Abstract
Amniotic fluid-derived stem cells (AFSC) have been shown to be broadly multipotent and non-tumorogenic. Previous studies of direct mixing of AFSC and neonatal rat ventricle myocytes indicated evidence of AFSC cardiogenesis. In this study, we examined human AFSC cardiogenic potential in indirect co-culture with human cardiac cells in conditions that eliminated the possibility of cell fusion. Human AFSC in contact with human cardiac cells showed expression of cardiac troponin T (cTnT) in immunohistochemistry, and no evidence of cell fusion was found through fluorescent in situ hybridization. When indirectly co-cultured with cardiac cells, human AFSC in contact with cardiac cells across a thin porous membrane showed a statistically significant increase in cTnT expression compared to non-contact conditions but lacked upregulation of calcium modulating proteins and did not have functional or morphological characteristics of mature cardiomyocytes. This suggests that contact is a necessary but not sufficient condition for AFSC cardiac differentiation in co-culture with cardiac cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart defects (CHD) are the most common birth defects and are the leading cause of birth defect-related death in the United States.10,17 Cardiac patches are used in repairs of some congenital heart defects including Tetralogy of Fallot (ToF). ToF is the most common type of cyanotic CHD.23 In surgical repair of ToF, the right ventricular out flow tract (RVOT) is often reshaped with a patch material typically made from polymers (PTFE, Dacron, etc.), fixed xenograft (e.g., bovine pericardium), or fixed autologous pericardium. The common shortcoming of these acellular polymers or fixed pericardium tissues is the lack of electrical and mechanical integration with the native tissue; this leads to potential risk of arrhythmia and loss of function.7,11,25 A tissue engineered patch encapsulated with cardiomyocytes (CM) and support cells, such as fibroblasts, smooth muscle cells, and endothelial cells, with properties and functions similar to native tissues would integrate with the healthy tissues to avoid the risk of arrhythmia and restore functions.31 The proliferation of CM is limited outside of specific conditions. A major portion of CM is formed during cardiac development stages from embryonic through neonatal periods.21,28 Naqvi et al., have shown significant amount of CM proliferation also occured in stage specific periods during adolescence.18 Furthermore, CM have been found to proliferate in certain post-infarction repairs, such as the ability of neonatal mice to regrow resected cardiac tissue.12 An autologous source of CM for heart defect repair in infants requires a tissue engineered solution.
Amniotic fluid derived stem cells (AFSC) have been shown to possess osteogenic, myogenic, and adipogenic potentials similar to mesenchymal stem cells.6 AFSC, as a potential therapeutic component, offers many advantages, especially in congenital defect repairs. When defects are diagnosed during pregnancy, amniotic fluid can be collected and AFSC isolated. These autologous AFSC pose low risk of immune rejection and could be an ideal implantable cell source for regenerative therapy in neonates with CHD if differentiate to CM occur in vitro prior to implantation or in vivo post implantation. To date, AFSC have been shown to possess multipotency,26 but yet to be shown to differentiate into mature CM.
Co-culturing AFSC with cardiac cells have been used as models to explore direct injection of AFSC for treating cardiac diseases as well as pre-differentiating AFSC prior to implantation through cell induced cardiogenesis. Previous studies of AFSC and neonatal rat ventricular myocytes (NRVM) co-cultured by direct mixing found cells expressing both human and cardiac markers.4,9 However, the cardiac proteins found in AFSC could be result of cell–cell fusion with NRVM.19 Using a xenogeneic cell source also makes therapeutic application difficult as the resulting cells could be vectors of xenotropic transmittable diseases and are at risk for immune rejections.
Previous studies from our lab have shown that AFSC in transmembrane co-culture with NRVM form functional gap junctions.5 In this study, we investigated the effects of cell–cell contact in transmembrane co-culture of human AFSC and human cardiac cells to initiate cardiac differentiation of AFSC. We hypothesized that co-culture with human cardiac cells will induce cardiac differentiation in AFSC, and cell–cell contact is a necessary component of such induction. We tested this by indirectly co-culturing these cells in conditioned media, shared media, and transmembrane systems.
Materials and Methods
Primary Human AFSC Isolation
AFSC were collected as previously described.2 Briefly, amniotic fluid was collected from amnioreduction procedures for treatment of twin–twin-transfusion syndrome during the second trimester according to protocols approved by Baylor College of Medicine and Rice University Institutional Review Boards (IRB). Cells from the fluid sample that adhered to untreated polystyrene petri dishes after 7 days in culture were sorted for c-kit expression (CD117/c-Kit antibody, BD Biosciences, Bedford, MA, USA) by fluorescence assisted cell sorting (Dako MoFlo cell sorter). C-kit positive AFSC were further expanded in maintenance media (63% aMEM (HyClone, Logan, UT, USA), 18% Chang Basal Medium (Irvine Scientific, Santa Ana, CA, USA), 2% Chang C supplement (Irvine Scientific), 15% fetal bovine serum (FBS; PAA Laboratories, Dartmouth, MA, USA), 1% GlutaMAX (Invitrogen, Carlsbad, CA, USA) and penicillin and streptomycin (Lonza, Houston, TX, USA), and characterized and monitored through karyotyping and flow cytometry for embryonic stem cell markers SSEA4 and Sox2; mesenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105; hematopoietic markers CD31 and CD45; and the immunological markers HLA-ABC and HLA-DR (BD Biosciences). For this study, AFSC isolated from an amniotic fluid sample of a single patient were used.
Primary Human Cardiac Cells Isolation
Cardiac cells were isolated from RVOT tissue samples, collected from pediatric surgeries according to protocols approved by Baylor College of Medicine and Rice University IRB. Enzymatic digestion of the samples was adapted from literature.3,14 Briefly, tissue samples were incubated in supplemented Krebs–Ringer solution (10 mM HEPES (Sigma-Aldrich, St. Louis, MO, USA), 129 mM NaCl (Fisher Scientific, Waltham, MA, USA), 4.7 nM KCl (Fisher Scientific), 1.2 mM KH2PO4 (Sigma-Aldrich), 1.2 mM MgSO4·7H2O (Sigma-Aldrich), 5 mM NaHCO3 (Sigma-Aldrich), 5.5 glucose (Sigma-Aldrich), 2% BSA (EMD Chemicals, Gibbstown, NJ, USA), 20 mM taurine (Sigma-Aldrich), 2 mM l-carnitine (Sigma-Aldrich), 5 mM creatine (Sigma-Aldrich)) with neutral protease (Worthington, Lakewood, NJ, USA). The protease solution was then brought to 37 °C for 15 min in a stir flask. The tissue was resuspended in supplemented Krebs–Ringer solution with collagenase (Type 2, Worthington) and hyaluronidase (Worthington) for 20 min in a stir flask. Cells were collected from further digestion in collagenase containing supplemented Krebs–Ringer solution in the stir flask at 15 min intervals. The cells were cultured in cardiac media (M199 (HyClone), 20 mM taurine, 2 mM l-carnitine, 5 mM creatine, 2% BSA, 1% PenStrep, 2% FBS) for 7 days and characterized by immunohistochemistry for cardiac specific markers and flow cytometry for cardiac progenitor markers including SSEA-4 and Isl1 (BD Biosciences).
Co-culture of AFSC and Human Cardiac Cells
AFSC were cultured in control conditions and co-culture conditions with cardiac cells (Fig. 1). AFSC cultured in maintenance media served as negative control, and AFSC cultured in cardiac media served as media control. AFSC in the conditioned media group were fed conditioned media collected from cardiac cell cultures. The conditioned media was not diluted with fresh media. Our group found no significant difference between AFSC cultured with 100% conditioned media and 50% conditioned media in experiments with NRVM.4 For transmembrane co-culture AFSC and cardiac cells were cultured together in a transmembrane system (Transwell, Corning, Lowell, MA, USA) with 0.4 μm pores used in previous studies.5,13 In shared media co-cultures, AFSC were cultured in the lower well and cardiac cells were cultured in the upper well of the Transwell plate. In the transmembrane co-cultures, AFSC were cultured on the underside of the Transwell membrane and cardiac cells were cultured on the upside of the membrane. Cardiac cells were seeded at the same density for shared media and contact co-culture groups for each run of the experiment. Direct mixing of AFSC and cardiac cells tracked by membrane bound fluorescent markers (RFP-PKH26 and GFP-PKH26, Sigma-Aldrich) served as a positive control for co-culture induced AFSC cardiac differentiation. The conditions were designed to identify whether secreted factors lead to cardiac differentiation in the conditioned media and shared media groups, and further distinguished whether the signals could be affected by concentration, degradation, and cross talk in the conditioned media group compared to shared media. Factors released by cardiac cells would be constantly renewed in the shared media condition whereas the concentration of these factors in conditioned media will decrease between media changes due to degradation. The transmembrane co-culture tested whether contact between AFSC and cardiac cells was needed while eliminating the possibility of cell fusion.
Experimental setup. AFSC were cultured in AFSC maintenance media (shaded) as negative control (group 1) and in cardiac cell media (solid) as media control (group 2). AFSC were cultured with cardiac cells in conditioned media culture (group 3), shared co-culture (group 4), transmembrane co-culture (group 5), and direct co-culture (group 6). AFSC in the conditioned media group were fed conditioned media collected from cardiac cell cultures. For transmembrane co-culture AFSC and cardiac cells were cultured together in a transmembrane system (Transwell, Corning, Lowell, MA, USA) with 0.4 μm pores used in previous studies.4,10 In the shared media co-culture AFSC were cultured in the lower well and cardiac cells were cultured in the upper well of the Transwell plate. In the contact co-culture AFSC were cultured on the underside of the Transwell membrane and cardiac cells were cultured on the upside of the membrane
Fluorescence In Situ Hybridization
After 7 days of culture, heterosexual, direct mixing cultures were subjected to fluorescence in situ hybridization analyses using probes specific for the X chromosome centromere and the Yq12 region of the Y chromosome.
Analysis of AFSC Differentiation
Cell cultures were monitored daily using phase contrast microscopy. After 7 days of culture, RNA samples were collected using an RNA collection kit following manufacturer protocol (Applied Biosystems, Carlsbad, CA, USA). mRNA samples were then reverse transcribed to DNA using a cDNA kit following manufacture protocol (Applied Biosystems). The resulting DNA samples were analyzed with quantitative real-time polymerase chain reaction (qRt-PCR) following manufacturer protocol (Applied Biosystems). mRNA sequences of interest included oct 4, sox2, connexin 43, GATA4, cardiac troponin T (cTnT), ryanodine receptor 2 (RYR2), L-type calcium channel subunit alpha-1c, and GAPDH (Applied Biosystems).
After 7–14 days of culture, cell cultures were also fixed with paraformaldehyde (Alfa Aesar, Ward Hill, MA, USA) at 4 °C for 20 min. Fixed cells were incubated with antibodies against GATA4 (rabbit polyclonal, Sigma-Aldrich) and cardiac troponin T (mouse polyclonal; Abcam, Cambridge, MA, USA), then in DyLight-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and DAPI with VectaShield (Vector, Burlingame, CA, USA). The cell were imaged using an epifluorescence microscope (DMI 6000B, Lieca Microsystems, Bannockburn, IL, USA).
Statistics
Statistical analyses on quantitative results were done in SigmaPlot using ANOVA analysis with post hoc Bonferroni corrections. p < 0.05 was considered significant. Results are presented with ± standard deviation and the numbers of samples/trials are indicated in captions.
IRB Statement
Patient samples were collected according to protocols approved by Baylor College of Medicine and Rice University IRB. Informed consent was obtained from all subjects, and the research was carried out according to the World Medical Association Declaration of Helsinki.
Results
Characterization of Primary Human AFSC
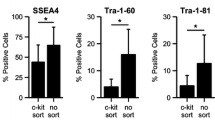
c-kit positive AFSC isolated from second trimester amniotic fluid were found to express mensenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105 at passage 6 by flow cytometry assay in agreement with literature and previous studies.2,5,29 AFSC did not express endothelial cell markers CD31 and CD45, and expressed HLA-ABC but not HLA-DR. The karyotype of AFSC was of a normal diploid human female (Fig. 2). We analyzed the karyotype of higher passage cells to assure the maintenance of normal karyotypes for the lower passage cells used in the experiments.
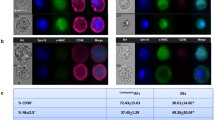
(a) Flow cytometry analysis of Passage 6 AFSC. Populations of cells expressed mesynchymal stem cell markers CD29, CD44, CD73, CD90, and CD105; and pluripotency markers CD117 (c-kit), SSEA-4, and Sox-2. The cells expressed human leukocyte antigen HLA-ABC (HMC class I) but showed limited expression of HLA-DR (MHC class II). The cells did not express hematopoietic markers CD31 and CD45. (b) Passage 6 AFSC showed normal karyotype in metaphase spreads
Characterization of Primary Human Cardiac Cells
Pediatric RVOT samples from patients between 93 and 242 days in age (average 187 ± 57 days) were collected. All procedures were the first operation for CHD treatment. Tissue samples were typically between 20 and 80 mg in size, and approximately 10,000 cells were isolated per mg of tissue. Cells isolated from these samples initially contained rod shaped cells with sarcomere bands, but no rod shaped cells remained after 7 days of culture. After 7 days, the cells were stained for cTnT and GATA4 (Fig. 3). A population (36 ± 8%) of potential cardiac progenitor were found to express cTnT with organizations resembling myofilaments with GATA4 localized in the nucleus, and exhibiting various morphologies (Fig. 3). Flow cytometry analysis of cultured cardiac cells against stem cell marker SSEA-4 and cardiac progenitor cell marker Isl1 showed a population (0.23%) of the cultured cardiac cells is positive for both cardiac progenitor markers.8,15
(a) Representative immunohistochemistry images of cultured cardiac cells. Stained for DNA (blue), cTnT (green), and GATA4 (orange). cTnT, was found throughout a population (36 ± 8%) of the cells. GATA4, a transcription factor, was localized near the nuclei. Scale bar = 50 μm. (b) Flow cytometry results of cultured cardiac cells against stem cell marker SSEA4 and cardiac progenitor cell marker Isl1. A population (0.23%) of the cultured cardiac cells is positive for both markers
Characterization of AFSC Gene Expression During Co-culture
In the direct mixing co-culture controls, AFSC in contact with cardiac cells were found to express cTnT (Fig. 4). In the indirect co-culture groups, GATA4 stained positive in all test and control groups while cTnT stained positive in the conditioned media, shared media, and transmembrane co-culture groups (Fig. 5). After 7 days, 38 ± 7% cells stained positive for GATA4 in the negative control, 38 ± 11% in the media control, 48 ± 12% in the conditioned media co-culture, 82 ± 7% in the shared media co-culture, and 77 ± 6% in the transmembrane co-culture. Also after 7 days, cTnT stained positive in conditioned media, shared media, and transmembrane co-culture groups; 20 ± 4% cells stained positive for cTnT in the conditioned media co-culture, 16 ± 9% in the shared media co-culture, and 29 ± 8% in the contact co-culture (Fig. 6). After 14 days, 47 ± 11% cells stained positive for GATA4 in the negative control, 53 ± 5% in the media control, 79 ± 8% in the conditioned media co-culture, 62 ± 9% in the shared media co-culture, and 79 ± 14% in the transmembrane co-culture. Also after 14 days, 20 ± 8% cells stained positive for cTnT in the conditioned media co-culture, 35 ± 10% in the shared media co-culture, and 35 ± 13% in the contact co-culture (Fig. 6). In both 7 and 14 day experiments, the populations of GATA4 positive cells were statistically significantly higher in the conditioned media, shared media, and transmembrane co-culture groups compared to the negative and media controls. In 7 day experiments, the populations of GATA4 positive cells were statistically significantly higher in the shared media and transmembrane co-culture groups compared to the conditioned media groups. In 7 day experiments, the populations of cTnT positive cells were statistically significantly higher in the transmembrane co-culture groups compared to the conditioned media and shared media groups. In 14 day experiments the populations of cTnT positive cells were statistically significantly higher in the transmembrane co-culture and shared media groups compared to the conditioned media groups.
Representative immunohistochemistry images of day 7 negative control, media control, conditioned media, shared media, and transmembrane co-culture groups; and day 14 negative control, media control, conditioned media, shared media, and transmembrane co-culture groups. Day 0 AFSC image included for comparison. Cells were stained for cTnT (green,) GATA4 (orange,) and DAPI (blue.) GATA4 stained positive in all groups. cTnT stained positive in conditioned media, shared media and contact co-culture groups at day 7 and day 14. Scale bar = 50 μm
Quantitative comparison of gene expression between experiment groups at day 7: First row: Oct-4 expression and Sox2 were not significantly different between all groups, indicating similar level of self-renewal abilities. n = 5 for each co-culture condition. cTnT expression was significantly increased (* p < 0.05) in contact co-culture groups, but L-type calcium channel subunit expression were not significantly different between groups. No ryanodine receptor 2 expression (not shown) was detected n = 5 for each co-culture condition. Second row: Cells were counted in immunohistochemistry for GATA4 and cTnT, horizontal lines denote groups with significant statistical differences from other groups (p < 0.05, cells were counted from images, more than 100 cells were counted per group). Bars represent means with standard deviation
After 7 days of co-culture conditions, AFSC were tested for RNA expression of the stem cell markers oct-4 and sox2; and cardiac genes GATA4, cTnT, L-type calcium channel subunits alpha-1c (CACNA1c) and beta-2 (CACNB2), and ryanodine receptor 2 (Ryr2). The expression of the stem cell markers oct-4 and sox2 were not statistically significantly different between all groups (Fig. 6). Cxn43 and GATA4 expression were not significantly different between all groups (not shown). cTnT expression was significantly higher in the contact co-culture group than the controls and all other co-culture groups (Fig. 6). L-type calcium channel subunits alpha-1c and beta-2 expression were not significantly different between the groups, and no expression of ryanodine receptor 2 was detected in any groups (Fig. 6).
Fluorescence In Situ Hybridization
Combined results from two direct mixing co-culture trials showed an XX pattern (female) in 94 interphase cells and an XY (male) pattern in 211 cells out of 310 interphase cells analyzed. No cells with an XXXY pattern were detected, indicating an absence of detected cell fusion.
Discussion
CM are characterized by their distinct morphology and electromechano-properties. Mature CM possessing rod-like shapes with segmented sarcomere structure were observed in the initial isolates from RVOT tissues. However, most of these mature CM did not attach in 2D culture substrates and were not found in culture after 2 to 3 days. After 7 days of culturing, a population of cells positive for progenitor markers were identified through flow cytometry.8,15 Furthermore, another population of the cardiac cells stained positive for cTnT in clear tubule-like structures in astral projections similar to those found in early stage CM similar to progenitor cell populations identified in other literature.16,20 These potential progenitor cells did not spontaneously beat during the culturing process and did not respond to electrical stimulation. Implantation of cardiac progenitor cells has been shown to help recover cardiac functions in infarct models.15 Cardiac progenitor cells have been identified through various means, including c-kit expression, cardiosphere culture, and substrate attachment.8,15 Cardiac progenitor cells possess the ability to proliferate while maintaining an immature phenotype and to differentiate into mature cardiac lineages with the appropriate culturing conditions and stimuli.8,15 Additionally, cardiac progenitor cells have been shown to be more abundant in pediatric sources, especially from patients less than one year old, compared to adult sources.15 As shown in previous studies, extended culture of cardiomyocytes is difficult.3 The presence of supporting cells, such as cardiac fibroblasts, smooth muscle cells, and endothelial cells, could be beneficial to the survival of the culture and would represent a more physiologically-relevant environment compared to native heart tissue. Cardiac fibroblast, smooth muscle cells, and endothelial cells within the culture could have directed AFSC differentiation through secreted factors and mechanotransduction separately from the progenitor populations. The differentiation could be both cardiomyogenic and non-cardiomyogenic.24 Pedrotty, et al., has shown paracrine factors released by cardiac fibroblasts significantly reduced electrophysiological properties of neonatal rat cardiomyocytes.22 Similarly, the presence of cardiac fibroblasts could have hindered AFSC CM differentiation in this study. The role of cardiac fibroblasts on directing AFSC differentiation should be examined in future studies.
Previous studies of direct mixing of AFSC and NRVM suggested potential differentiation into a cardiac lineage.4,9 Similar studies of bone marrow derived stem cells heterosexually co-cultured with CM have shown actively dividing cells containing both XX and XY chromosomal pairs, suggesting cell fusion.19 However, no such evidence was observed in direct mixing co-culture between AFSC and human cardiac cells in our positive control. This study was designed to test human–human co-culture in a controlled non-fusion environment to determine the configurations of co-culturing to replicate such differentiation with the goal of viable production for therapeutic needs. Immunohistochemistry results showed that GATA4 expression in the test groups were upregulated compared to the controls, and cTnT expression were also upregulated in the test groups, especially in the transmembrane test groups in both 7 and 14 day experiments and in the shared media test groups in 14 day experiments. However, RT-qPCR results indicated no statistically significant differences were found in cTnT mRNA expression between conditioned media or shared media groups and the controls. This indicated that the media effect and cytokine signaling were not able to induce significant cardiomyocyte gene expression in AFSC. Contact co-culture resulted in significantly higher expression of cTnT mRNA compared to the controls and other co-culture conditions. This suggests cell–cell contact improved the upregulation of cardiac genes in AFSC population exhibiting cardiac differentiation, but does not affect the induction of differentiation. As a comparison, AFSC in close proximity to cardiac cells in direct mixing co-culture exhibited similar population of cTnT positive AFSC.
Neither highly organized sarcomere structure nor rod shape cells were observed in AFSC in any of the experimental groups. AFSC did not spontaneously beat after 7 days of co-culture in any group, similar to direct mixing studies.4,9 These observations agree with the lack of changes in the L-type calcium channel subunit expression and the lack of ryanodine receptor channel expression in the transmembrane co-culture groups in qRT-PCR. L-type calcium channel is needed to generate membrane action potential characteristic of CM and ryanodine receptors participate in controlling the release of calcium stored in the sarcomere. The upregulation of cardiac sarcomere components, however, was not followed by the formation of sarcomere organization typical of functional CM. Furthermore, the cells lacked key calcium handling components and electrical signaling proteins. Both the contractile apparatus and the calcium signaling system are necessary components of a functional CM.
Cell–cell contact increases electrochemical signaling and mechanotransduction, both of which have important roles in differentiation and maintenance of cell phenotype. Electrical stimulation and changes in local membrane resting potential could help induce myocyte differentiation.30 Contractile forces generated by neighboring cells also have been shown to aid in cardiogensis of developing cardiomyocytes.27 Furthermore, mitochondrial transfer could also allow contacting cells to influence each other’s differentiation, as shown in mesenchymal stem cells reprograming CM to progenitor cells.1 It would be beneficial to consider cell contacts in future designs of co-culture based cardiac differentiation systems. Such strategies include incorporating indirect contact through porous membranes similar to this study, embedding surface factors in culturing substrates, customizing mechanical properties of the substrate to mimic cell contacts, and 3 dimensional culturing systems.
When cultured with cardiac cells for 7 days, human AFSC in contact with human cardiac progenitor cells showed a statistically significant increase in cTnT compared to non-contact conditions but lack some components of the excitation–contraction coupling system and did not have functional or morphological characteristics of CM. This suggests that contact is a necessary but not sufficient condition for AFSC cardiac differentiation in co-culture with cardiac cells.
Abbreviations
- AFSC:
-
Amniotic fluid derived stem cells
- CHD:
-
Congenital heart defects
- RVOT:
-
Right ventricular out flow tract
- CM:
-
Cardiomyocytes
- NRVM:
-
Neonatal rat ventricular myocytes
References
Acquistapace, A., T. Bru, P.-F. Lesault, F. Figeac, A. E. Coudert, O. le Coz, C. Christov, X. Baudin, F. Auber, R. Yiou, J.-L. Dubois-Randé, and A.-M. Rodriguez. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells (Dayton, Ohio) 29:812–824, 2011.
Benavides, O., and J. Petsche. Evaluation of endothelial cells differentiated from amniotic fluid-derived stem cells. Tissue Eng. Part A 18:1123–1131, 2012.
Bistola, V., M. Nikolopoulou, A. Derventzi, A. Kataki, N. Sfyras, N. Nikou, M. Toutouza, P. Toutouzas, C. Stefanadis, and M. M. Konstadoulakis. Long-term primary cultures of human adult atrial cardiac myocytes: cell viability, structural properties and BNP secretion in vitro. Int. J. Cardiol. 131:113–122, 2008.
Chiavegato, A., S. Bollini, M. Pozzobon, A. Callegari, L. Gasparotto, J. Taiani, M. Piccoli, E. Lenzini, G. Gerosa, I. Vendramin, E. Cozzi, A. Angelini, L. Iop, G. F. Zanon, A. Atala, P. De Coppi, and S. Sartore. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J. Mol. Cell. Cardiol. 42:746–759, 2007.
Connell, J. P., E. Augustini, K. J. Moise, A. Johnson, and J. G. Jacot. Formation of functional gap junctions in amniotic fluid-derived stem cells induced by transmembrane co-culture with neonatal rat cardiomyocytes. J. Cell Mol. Med. 17:774–781, 2013.
De Coppi, P., G. Bartsch, M. M. Siddiqui, T. Xu, C. C. Santos, L. Perin, G. Mostoslavsky, A. C. Serre, E. Y. Snyder, J. J. Yoo, M. E. Furth, S. Soker, and A. Atala. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25:100–106, 2007.
Du, Z. D., Z. M. Hijazi, C. S. Kleinman, N. H. Silverman, and K. Larntz. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J. Am. Coll. Cardiol. 39:1836–1844, 2002.
French, K. M., A. V. Boopathy, J. A. DeQuach, L. Chingozha, H. Lu, K. L. Christman, and M. E. Davis. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 8:4357–4364, 2012.
Guan, X., and D. Delo. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J. Tissue Eng. Regen. Med. 5:220–228, 2011. doi:10.1002/term.
Hamilton, B. E., D. L. Hoyert, J. A. Martin, D. M. Strobino, and B. Guyer. Annual summary of vital statistics: 2010–2011. Pediatrics 131:548–558, 2013.
Harris, L., and S. Balaji. Arrhythmias in the adult with congenital heart disease. In: Diagnosis and Management of Adult Congenital Heart Disease, edited by M. Gatzoulis, G. Webb, and P. E. Daubeney. Philadelphia: Elsevier Health Sciences, 2003, pp. 105–113.
Heallen, T., M. Zhang, J. Wang, M. Bonilla-Claudio, E. Klysik, R. L. Johnson, and J. F. Martin. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science (New York, N.Y.) 332:458–461, 2011.
Jacot, J., and J. Wong. Endothelial injury induces vascular smooth muscle cell proliferation in highly localized regions of a direct contact co-culture system. Cell Biochem. Biophys. 52:37–46, 2008.
Kehat, I., and D. Kenyagin-Karsenti. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 108:363–364, 2001.
Mishra, R., K. Vijayan, E. J. Colletti, D. A. Harrington, T. S. Matthiesen, D. Simpson, S. K. Goh, B. L. Walker, G. Almeida-Porada, D. Wang, C. L. Backer, S. C. Dudley, L. E. Wold, and S. Kaushal. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation 123:364–73, 2011.
Moretti, A., L. Caron, A. Nakano, J. T. Lam, A. Bernshausen, Y. Chen, Y. Qyang, L. Bu, M. Sasaki, S. Martin-Puig, Y. Sun, S. M. Evans, K.-L. Laugwitz, and K. R. Chien. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165, 2006.
Murphy, S., J. Xu, and K. Kochanek. Deaths: final data for 2010. National Vital Statistics Reports 60, 2013.
Naqvi, N., M. Li, J. W. Calvert, T. Tejada, J. P. Lambert, J. Wu, S. H. Kesteven, S. R. Holman, T. Matsuda, J. D. Lovelock, W. W. Howard, S. E. Iismaa, A. Y. Chan, B. H. Crawford, M. B. Wagner, D. I. K. Martin, D. J. Lefer, R. M. Graham, and A. Husain. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 157:795–807, 2014.
Nygren, J. M., S. Jovinge, M. Breitbach, P. Säwén, W. Röll, J. Hescheler, J. Taneera, B. K. Fleischmann, and S. E. W. Jacobsen. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat. Med. 10:494–501, 2004.
Oh, H., S. B. Bradfute, T. D. Gallardo, T. Nakamura, V. Gaussin, Y. Mishina, J. Pocius, L. H. Michael, R. R. Behringer, D. J. Garry, M. L. Entman, and M. D. Schneider. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. U.S.A. 100:12313–12318, 2003.
Olson, E. N., and D. Srivastava. Molecular pathways controlling heart development. Science 272:671–676, 1996.
Pedrotty, D. M., R. Y. Klinger, R. D. Kirkton, and N. Bursac. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc. Res. 83:688–697, 2009.
Reller, M., and M. Strickland. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatrics 153:807–813, 2008.
Schuldiner, M., O. Yanuka, J. Itskovitz-Eldor, D. A. Melton, and N. Benvenisty. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. U.S.A. 97:11307–12, 2000.
Steeds, R. P., and D. Oakley. Predicting late sudden death from ventricular arrhythmia in adults following surgical repair of tetralogy of Fallot. QJM 97:7–13, 2003.
Tsai, M.-S., J.-L. Lee, Y.-J. Chang, and S.-M. Hwang. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. (Oxford, England) 19:1450–1456, 2004.
Wozniak, M. A., and C. S. Chen. Mechanotransduction in development: a growing role for contractility. Nature reviews. Mol. Cell Biol. 10:34–43, 2009.
Xin, M., Y. Kim, L. B. Sutherland, M. Murakami, X. Qi, J. McAnally, E. R. Porrello, A. I. Mahmoud, W. Tan, J. M. Shelton, J. A. Richardson, H. A. Sadek, R. Bassel-Duby, and E. N. Olson. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. U.S.A. 110:13839–13844, 2013.
Zhang, P., J. Baxter, K. Vinod, T. N. Tulenko, and P. J. Di Muzio. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 18:1299–1308, 2009.
Zhou, B., Q. Ma, S. Rajagopal, S. M. Wu, I. Domian, J. Rivera-Feliciano, D. Jiang, A. von Gise, S. Ikeda, K. R. Chien, and W. T. Pu. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454:109–113, 2008.
Zimmermann, W.-H., I. Melnychenko, G. Wasmeier, M. Didié, H. Naito, U. Nixdorff, A. Hess, L. Budinsky, K. Brune, B. Michaelis, S. Dhein, A. Schwoerer, H. Ehmke, and T. Eschenhagen. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 12:452–458, 2006.
Acknowledgments
This project is supported by the American Heart Association (Beginning Grant-in-Aid to JGJ.) Amniotic fluid samples were provided by the Fetal Center at Texas Children’s Hospital Pavilion for Women. Pediatric cardiac tissue samples were provided by Division of Congenital Heart Surgery at Texas Children’s Hospital through the Heart Center Biorepository.
Disclosures
There are no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael S. Detamore oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Gao, Y., Connell, J.P., Wadhwa, L. et al. Amniotic Fluid-Derived Stem Cells Demonstrated Cardiogenic Potential in Indirect Co-culture with Human Cardiac Cells. Ann Biomed Eng 42, 2490–2500 (2014). https://doi.org/10.1007/s10439-014-1114-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1114-5