniec, M., Choma, J., Gorka, J., Zawislak, A.: Colloidal silica templating synthesis of carbonaceous monoliths assuring formation of uniform spherical mesopores and incorporation of inorganic nanoparticles. Chem. Mater. 20, 1069–1075 (2008)
Jaroniec, M., Gorka, J., Choma, J., Zawislak, A.: Synthesis and properties of mesoporous carbons with high loadings of inorganic species. Carbon 47, 3034–3040 (2009)
Jaroniec, M., Madey, R., Choma, J., McEnaney, B., Mays, T.J.: Comparison of adsorption methods for characterizing the microporosity of activated carbons. Carbon 27, 77–83 (1989)
Jun, S., Joo, S.H., Ryoo, R., Kruk, M., Jaroniec, M., Liu, Z., Ohsuna, T., Terasaki, O.: Synthesis of new, nanoporous carbon with hexagonally ordered nanostructure. J. Am. Chem. Soc. 122, 10712–10713 (2000)
Kalbour, H., Baumann, T.F., Satcher, J.H., Saulnier, A., Ahn, C.C.: Toward new candidates for hydrogen storage: high-surface area carbon aerogels. Chem. Mater. 18, 6085–6087 (2006)
Kruk, M., Jaroniec, M., Gadkaree, K.P.: Nitrogen adsorption studies of novel synthetic active carbons. J. Coll. Interface Sci. 192, 250–256 (1997a)
Kruk, M., Jaroniec, M., Sayari, A.: Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 13, 6267–6273 (1997b)
Lee, J., Han, S., Hyeon, T.: Synthesis of new nanoporous materials using nanostructured silica materials as templates. J. Mater. Chem. 14, 478–486 (2004)
Li, Z., Jaroniec, M.: Colloidal imprinting: a novel approach to the synthesis of mesoporous carbons. J. Am. Chem. Soc. 123, 9208–9209 (2001)
Liang, C., Dai, S.: Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J. Am. Chem. Soc. 128, 5316–5317 (2006)
Liang, C., Dai, S., Guiochon, G.: A graphitized-carbon monolithe column. Anal. Chem. 75, 4904–4912 (2003)
Liu, L., Deng, Q.F., Ma, T.Y., Lin, X.Z., Hou, X.X., Liu, Y.P., Yuan, Z.Y.: Ordered mesoporous carbons: citric acid—catalyzed synthesis, nitrogen doping and CO2 capture. J. Mater. Chem. 21, 16001–16009 (2011)
Lu, A.H., Spliethoff, B., Schuth, F.: Aqueous synthesis of ordered mesoporous carbon via self-assembly catalyzed by amino acid. Chem. Mater. 20, 5314–5319 (2008)
Meng, Y., Gu, D., Zhang, F., Shi, Y., Cheng, L., Feng, D., Wu, Z., Chen, Z., Wan, Y., Stein, A., Zhao, D.: A family of highly ordered mesoporous polymer resin and carbon structures from organic–organic self-assembly. Chem. Mater. 18, 4447–4464 (2006)
Meng, Y., Gu, D., Zhang, F., Shi, Y., Yang, H., Li, Z., Yu, C., Tu, B., Zhao, D.: Ordered mesoporous polymers and homologous carbon frameworks: amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 44, 7053–7059 (2005)
Ryoo, R., Joo, S.H., Jun, S.: Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 103, 7743–7746 (1999)
Ryoo, R., Joo, S.H., Kruk, M., Jaroniec, M.: Ordered mesoporous carbons. Adv. Mater. 13, 677–681 (2001)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
Wang, X., Liang, C., Dai, S.: Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol–formaldehyde and block copolymers under highly acidic conditions. Langmuir 24, 7500–7505 (2008)
Yoon, S.B., Chai, G.S., Kang, S.K., Yu, J.S., Gierszal, K.P., Jaroniec, M.: Graphitized pitch-based carbons with ordered nanopores synthesized by using colloidal crystals as templates. J. Am. Chem. Soc. 127, 4188–4189 (2005)
Zakhidov, A.A., Baughaman, R.H., Iqbal, Z., Cui, C., Khayrullin, I., Dantas, S.O., Marti, J., Ralchenko, V.G.: Carbon structures with three-dimensional periodicity at optical wavelengths. Science 282, 897–901 (1998)
Zhang, F., Meng, Y., Gu, D., Yan, Y., Yu, C., Tu, B., Zhao, D.: A facile aqueous route to synthesize highly ordered mesoporous polymers and carbon frameworks with Ia3d bicontinuous cubic structure. J. Am. Chem. Soc. 127, 13508–13509 (2005)
Acknowledgments
MJ acknowledges the National Science Foundation for support of this research under CHE-0848352 grant. JC acknowledges the National Science Centre for support of this research under Grant UMO 2011/03/N/ST5/04444. KJ acknowledges the Ministry of Science and Higher Education (Poland) for support of this research under Grant BS 038/2012. KJ—is a fellow under the program “Scholarships for Graduate Students of Majors Relevant to Regional Development” founded through Humane Capital Operational Program. The TEM data were recorded using the (cryo) TEM facility at the Liquid Crystal Institute, Kent State University, supported by the Ohio Research Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choma, J., Jedynak, K., Marszewski, M. et al. Organic acid-assisted soft-templating synthesis of ordered mesoporous carbons. Adsorption 19, 563–569 (2013). https://doi.org/10.1007/s10450-013-9479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9479-6