Abstract
Mesoporous carbons (MCs) with controllable pore structure and pore size have been prepared by direct carbonization of composites of reverse compolymer-phloroglucinol/formaldehyde. The microstructure of the MCs was analyzed by small-angle X-ray scattering, nitrogen adsorption isotherms and transmission electron microscope. The results showed the pore size of the obtained MCs can be easily tuned from 6.4 to 11.4 nm. Furthermore, the inkbottle-like or cylindrical pore structure of MCs can be obtained by simply adjusting the ratios of formaldehyde and phloroglucinol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mesoporous carbons (MCs), owning to their unique high surface area [1], catalytic [2], conductive and adsorptive properties [3], have inspired tremendous attention over the past decades and have been widely used in diverse areas ranging from macromolecular adsorption and separation, energy storage and conversion, catalysis support, etc. Traditionally, MCs through carbon arogel synthesized by the poly-condensation of resorcinol (R)/formaldehyde (F) [4], phenol/furfural [5], and melamine/formaldehyde via a sol–gel process [6]. However, carbon aerogel preparation is time consuming and has its advantage such as tunability of pore size, which limits the large-scale application [7]. Some studies have tried to simplify the preparation process by ambient drying instead of conventional supercritical drying [8]. Very recently, organic–organic self-assembly method is applied to synthesize MCs. Compared with hard-templating strategy, this technique does not require tedious post-processing such as template-removal by NaOH or HF, which would lead to environment polluted [9, 10]. Therefore, significant efforts involving various routes based on the soft-template have been devoted to prepare MCs since Liang et al. successfully prepared MCs with channel pore structures using resorcinol/formaldehyde as carbon sources and the diblock copolymer poly(styrene)-b-poly(4-vinylpy-ridine) (PS-b-P4VP) as a template [11]. Tanaka et al. also obtained the similar results by employing the same carbon sources and triblock copolymers F127 (EO106–PO70–EO106) as template with triethyl orthoacetate (EOA) was added to decrease the polymerization of resorcinol and formaldehyde [12]. More recently, Zhao et al. apply the evaporation induced self-assembly method (EISA) to synthesize a series of MCs with high surface area and uniform pore size through self-assembly of low molecular, soluble in ethanol phenolic resins and triblock copolymers [13]. For all the work mentioned above, the synthesis of carbon precursor usually needs a very long time which is possibly a barrier to the industrial application. Furthermore, tuning the pore size of MCs is challenging [14, 15].
As is well-known, phloroglucinol can react with formaldehyde easily to generate multi-hydroxyl composites [16, 17]. If they are adopted as reactants to synthesize carbon precursors, lots of time can be save compared with of that if phenol or resorcinol and formaldehyde are used. Moreover, different amounts of formaldehyde while keeping the other reactants constant might form various composites which can lead to different interactions with copolymer. So herein we try to reduce the time of synthesis of carbon precursor and to control the pore structure and size of MCs (see Fig. 1). As highlighted here, MCs with pore diameters up to 11.4 nm and controllable pore structure can be successfully obtained.
2 Experimental
2.1 Chemicals
Poly(propyleneoxide)-poly(ethyleneoxide)-poly(propyleneoxide)triblock copolymer (PO97EO186PO97, MW = 19,500) was purchased from by Nanjing Chem Corp Limit. Phloroglucinol, formalin solution (37–40 wt%), NaOH and ethanol were purchased from Tianjin Chem Corp. All chemicals were used as received without further purification.
2.2 Preparation of MCs
The sample was prepared as described by a previous report with some modifications [16]. The typical synthetic process of MCs proceeds as follows: PO97–EO186–PO97 (5 g) was dissolved in ethanol (24 g) under magnetic stirring at 30 °C. Then, the resin solution was synthesized as follows, 3 g of phloroglucinol and 0.03 g solid NaOH were dissolved in ethanol (24 g), followed by addition of a certain amount of 37% formaldehyde and 8 g of ethanol with stirring at 30 °C for 10 min. Subsequently, the resin solution was added into the ethanol solution containing PO97EO186PO97, and stirred for 10 min to form a homogeneous solution. A brown film was obtained by pouring the solution into a dish and allowing the ethanol to evaporate at room temperature for about 24 h, then heating in an oven at 100 °C for 24 h. MCs were obtained by direct carbonization of the as synthesized samples at 700 °C for 3 h at a heating rate of 1 K/min under N2 (here the samples can be denoted as MCs-X, where X = 1, 2, 3 and 4 corresponding to the weight content of the formaldehyde 1.0, 1.2,1.4 and 1.6 g, respectively).
2.3 Characterization of MCs
Small-angle X-ray scattering (SAXS) recorded by using an imaging plate with X-ray wavelength of λ 1.38 Å at beam line 4B9A of the Beijing Synchrotron Radiation Facility (BSRF). TEM measurement was conducted by using a Hitachi H-800. The samples were prepared by dispersing the products in ethanol with an ultrasonic bath for 30 min and then a few drops of the resulting suspension were placed on a copper grid. Nitrogen adsorption–desorption isotherms were performed at 77 K on a TriStar 3000 volumetric adsorption system. The specific surface area was calculated from the adsorption data in the relative pressure interval from 0.05 to 0.35 using the Brunauer–Emmett–Teller (BET) method. The pore size distribution curve was obtained from adsorption branch by using Barrett–Joyner–Halenda (BJH) method. The total pore volume (Vtotal) was calculated at the relative pressure of 0.99. The micropore volume (Vmicro) was determined by t-plot model, and the mesoporous volume (Vmeso) was calculated by the difference of Vtotal and Vmicro.
3 Results and discussion
The SAXS patterns of MCs-X are shown in Fig. 2. It can be observed the patterns alter as the content of formaldehyde changes. For example, when the content is low such as 1 or 1.2 for MCs-1-2, both samples show wide peaks in small angle areas suggesting that the MCs obtained have wormlike mesopores [5]. While for the high content 1.4 or 1.6 of MCs-3-4, the peak in small angle area is very unconspicuous. This indicates that the arrangement of mesopore becomes enen more disordered when the content of formaldehyde is increasing.
The porosity and textural parameters of samples are explored by N2 sorption (Fig. 3). All samples show typical type-IV curves and have hysteresis loops which are ascribed to the capillary of condensation effects and reveals of presence of mesopores (Fig. 3a) [18]. It is interesting that it can be found with the ratios of formaldehyde/template altering, the hysteresis loops of MCs are obviously different which is very similar to the results of previously report [16]. However, it can be noticed that It can also be noticed that the adsorption of at lower P/P0 at about 0–0.1 suggest the MCs have less amount of micropore compared to with that of MCs synthesized by phenol or resorcinol/formaldehyde carbon precursors. When the content is 1 or 1.2, the hystersis loops are H1 type at about relative pressure (P/P0) between 0.6 and 0.9, ie, the adsorption and desorption branches of the isotherm are nearly parallel, which is typical of mesoporous solids consisting of very large mesopores with a large hysteresis indicating an interconnected cylinder pore system. While when the content is 1.4 or 1.6, the hystersis loops are H2 type at about relative pressure (P/P0) between 0.4 and 0.7, which implied small mesopore of the samples with a cage-like type pore, i.e., to the pores with the narrower orifice and the broader inner part. The pore size distribution of MCs is shown in Fig. 3b. The pore size distribution of the four samples is centralized at about 11.4, 9.3, 4.3 and 4.3 nm, respectively. Their pore volumes of MCs-1-2 are larger than that MCs-3-4 possibly because of the nature of pore structure and size (as shown in Table 1). From the pore size distributions of samples (Fig. 3b), it could be clearly seen that the pore size of MCs can be tailored from 4.3 to 11.4 nm with the changing content of formaldehyde.
Table 1 exhibits the parameters of the MCs-X, it can be observed that all samples have great pore volumes and high surface areas. Noticeably, all samples have higher mesopore ratio despite the pore-type of cage-like of MCs-3-4, which is very different from the previously reported of long-time synthesis MCs [16]. This is maybe because that the less time may reduce the polymerization of phloroglucinol and formaldehyde, which results in the weak reactivity of phloroglucinol/formaldehyde composites.
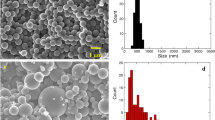
Figure 4 shows the typical TEM images of both MCs-1 and MCs-2 samples, it can be obviously visible that the microstructure of the obtained samples is disordered (Fig. 4a for MCs-1 and Fig. 4c for MCs-3, respectively) which is consistent with the results of SAXS patterns described above. The differences of pore type can not be directly observed maybe because of the wormlike pore structure of samples. High-resolution transmission electron microscopy demonstrated the amorphous nature of carbon lattice which is for most of mesoporous materials (Fig. 4b for MCs-1 and Fig. 4d for MCs-3, respectively).
4 Conclusion
MCs with controllable pore size and structure have been successfully prepared by using a facial and mild strategy so-called EISA method. Pore size of MCs can be tuned from 4.3 to 11.4 nm only by adjusting the content of formaldehyde while the amount of the other reactants was kept constant. Moreover, the pore structure also could be controlled as cylinder or cage-like type. In addition, the synthesis is easy, inexpensive and suitable for large-scale production. These MCs should find applications in chemical catalysis, adsorption and/or selective molecular separations.
References
W. Zhou, K. Zhou, X. Liu, R. Hu, H. Liu, S. Chen, J. Mater. Chem. A 2, 7250–7255 (2014)
A. Zahoor, M. Christy, Y.J. Hwang, Y.R. Lim, P. Kim, K.S. Nahm, Appl. Catal. B 147, 633–641 (2014)
G. Zu, J. Shen, L. Zou, F. Wang, X. Wang, Y. Zhang, X. Yao, Carbon 99, 203–211 (2016)
G. Ramos-Fernandez, M. Canal-Rodríguez, A. Arenillas, J.A. Menendez, I. Rodríguez-Pastor, I. Martin-Gullon, Carbon 126, 456–463 (2018)
M. Enterría, J.L. Figueiredo, Carbon 108, 79–102 (2016)
J. Gu, Z. Du, C. Zhang, S. Yang, Adv. Energy Mater. 6, 1600917–1600923 (2016)
D. Carriazo, M.C. Gutierrez, M.L. Ferrer, F. del Monte, Chem. Mater. 22, 6146–6152 (2010)
S.H. Chai, Z.M. Liu, K. Huang., S. Tan, S. Dai, Ind. Eng. Chem. Res. 55, 7355–7361 (2016)
H. Funabashi, S. Takeuchi, S. Tsujimura, Sci. Rep. 7, 45147–45155 (2017)
K. Zhu, K. Egeblad, C.H. Christensen, Eur. J. Inorg. Chem. 25, 3955–3960 (2007)
C. Liang, Z. Li, S. Dai, Angew. Chem. Int. Ed. 47, 3696–3717 (2008)
S. Tanaka, N. Nishiyama, Y. Egashira., K. Ueyama, Chem. Commun. 16, 2125–2127 (2005)
M. Florent, C. Xue, D. Zhao, D. Goldfarb, Chem. Mater. 24, 383–392 (2012)
J. Hou, K. Jiang, M. Tahir, X. Wu, F. Idrees, M. Shen, C. Cao, J. Power Sources 371, 148–155 (2017)
Y. Zhu, Y. Liu, Y. Liu, T. Ren, T. Chen, Z. Yuan, ChemCatChem 7, 2903–2909 (2015)
P. Li, Y. Song, Z. Tang, G. Yang, J. Yang, J. Sol-Gel. Sci. Technol. 69, 47–51 (2013)
J. Zhang, Z. Qiao, S.M. Mahurin, X. Jiang, S.H. Chai, H. Lu, K. Nelson, S. Dai, Angew. Chem. Int. Ed. 54, 4582–4586 (2017)
C.M. Wu, S.Y. Lin, J. Phys. Chem. C 119, 12335–12344 (2015)
Acknowledgements
The research was supported by the financial support of the Shanxi Province Foundation for Youths (2015021072), the Program for the Innovative Talents of Taiyuan Institute of Technology (TITXD201403), Special Youth Science and Technology Innovation (QKCZ201635), National Training Programs of Innovation and Entrepreneurship for Undergraduates (201614101004). Greatly thanks Fund of Testing from Institute of High Energy and Physics for SAXS measurements assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Li, P., Ma, X. et al. Facile synthesis of mesoporous carbons with tailored porosity by a fast polymerization. J Porous Mater 26, 561–565 (2019). https://doi.org/10.1007/s10934-018-0652-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0652-x