Abstract
The functional regeneration of thick vascularized tissues such as bone and muscle is complicated by the large volume of lost tissue, challenging biomechanical environment, and the need to reproduce the highly organized structure of both the native tissue extracellular matrix and its vascular support system. Stem cell or progenitor cell delivery approaches, for example, continue to be plagued by low viability and engraftment in part due to the initial absence of a vascular supply. Recognition of diffusion limitations in thick tissues has prompted regenerative strategies that seek to accelerate establishment of a functional vasculature. The successful design of robust regeneration strategies for these challenging clinical scenarios will rely on a thorough understanding of interactions between construct design parameters and host biological and biomechanical factors. Here, we discuss the critical role of vascularization in normal bone tissue homeostasis and repair, vascular network adaptation to the local biomechanical environment, and the future directions of revascularization approaches being developed and integrated with bone regeneration strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascularization plays a critical role in the healing and functional regeneration of many types of damaged or diseased tissues. Nonhealing bone and muscle defects, for example, typically consist of large three-dimensional regions that lack initial vascularization, creating a locally hypoxic environment. The survival of cells injected into such defects or implanted within biomaterial constructs is remarkably poor due in part to diffusion limitations. This is not surprising given that bone cells are typically located within a few hundred microns of a vascular supply. Perfusion bioreactors can successfully overcome diffusion limitations and maintain cell viability throughout porous biomaterial constructs in vitro.90 However, if not adequately supported by a vascular network that can rapidly connect with host vessels and perfuse even the center of such thick constructs, a loss of cells within the construct interior upon transplantation in vivo is inevitable. Though promoting cell viability is a driving motivation for developing effective revascularization strategies, it is important to recognize that vascular perfusion also plays a key role in the recruitment of local and circulating progenitor cells to the site of healing. Several strategies are being developed to promote rapid revascularization of large tissue defects, including delivery of angiogenic growth factors, co-transplantation of vascular cells, and cultivation of pre-vascularized constructs.46 While the primary goal is to satisfy tissue metabolic and oxygen demands during regeneration, it must be recognized that early vascular structures will adapt and remodel over time in response to functional demands. For mechanically loaded tissues such as those in the musculoskeletal system, both local tissue strains and hemodynamic factors will induce adaptive changes in the vascular network structure and newly-formed tissue organization. Vascularization strategies must therefore permit adaptation of neovascular structures to the dynamic mechanical microenvironment. A deeper understanding of the relationship between vascularization and tissue adaptation is therefore essential for the design of future revascularization strategies to promote and accelerate the functional regeneration of bone and muscle tissues.
This review discusses a forward-looking framework of new vascularization approaches for bone regeneration. We first discuss the homeostatic and regenerative relationships between circulatory and soft tissue support, mechanical loading, and bone regeneration. This framework provides the platform to discuss new strategies to promote revascularization and regeneration of large bone defects: delivery of growth factors, transplantation of cells, and cultivation of pre-vascularized constructs. Vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP-2) are discussed as potent examples of bioactive factors used in bone regeneration. Other reviews have focused in great detail on cell and scaffold based vascularization or bone regeneration strategies.2,51,70 Here, we limit the discussion to progenitors and cells commonly used in clinical and animal studies including bone marrow and adipose derived cells, endothelial cells, and periosteal cells. Several unanswered questions exist in tissue vascularization approaches: the choice of cells, the timing of their addition, the use of pre-formed vessel elements, or the influence of a dynamic mechanical environment pre- or post-implantation. In this review we establish a broad framework to consider these questions as they pertain to bone regeneration strategies. This is an expansive field with many new and interesting directions that are being explored on many scales—this review largely focuses on new developments that have been tested in vivo.
Circulatory Support and Mechanical Loading in Bone Homeostasis and Repair
Calcium homeostasis and structural stability are the two most important functions of bone. Remodeling is a continuous process in the adult skeleton that exists in a balance between resorption and new bone deposition. Bone tissue is extremely well vascularized providing an intraosseous blood flow rate as high as 5–20 mL/min per 100 g bone.54 Blood vessels of the skeletal system have highly defined topologies and are critical for maintenance of bone function. Minor injuries to bone such as the majority of simple fractures typically heal well, however defects beyond a critical size of approximately 2–3 cm in humans require augmentative treatment and represent a significant challenge for orthopaedic surgeons. Several factors like local vascular support from the surrounding muscle tissue, periosteum, and mechanical loading, play a critical role in bone function and repair.
Bone Blood Supply and Homeostasis
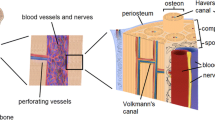
Long bones have overlapping anastomotic circulations to maintain homeostatic blood flow and support regeneration even when faced with interruptions to flow. In addition to the main diaphyseal nutrient arteries, long bones are supplied by epiphyseal, metaphyseal, and periosteal circulations that extensively anastomose with the muscle vasculature (Fig. 1).54 Osteons, the basic unit of cortical bone, consist of a haversian canal with a centralized vascular supply and concentric mineralized lamellae containing highly networked osteocytes. Along with transport of nutrients and metabolites, blood vessels function as conduits for inflammatory and regenerative cells,40 and vessel associated pericytes have been shown to be multipotent.20 It is thus reasonable to expect that dynamic adaptations of bone to environmental changes are associated with changes in its vascularity.
Arterial blood supply of a typical long bone showing the major arteries and their possible anastomotic areas. From Laroche et al. 54 Copyright © 2002 Elsevier Masson SAS. All rights reserved
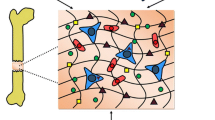
In health, the adult skeleton maintains a balance between resorption and deposition of new bone. Multiple cells like osteoblasts, osteoclasts, and vascular endothelial cells of the terminal vessel structure called a vascular bud take part in the formation of structures called basic (bone) multicellular units (BMUs) with a capillary at its center (Fig. 2). Their proximity allows for communication between endothelial cells, osteoblasts, and osteoclasts and facilitates an intricate coupling mechanism between the processes of bone resorption and formation.73 Continuous mechanical regulation of the mineralized extracellular matrix density and organization is another integral component of bone homeostasis. The dependence and adaptation of bone to dynamic load bearing is well established. Osteocytes within the lacunae-canalicular structures serve as bone’s mechanosensors that ultimately signal to osteoblasts and osteoclasts to guide the deposition and resorption of bone.29,86
BMU and coupling of endotheilal cells, osteoclasts and osteoblasts. Endothelial cells (1–6) at the advancing end of the vascular bud and the stalk cells maintain a differential gene expression profile allowing for osteoclastic (Oc) resorption, proliferation and activation of osteoblasts (Ob), followed by quiescence when endothelial cells are in proximity to the osteoblasts. Panels a–c describe a snapshot in the progression of this differential control. Rv.Z—reversal zone. From Parfitt et al. 73
Vascular Response to Injury and Fracture Healing
Vascular adaptation to mechanical factors and injury are not well understood, but it is clear that the ensuing hypoxia is a major regulator of both the revascularization and tissue healing response. After moderate to severe blunt trauma, there is a rapid reduction in functional capillary density, local vasoconstriction, and an increase in capillary permeability.3 An acute ischemic environment is established during the intervening period from injury to re-establishment of adequate tissue perfusion. The close connections between the bone and muscle vascular systems ensure that many collaterals and anastomoses contribute to the blood supply to the healing bone post injury. Though bone is considered to have a high tolerance to transient hypoxia,4,56 a continuing or pervasive lack of vascular support is severely detrimental to bone healing as evidenced by the high incidence of amputations in lower limb fractures with vascular injuries.35
Fractures, surgical osteotomies, or distraction osteogenesis are normally accompanied by a large transient increase in local blood flow, which stays elevated for several weeks post injury, especially in the healing callus.5,39 This increase in blood flow results from both angiogenic and arteriogenic vascular growth mechanisms within the overlapping parts of the vascular plexuses perfusing the bone.10,76 Hence, it is not surprising that bone trauma with concomitant vascular injury has a higher risk of non-union as evident from a mouse model of non-stabilized tibial fracture and hind limb ischemia (Fig. 3).63 Further, anti-angiogenic therapy inhibiting the early vascular response results in fibrous unions without significant endochondral ossification or periosteal reaction.41,81 A lack of vascularization within the first week can result in non-union after osteotomy and even subtle reductions in blood within the healing callus negatively influence the mechanical properties of the healing bone, suggesting that the timing of revascularization and vascular remodeling are critical in bone regeneration.14,39
Microfil vascular perfusion casts in a non-stabilized tibial fracture model with hind-limb ischemia in mice. After 10 days of injury, microfil perfused vessels were not seen at the fracture site. An increase in number of vessels was noted at 14 and 21 days post injury, but fracture healing was impaired compared to controls. Scale bar: 2 mm (bright yellow—microfil containing vessels). From Lu et al. 63
Influence of Surrounding Soft Tissue on Bone Regeneration
Functional interactions between bone and muscle are not fully understood but muscle clearly plays a critical role in bone homeostasis and regeneration. In addition to being a source of vascularizing elements, myoblasts from neighboring muscle tissue can differentiate down an osteogenic lineage and contribute to bone repair, especially in open fractures with bone and muscle injury.61,62 Not surprisingly, transposition of muscle tissue over injured bone improves healing in tibial fractures,88 while volumetric loss of adjacent muscle tissue severely impairs bone regeneration and compromises the end stage biomechanical properties of the regenerated bone.94 Recent evidence from a distraction osteogenesis model showed an increase in expression of the arterial marker Ephrin B2 (EphB2) in the defect and the surrounding muscle which parallels BMP-2 expression.64 This apparent increase in EphB2 may be a function of the hemodynamic status as a result of the increased vascular volume or simply indicate a shift to a more mature network after the increase in vascular volume after the distraction process. Importantly, vascular cells at the osteotomy site and the surrounding muscle expressed BMP-2.64 Thus, a larger and more complex synergistic interaction between bone and muscle tissue can be envisaged that includes stimulating angiogenesis, supporting the formation of collaterals that can anastomose with intraosseous circulation, and contributing progenitors and growth factors to support bone regeneration.
Mechanical Environment Guiding Bone Regeneration
The importance of the mechanical environment in bone repair and regeneration has long been recognized. Ambulatory rodents, with medullary pin stabilized femoral diaphyseal fractures, show earlier periosteal response and improved biomechanical properties compared to immobilized controls.79 As early as 2 weeks after tibial osteotomies, regional blood flow increases to 19–50 mL/min per 100 g of bone when stabilized by semi-rigid fixators. This is four-fold higher than that for rigid fixators, and is accompanied by a higher periosteal callous reaction at 6 weeks.91 Such observations have shifted the paradigm in treatment of fractures from prolonged rigid immobilization to techniques involving limited loading and early ambulation (‘functional weight-bearing’). The time dependent effects of mechanical loading on bone repair have also been observed in critically sized defects and may be related to the time course of vascular ingrowth. Defects treated with a healing dose of BMP-2 progress to non-union when subjected to early axial functional loading. In contrast, a delay in loading of just 4 weeks stimulates improved healing both in terms of bone formation and biomechanical function.12,13 Interestingly, these adaptive responses are accompanied by spatial differences in bone formation that may be correlated to patterns of vascular growth between the proximal and distal ends of the defect seen even in defects stabilized by a stiff plate (Fig. 4).10,13
MicroCT image of microfil perfused vasculature in rat things showing both bone and vessels (a) Control femur with surrounding vessels, (b) 8 mm femoral segmental defect (#—marks defect limits in femur) and vasculature at 3 weeks post surgery. (c) A closer view of the spatial inhomogeneity of vessels in the defect region between the proximal and distal regions of the healing segmental defects stabilized by a stiff plate showing higher vascular volume and connectivity at the proximal end. From Boerckel et al. 13
Vascularization Strategies in Bone Regeneration
Early restoration of functional vasculature can have many advantages in bone regeneration, including rapid access to metabolites, inflammatory cells, and stem cells.28 Vascularized autologous bone grafts are advantageous for bone repair as they heal rapidly at the graft-host interface allowing for early integration and structural strength at the defect site97; however, they have very limited availability necessitating alternative interventional techniques. Larger defects benefit from combined use of devitalized bone with vascular pedicle bone grafts as this combined treatment option still has the benefit of enhanced neovessel formation and bone defect healing.68 For even more complicated multi-tissue injuries with open fractures, muscle or fasciocutaneous flaps can be used to cover the fracture site16; however, the accompanying morbidity and lack of adequately accessible flaps have similarly prompted the need for alternate strategies. Future directions to enhance vascularization in bone and muscle regeneration are largely aimed at enhancing early vascular support by the delivery of pro-angiogenic growth factors, stem cells, or pre-vascularization of tissue engineered constructs. Since the vascularization and regeneration progresses in a mechanically active environment, adequate consideration must be given to the role of biomechanical stimuli in these processes.
Growth Factors and Other Biologics
Growth factor delivery to treat bone injuries has been used to promote combined angiogenesis and osteogenesis. Several growth factors, such as VEGF, FGF, IGF, BMP-2, and PDGF, have been used as therapeutic agents to induce angiogenesis and improve fracture healing.42,47 The angiogenic growth factor VEGF has been the focus of many studies aimed at enhancing angiogenesis. Delivery of VEGF via scaffolds or gene transfer has been shown to increase local vascularity and improve fracture repair, whereas inhibition of VEGF was shown to impair bone regeneration.40,85 The timing of delivery and availability of angiogenic factors is a critical consideration for successful tissue regeneration. Whereas prolonged release of VEGF at relatively low levels from an alginate scaffold can promote functional neovascularization,57 constant high levels of VEGF can induce leaky vessels with limited progression to mature networks.58,98 While it is clear that VEGF can enhance angiogenesis which is necessary for bone regeneration, it can also have a direct effect on osteogenesis by recruiting and promoting both osteoblast and osteoclast activity.23,84
There is also an interdependent relationship between VEGF and the osteogenic growth factor, BMP-2. BMP-2 is commonly delivered in bone repair and acts as a potent mitogen and morphogen that can recruit stem cells and induce osteogenic differentiation; in addition, it is a potent inducer of VEGF production in osteoblasts.24 Co-delivery of both growth factors, BMP-2 and VEGF, has been shown to accelerate the early healing phase and enhance bone formation.74 The BMP–VEGF axis in bone healing seems connected by a pattern of temporal feedback between the bone healing and vascularization growth factor signal cascades. Further understanding of this relationship and optimization of the control over this relationship will continue to be a direction for future work.
Synthetic peptides, offer an alternative, yet promising direction to exploit the relationship between angiogenesis and osteogenesis. Of particular interest is TP508, a 23 amino acid synthetic peptide representing a portion of the receptor-binding domain of the human thrombin molecule, which may competitively bind to high-affinity non-proteolytically activated receptor components to exert its signaling effects.36 This peptide is currently in clinical trials but animal studies have already shown TP508 to increase the number and size of local vessels, promote an earlier resolution of inflammatory phase, and improve fracture healing.60,93 The modulation of inflammation and pro-angiogenic roles of TP508 are thought to improve the early response to injury, including cell recruitment, to promote the natural healing and regenerative response.78,93
Regardless of the specific bioactive molecule the idea to modulate host or implanted cell function via controlled spatiotemporal delivery strategies has gained considerable recent momentum.27,44 This structured release of factors is involved in maintaining the chemotactic gradient driving cell migration, proliferation, and phenotypic maturation.6,74 Large scale use of single or multiple growth factor regimes remains hamstrung by several shortcomings including: the lack of clear dose–effect ratio demonstrations, the choice of appropriate factors or combinations thereof, the timing of delivery, regulatory approval hurdles, and finally identification of optimal delivery vehicles. The use of implanted cells, even to act as a source of these growth factors, can be an alternate strategy to growth factors alone due to the ability of the cells to participate effectively in the regulatory feedback control mechanisms.
Cell based Therapeutics
Stem cell-based therapies are another promising avenue for the treatment of bone defects. In clinical scenarios of bone injury with the need for volumetric tissue replacement, stem cell therapies offer the potential to regenerate both bone and vascular tissue. The two natural choices for cells in rebuilding large bone defects are the cells found in physiological proximity during normal repair—bone marrow derived stem cells (BMSCs) and periosteum derived cells. Culture expanded BMSC seeded scaffolds have been used in numerous animal models and been shown to promote bone formation.1,65,67 These BMSCs have also been combined with an osteoinductive scaffold to successfully treat a challenging clinical case of established tibial non-union.7 Similarly, periosteum derived cells can be used in scaffolds to achieve bridging of large bone defects,45,75 including a clinical case of delayed repair.89 In addition to directly participating in the formation of bone and vascular structures, the delivered cells can recruit vascular and other support cells to the region by paracrine influence.84
Different cell sources have been used with varying success in bone regeneration15 but one roadblock to the wider use of cell-based scaffolds is the question of scale. Large numbers of cells are required for replacement constructs that are of a clinically relevant size. BMSCs and periosteal cells typically require culture expansion for in vivo use and questions about the proliferation and differential potential of BMSCs persist.26,89 Because of these potential limitations there has been considerable interest in the more numerous and easy to harvest, adipose derived stem (stromal) cells (ADSCs). ADSCs can be differentiated down the adipogenic, endothelial, myogenic, chondrogenic, and osteoblastic lineages under appropriate conditions.32,34 While ADSCs are inferior to BMSCs in their osteogenic potential,69 they have remarkable potential for revascularization.50 Future directions may focus on exploiting the revascularization potential of ADSCs and combine it with osteogenic stimuli.
Large bone injuries often have poorly defined spatial boundaries, which create a particular challenge for retention of cells after delivery. Insights from cardiovascular cell delivery studies reveal that scaffold-free direct cell delivery is associated with cell death, poor engraftment, and localization in other unintended sites.95 The use of scaffolds for cell delivery enhances engraftment, but is still limited by cell death, local inflammation, and hypoxia. Under conditions of inflammation and hypoxia only a small percentage of the delivered cells remain at the implanted site after extended durations; however, it is unclear whether this is due to cell death or host niche adaptations. While survival of these stem cells (BMSCs) may be limited, this cell-seeded scaffold therapy is sufficient to induce new bone formation.33
Providing endothelial cells (EC), vascular support cells and other regenerative cells together within cell seeded scaffolds can serve to establish a stable vascular network to support bone and muscle regeneration. For example, EC and MSC co-cultures implanted in vivo have been shown to increase vascularized bone formation.31 It must also be noted that the choice of cells used to create such co-seeded scaffolds and the timing of their addition has a large bearing on the fate of the engineered microvasculature. In co-culture studies, different cell types are typically seeded together; however, the timing at which specific cell types are added to nascent vessel structures may have an equally important role. In collagen–glycosaminoglycan scaffolds, the delayed addition of MSCs to a culture of ECs, after the preliminary establishment of a nascent endothelial cell network, improved formation of vessel like structures.66 The production of PDGF by EC networks was suppressed by the delayed addition of MSCs; this suggested a shift towards early stabilization in the nascent networks and recapitulates some of the normal maturation signals. When implanted in vivo, the delayed co-cultures showed higher vascularity and retained their EC–perivascular cell relationship. In another study, scaffolds containing adipose derived microvessels co-seeded with astrocyte precursors, on implantation in vivo, resulted in microvessel networks with niche characteristics like reduced vascular permeability.72 Future approaches building on the ability to form and control the characteristics of vessel networks forming within the cell-seeded scaffolds may improve construct vascularization.
Prevascularized Constructs
The immediate challenge of hypoxia and limited vascular supply following injury may necessitate strategies that contain preformed vasculature and can rapidly re-establish perfusion. Perfluorocarbon compounds, due to the increased solubility of oxygen in them, find novel use in tissue engineering attempts at reduction of hypoxia.9,48,83 Combining these compounds with cell delivery vehicles can improve short term local oxygen availability, cell survival, and bone formation in bone tissue engineering applications. However, the direct effect of perfluorocarbon compounds versus any oxygen or carbon dioxide solubility mediated effects are still being investigated.83 Further, functional revascularization, especially in thick 3D tissue engineered constructs may not necessarily be established in the intervening period to sustain both the oxygen and nutrient demands. Even growth factor or cell seeded scaffold driven neovessel formation may take days to re-establish effective perfusion post-implantation in vivo. This is expected due to the time taken in either recruitment of new vessels to invade the implants or in the de novo assembly of seeded cells into perfusion capable vessels. An interesting alternative is to prevascularize the implants in vivo and subsequently transfer them to the site of interest, like the myocardium, to improve graft integration and function.30 This technique has also been adapted to create vascularized bone graft substitutes.8,19,49 Alternatively, in vitro prevascularization approaches allow for vascular networks to be formed prior to implantation and can thus limit the need for repeated surgical interventions and can accelerate construct perfusion.
Strategies to create prevascularized tissue in vitro are primarily two-fold: in vitro culture of cell seeded scaffolds (ECs, MSCs, fibroblasts, myoblasts, etc.) to allow establishment of initial vascular network before implantation, or use of pre-formed microvessels rather than single cells to accelerate the angiogenic process. In vitro culture of 1–2 weeks can allow co-cultures to form early tubular networks,77 leading to early functional anastomosis and perfusion compared to cell–seeded constructs without a pre-culture period.18 When implanted in vivo, such perfusion capable networks may thus serve to maintain the viability of cells co-implanted or co-cultured within this scaffold.59 Establishing perivascular cell coverage can stabilize nascent vessel structures. Stable vessel structures can be created in vitro by delayed addition of MSCs to an EC network and can provide better vascularization when implanted in vivo compared to ECs alone.66 Using existing microvascular elements to generate prevascularized constructs may thus have an advantage in re-establishing perivascular cell coverage and provide arterioles, venules, and capillary structures to participate in the formation of this new network.43,71,80 Irrespective of the differing approaches, the two strategies converge to a final perfusion capable vessel network that pervades the thick tissue replacement constructs, prior to implantation.
An important determinant in our choice of vascularization strategies must be the rapidity of reperfusion and connection with the host circulation. The extent of host vessel penetration or of implanted vessel structures moving across the construct boundaries is not well understood. From the limited evidence available, it appears more likely that vascular penetration into or out of a construct is limited to short distances from the construct boundaries.77,80,92 When collagen constructs made with freshly harvested microvessels were immediately implanted there was progressive graft perfusion as well as vessel remodeling.71 However, when a nascent network was first formed by a period of pre-culture and subsequently implanted, a clearly identifiable and well-perfused vascular tree was observed within 2–3 days following implantation.55,80 Interestingly, a loss in smooth muscle cells from preformed microvessels with short culture periods may produce an accelerated angiogenic phase post implantation.55 Recently, it was demonstrated that prevascularization of engineered bone tissue grafts can increase new bone formation compared to non-prevascularized grafts.92 Further work with prevascularized constructs represents a promising avenue in bone tissue engineering.
Biomechanical Influences on Vascularization and Bone Regeneration Strategies
The relationship between angiogenesis, neovessels, and the biomechanical environment is often overlooked in vascularization strategies. The regenerating musculoskeletal environment is dynamic and may include angiogenesis, vascular remodeling, and changes in ECM mechanical properties by angiogenesis, mineralization or external loading. For example, collagen scaffolds seeded with microvessels have an initial decrease in stiffness followed by a subsequent increase during in vitro angiogenesis.52 When these principles are extrapolated to an in vivo scenario, it suggests that vascular ingrowth into healing tissues or scaffolds in vivo may potentially alter local tissue mechanics thereby affecting function and recovery. Further, mechanical loading can also dictate the orientation of the newly formed vessels and the distribution of arterioles, venules, and capillaries within the vascular bed after the onset of blood flow (Fig. 5).17,53 Thick prevascularized grafts intended for use in bone or muscle reconstruction are likely to experience similar mechanical perturbations in the form of tensile or compressive loading and interstial fluid flow. Though not fully understood, the interactions between angiogenesis, microvascular networks, and the mechanical environment may have a significant impact on the regenerative potential of vascularization efforts for bone and muscle regeneration.
Fate of microvascular constructs with aligned microvessels (anchorage induced) after 30 days of subcutaneous implantation. The schematic shows the microvascular constructs anchored by the long axis in a metal frame during in vitro culture and the two configurations for implantation subcutaneously. (a) Aligned vascular constructs implanted subcutaneously without the anchoring frame lost the imparted pattern, were perfused and showed good smooth muscle coverage (left column). (b) Constructs implanted subcutaneously with the anchoring frame maintained their alignment but showed differences in microvessel type (arterioles, venules, and capillaries) distribution (right column). From Chang et al. 17 Copyright: The American Heart Association, Inc
Axial mechanical loading in healing bone defects may have a synergistic role with hemodynamic and interstitial changes associated with the angiogenesis supporting bone regeneration. Improvement in bone healing by axial loading is associated with an early increase in both cortical and medullary vasculature, implicating the vasculature as a critical effector of this improved regeneration.37,91 Further, during rigid or distraction osteogenesis processes there is first an increase in the size of the surrounding vessels followed by formation of smaller vessels.64 Changes in luminal shear, hoop stresses, and interstitial shear would result as direct consequences of changes in the local blood flow in maturing and remodeling networks. In turn, endothelial cells exposed to shear or stretch would have a subsequent increase in VEGF expression, which is normally limited to the arterial side in vivo.25,38 As described earlier, VEGF can promote osteoblast survival and increase mineralization.81,82,96 The correlation of heightened expression of EphB2, an arterial marker, and of BMP2, observed during regeneration in distraction osteogenesis supports the co-regulation of bone and vascular tissue development.64
There is an apparent role of mechanical stimuli in the expression of osteogenic signals by vascular endothelial cells. Though the role of hemodynamic forces in the expression of BMPs by vascular cells is not clearly understood, it is known that high pressure can induce BMP2 expression in arteries, suggesting a role for mechanical factors like hemodynamic changes.21,22 Surprisingly, in a rodent model that combined a critically sized segmental bone defect with concomitant hind limb ischemia, there was still BMP-2 mediated mineralization and functional healing.87 Though the total vascularity was unchanged between the groups with and without ischemia at 12 weeks, there were more interconnected networks with smaller diameter vessels in the thigh with concomitant ischemia. More importantly, when the defect was treated with a previously established non-healing dose of BMP-2, the concomitant hindlimb ischemia actually produced consistent bridging of critical sized femoral defects (Fig. 6).11,87 Interpreted in the light of evidence from distraction osteogenesis showing BMP2 expression by vascular cells,64 a more complex interaction begins to emerge in the osteogenic response relating angiogenesis, collateral vessel formation, and mechanical forces including hemodynamic changes.
Effect of hind limb ischemia (HLI) on bone regeneration in a rat femoral segmental defect model. (a) Digital radiographs and uCT reconstructions with mineral density mapping (color cross-sections) (b) of segmental defects (#—marks defect limits in femur) treated with 0.5 μg (non-healing dose) of BMP-2 with or without hind limb ischemia (HLI). Mineralized bridging was seen at 12 weeks in the HLI group even with the low non-healing dose of BMP-2. (c) At 12 weeks, terminal vascular perfusion with microfil revealed highly interconnected vasculature in the thigh region (at the level of the defects) with hind limb ischemia though total vascular volumes were comparable. From Uhrig et al. 87
Early vascularization is a critical step in effective bone regeneration and is responsive to the mechanical environment. It has been shown that early axial loading (compressive) of critically sized femoral defects that were treated with a mineralizing dose of BMP-2, resulted in a reduction of the vascular volume and vessel inter-connectivity within the defect space even as late as 3 weeks post-surgery.12,13 Not surprisingly, the associated bone volume followed similar trends and was lower in the axially loaded animals. This study, for the first time, presented a clear association in which early axial loading reduced defect region vascularization and impaired osteogenesis. When the application of loading was delayed in the same model—allowing for the early angiogenesis and mineralized bridging phases to progress—it was found that the loading improved bone formation compared to unloaded controls. Interestingly, this delayed loading produced a significant reduction in vascular connectivity with increased vessel thickness and reduction in smaller vessels.13 These studies demonstrate the significant healing stage dependent interactions that exist among the in vivo biomechanical environment, vascular ingrowth, and functional tissue regeneration.
Conclusions and Future Directions
Bone is a remarkably resilient material that continuously remodels and repairs throughout life. When a bone is fractured, a cascade of biological events is initiated that typically progresses through inflammation, vascularization, and callous formation to successfully heal the bone and restore biomechanical function; however, when a defect is too large in a thick vascularized tissue, such as bone, the endogenous responses alone fail to repair and regenerate functional tissue in this defect gap. Under these circumstances, there is a need for interventional strategies that promote rapid vascular ingrowth and subsequent tissue regeneration. To this extent, progress has been made on several avenues of investigation to accelerate tissue revascularization. Growth factors and biologics are one of the more established techniques that have been used to induce both neovascularization and osteogenesis. Future directions will address the current need for better spatiotemporal release and dose control in the delivery of single or multiple growth factors. Cell based strategies have also been used to repair large volumetric tissue loss as these cells have the potential to provide both a source of growth factors and regenerative cells. New lines of investigation have utilized these stem cells, specifically bone marrow and adipose derived cells, for their potential to form prevascularized musculoskeltal tissues. In addition to serving as a source of growth factors, these cells can promote vessel assembly within implanted constructs. While delivery of growth factors and cells may serve to eventually promote tissue formation, these techniques do not address the fact that a delay in re-establishing blood perfusion leaves the centers of implanted construct highly susceptible to severe hypoxia. Prevascularized scaffolds offer an elegant solution by combining cell delivery with vascular networks that exists through the scaffold bulk. Upon implantation, the pre-developed vessels in the scaffold can rapidly inosculate with the host circulation to establish blood flow and maintain implant viability and function. Regardless of the revascularization strategy used, adequate consideration of the local biomechanical environment is paramount to the design of effective tissue regeneration approach as mechanical forces have a clear influence on both vascularization and osteogenesis. Together these new approaches represent the promising frontiers in vascularization strategies, which will translate into effective therapies for challenging cases of bone regeneration.
References
Abukawa, H., M. Shin, W. B. Williams, J. P. Vacanti, L. B. Kaban, and M. J. Troulis. Reconstruction of mandibular defects with autologous tissue-engineered bone. J. Oral Maxillofac. Surg. 62:601–606, 2004.
Amini, A. R., C. T. Laurencin, and S. P. Nukavarapu. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 40:363–408, 2012.
Amon, M., M. W. Laschke, Y. Harder, B. Vollmar, and M. D. Menger. Impact of severity of local soft-tissue trauma on long-term manifestation of microcirculatory and microlymphatic dysfunctions. J. Trauma 61:924–932, 2006.
Aro, H., E. Eerola, A. J. Aho, and J. Niinikoski. Tissue oxygen tension in externally stabilized tibial fractures in rabbits during normal healing and infection. J. Surg. Res. 37:202–207, 1984.
Aronson, J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin. Orthop. Relat Res., 124–131, 1994. doi:10.1097/00003086-199404000-00020.
Bae, H., A. S. Puranik, R. Gauvin, F. Edalat, B. Carrillo-Conde, N. A. Peppas, and A. Khademhosseini. Building vascular networks. Sci. Transl. Med., 4:160ps23, 2012.
Bajada, S., P. E. Harrison, B. A. Ashton, V. N. Cassar-Pullicino, N. Ashammakhi, and J. B. Richardson. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J. Bone Joint Surg. Br. 89-B:1382–1386, 2007.
Beier, J. P., R. E. Horch, A. Hess, A. Arkudas, J. Heinrich, J. Loew, H. Gulle, E. Polykandriotis, O. Bleiziffer, and U. Kneser. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J. Tissue Eng. Regen. Med. 4:216–223, 2010.
Benjamin, S., D. Sheyn, S. Ben-David, A. Oh, I. Kallai, N. Li, D. Gazit, and Z. Gazit. Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Eng. Part A 19:748–758, 2013.
Berggren, A., A. J. Weiland, L. T. Ostrup, and H. Dorfman. Microvascular free bone transfer with revascularization of the medullary and periosteal circulation or the periosteal circulation alone. A comparative experimental study. J. Bone Joint Surg. Am. 64:73–87, 1982.
Boerckel, J. D., Y. M. Kolambkar, K. M. Dupont, B. A. Uhrig, E. A. Phelps, H. Y. Stevens, A. J. Garcia, and R. E. Guldberg. Effects of protein dose and delivery system on bmp-mediated bone regeneration. Biomaterials 32:5241–5251, 2011.
Boerckel, J. D., Y. M. Kolambkar, H. Y. Stevens, A. S. Lin, K. M. Dupont, and R. E. Guldberg. Effects of in vivo mechanical loading on large bone defect regeneration. J. Orthop. Res. 30:1067–1075, 2012.
Boerckel, J. D., B. A. Uhrig, N. J. Willett, N. Huebsch, and R. E. Guldberg. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc. Natl. Acad. Sci. USA 108:E674–E680, 2011.
Brownlow, H. C., A. Reed, and A. H. Simpson. The vascularity of atrophic non-unions. Injury 33:145–150, 2002.
Bueno, E. M., and J. Glowacki. Cell-free and cell-based approaches for bone regeneration. Nat. Rev. Rheumatol. 5:685–697, 2009.
Chan, J. K., L. Harry, G. Williams, and J. Nanchahal. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast. Reconstr. Surg. 130:284e–295e, 2012.
Chang, C. C., L. Krishnan, S. S. Nunes, K. H. Church, L. T. Edgar, E. D. Boland, J. A. Weiss, S. K. Williams, and J. B. Hoying. Determinants of microvascular network topologies in implanted neovasculatures. Arterioscler. Thromb. Vasc. Biol. 32:5–14, 2012.
Chen, X., A. S. Aledia, C. M. Ghajar, C. K. Griffith, A. J. Putnam, C. C. Hughes, and S. C. George. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. Part A 15:1363–1371, 2009.
Chung, Y. G., A. T. Bishop, G. A. Giessler, O. Suzuki, J. L. Platt, M. Pelzer, P. F. Friedrich, and T. Kremer. Surgical angiogenesis: a new approach to maintain osseous viability in xenotransplantation. Xenotransplantation 17:38–47, 2010.
Crisan, M., S. Yap, L. Casteilla, C. W. Chen, M. Corselli, T. S. Park, G. Andriolo, B. Sun, B. Zheng, L. Zhang, C. Norotte, P. N. Teng, J. Traas, R. Schugar, B. M. Deasy, S. Badylak, H. J. Buhring, J. P. Giacobino, L. Lazzari, J. Huard, and B. Peault. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313, 2008.
Csiszar, A., S. Lehoux, and Z. Ungvari. Hemodynamic forces, vascular oxidative stress, and regulation of Bmp-2/4 expression. Antioxid. Redox Signal. 11:1683–1697, 2009.
Csiszar, A., K. E. Smith, A. Koller, G. Kaley, J. G. Edwards, and Z. Ungvari. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappab activation by tumor necrosis factor-alpha, H2o2, and High Intravascular Pressure. Circulation 111:2364–2372, 2005.
Dai, J., and A. B. Rabie. Vegf: an essential mediator of both angiogenesis and endochondral ossification. J. Dent. Res. 86:937–950, 2007.
Deckers, M. M., R. L. Van Bezooijen, G. Van Der Horst, J. Hoogendam, C. Van Der Bent, S. E. Papapoulos, and C. W. Lowik. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143:1545–1553, 2002.
Dela Paz, N. G., T. E. Walshe, L. L. Leach, M. Saint-Geniez, and P. A. D’amore. Role of shear-stress-induced VEGF expression in endothelial cell survival. J. Cell Sci. 125:831–843, 2012.
Derubeis, A. R., and R. Cancedda. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann. Biomed. Eng. 32:160–165, 2004.
Discher, D. E., D. J. Mooney, and P. W. Zandstra. Growth factors, matrices, and forces combine and control stem cells. Science (New York, NY) 324:1673–1677, 2009.
Doherty, M. J., B. A. Ashton, S. Walsh, J. N. Beresford, M. E. Grant, and A. E. Canfield. Vascular pericytes express osteogenic potential in vitro and in vivo. J. Bone Miner. Res. 13:828–838, 1998.
Duncan, R. L., and C. H. Turner. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 57:344–358, 1995.
Dvir, T., A. Kedem, E. Ruvinov, O. Levy, I. Freeman, N. Landa, R. Holbova, M. S. Feinberg, S. Dror, Y. Etzion, J. Leor, and S. Cohen. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc. Natl. Acad. Sci. USA 106:14990–14995, 2009.
Fedorovich, N. E., R. T. Haverslag, W. J. Dhert, and J. Alblas. The role of endothelial progenitor cells in prevascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng. Part A 16:2355–2367, 2010.
Fraser, J. K., I. Wulur, Z. Alfonso, and M. H. Hedrick. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 24:150–154, 2006.
Giannoni, P., S. Scaglione, A. Daga, C. Ilengo, M. Cilli, and R. Quarto. Short-time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Eng. Part A 16:489–499, 2010.
Gimble, J. M., A. J. Katz, and B. A. Bunnell. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100:1249–1260, 2007.
Glass, G. E., M. F. Pearse, and J. Nanchahal. Improving lower limb salvage following fractures with vascular injury: a systematic review and new management algorithm. J. Plast Reconstr. Aesthet. Surg. 62:571–579, 2009.
Glenn, K. C., G. H. Frost, J. S. Bergmann, and D. H. Carney. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept. Res. 1:65–73, 1988.
Goodship, A. E., and J. Kenwright. The influence of induced micromovement upon the healing of experimental tibial fractures. J. Bone Joint Surg. Br. 67:650–655, 1985.
Gruden, G., S. Thomas, D. Burt, S. Lane, G. Chusney, S. Sacks, and G. Viberti. Mechanical stretch induces vascular permeability factor in human mesangial cells: mechanisms of signal transduction. Proc. Natl. Acad. Sci. USA 94:12112–12116, 1997.
Grundnes, O., and O. Reikeras. Blood flow and mechanical properties of healing bone. Femoral osteotomies studied in rats. Acta Orthop. Scand. 63:487–491, 1992.
Hankenson, K. D., M. Dishowitz, C. Gray, and M. Schenker. Angiogenesis in Bone Regeneration. Injury 42:556–561, 2011.
Hausman, M. R., M. B. Schaffler, and R. J. Majeska. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 29:560–564, 2001.
Hollinger, J. O., A. O. Onikepe, J. Mackrell, T. Einhorn, G. Bradica, S. Lynch, and C. E. Hart. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-bb and an injectable beta-tricalcium phosphate/collagen matrix. J. Orthop. Res. 26:83–90, 2008.
Hoying, J. B., C. A. Boswell, and S. K. Williams. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev. Biol. Anim. 32:409–419, 1996.
Huebsch, N., and D. J. Mooney. Inspiration and application in the evolution of biomaterials. Nature 462:426–432, 2009.
Hutmacher, D. W., and M. Sittinger. Periosteal cells in bone tissue engineering. Tissue Eng. 9(Suppl 1):S45–S64, 2003.
Kaully, T., K. Kaufman-Francis, A. Lesman, and S. Levenberg. Vascularization—the conduit to viable engineered tissues. Tissue Eng. Part B 15:159–169, 2009.
Kawaguchi, H., K. Nakamura, Y. Tabata, Y. Ikada, I. Aoyama, J. Anzai, T. Nakamura, Y. Hiyama, and M. Tamura. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 86:875–880, 2001.
Kimelman-Bleich, N., G. Pelled, D. Sheyn, I. Kallai, Y. Zilberman, O. Mizrahi, Y. Tal, W. Tawackoli, Z. Gazit, and D. Gazit. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials 30:4639–4648, 2009.
Kneser, U., E. Polykandriotis, J. Ohnolz, K. Heidner, L. Grabinger, S. Euler, K. U. Amann, A. Hess, K. Brune, P. Greil, M. Sturzl, and R. E. Horch. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 12:1721–1731, 2006.
Koh, Y. J., B. I. Koh, H. Kim, H. J. Joo, H. K. Jin, J. Jeon, C. Choi, D. H. Lee, J. H. Chung, C. H. Cho, W. S. Park, J. K. Ryu, J. K. Suh, and G. Y. Koh. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler. Thromb. Vasc. Biol. 31:1141–1150, 2011.
Krishnan, L., C. C. Chang, S. S. Nunes, S. K. Williams, J. A. Weiss, and J. B. Hoying. Manipulating the microvasculature and its microenvironment. Crit. Rev. Biomed. Eng. 41:91–123, 2013.
Krishnan, L., J. B. Hoying, H. Nguyen, H. Song, and J. A. Weiss. Interaction of angiogenic microvessels with the extracellular matrix. Am. J. Physiol. Heart Circ. Physiol. 293:H3650–H3658, 2007.
Krishnan, L., C. J. Underwood, S. Maas, B. J. Ellis, T. C. Kode, J. B. Hoying, and J. A. Weiss. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc. Res. 78:324–332, 2008.
Laroche, M. Intraosseous circulation from physiology to disease. Joint Bone Spine 69:262–269, 2002.
Laschke, M. W., H. Mussawy, S. Schuler, A. Kazakov, M. Rucker, D. Eglin, M. Alini, and M. D. Menger. Short-term cultivation of in situ prevascularized tissue constructs accelerates inosculation of their preformed microvascular networks after implantation into the host tissue. Tissue Eng. Part A 17:841–853, 2011.
Lee, C. M., D. C. Genetos, Z. You, and C. E. Yellowley. Hypoxia regulates PGE(2) release and EP1 receptor expression in osteoblastic cells. J. Cell. Physiol. 212:182–188, 2007.
Lee, K. Y., M. C. Peters, and D. J. Mooney. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J. Control Release 87:49–56, 2003.
Lee, R. J., M. L. Springer, W. E. Blanco-Bose, R. Shaw, P. C. Ursell, and H. M. Blau. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation 102:898–901, 2000.
Levenberg, S., J. Rouwkema, M. Macdonald, E. S. Garfein, D. S. Kohane, D. C. Darland, R. Marini, C. A. Van Blitterswijk, R. C. Mulligan, P. A. D’amore, and R. Langer. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23:879–884, 2005.
Li, G., J. T. Ryaby, D. H. Carney, and H. Wang. Bone formation is enhanced by thrombin-related peptide TP508 during distraction osteogenesis. J. Orthop. Res. 23:196–202, 2005.
Liu, R., O. Birke, A. Morse, L. Peacock, K. Mikulec, D. G. Little, and A. Schindeler. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskelet. Disord. 12:288, 2011.
Liu, R., S. L. Ginn, M. Lek, K. N. North, I. E. Alexander, D. G. Little, and A. Schindeler. Myoblast sensitivity and fibroblast insensitivity to osteogenic conversion by BMP-2 Correlates with the Expression of Bmpr-1a. BMC Musculoskelet. Disord. 10:51, 2009.
Lu, C., T. Miclau, D. Hu, and R. S. Marcucio. Ischemia leads to delayed union during fracture healing: a mouse model. J. Orthop. Res. 25:51–61, 2007.
Matsubara, H., D. E. Hogan, E. F. Morgan, D. P. Mortlock, T. A. Einhorn, and L. C. Gerstenfeld. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 51:168–180, 2012.
Mauney, J. R., C. Jaquiery, V. Volloch, M. Heberer, I. Martin, and D. L. Kaplan. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials 26:3173–3185, 2005.
Mcfadden, T. M., G. P. Duffy, A. B. Allen, H. Y. Stevens, S. M. Schwarzmaier, N. Plesnila, J. M. Murphy, F. P. Barry, R. E. Guldberg, and F. J. O’brien. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater. 9(12):9303–9316, 2013.
Mistry, A. S., and A. G. Mikos. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 94:1–22, 2005.
Muramatsu, K., K. Ihara, T. Miyoshi, K. Yoshida, R. Iwanaga, T. Hashimoto, and T. Taguchi. Stimulation of neo-angiogenesis by combined use of irradiated and vascularized living bone graft for oncological reconstruction. Surg. Oncol. 21:223–229, 2012.
Niemeyer, P., K. Fechner, S. Milz, W. Richter, N. P. Suedkamp, A. T. Mehlhorn, S. Pearce, and P. Kasten. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31:3572–3579, 2010.
Novosel, E. C., C. Kleinhans, and P. J. Kluger. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 63:300–311, 2011.
Nunes, S. S., K. A. Greer, C. M. Stiening, H. Y. Chen, K. R. Kidd, M. A. Schwartz, C. J. Sullivan, H. Rekapally, and J. B. Hoying. Implanted microvessels progress through distinct neovascularization phenotypes. Microvasc. Res. 79:10–20, 2010.
Nunes, S. S., L. Krishnan, C. S. Gerard, J. R. Dale, M. A. Maddie, R. L. Benton, and J. B. Hoying. Angiogenic potential of microvessel fragments is independent of the tissue of origin and can be influenced by the cellular composition of the implants. Microcirculation 17:557–567, 2010.
Parfitt, A. M. The mechanism of coupling: a role for the vasculature. Bone 26:319–323, 2000.
Patel, Z. S., S. Young, Y. Tabata, J. A. Jansen, M. E. Wong, and A. G. Mikos. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43:931–940, 2008.
Puelacher, W. C., J. P. Vacanti, N. F. Ferraro, B. Schloo, and C. A. Vacanti. Femoral shaft reconstruction using tissue-engineered growth of bone. Int. J. Oral Maxillofac. Surg. 25:223–228, 1996.
Rhinelander, F. W. The normal microcirculation of diaphyseal cortex and its response to fracture. J. Bone Joint Surg. Am. 50:784–800, 1968.
Rouwkema, J., J. De Boer, and C. A. Van Blitterswijk. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 12:2685–2693, 2006.
Ryaby, J. T., M. R. Sheller, B. P. Levine, D. G. Bramlet, A. L. Ladd, and D. H. Carney. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J. Bone Joint Surg. Am. 88(Suppl 3):132–139, 2006.
Sarmiento, A., J. F. Schaeffer, L. Beckerman, L. L. Latta, and J. E. Enis. Fracture healing in rat femora as affected by functional weight-bearing. J. Bone Joint Surg. Am. 59:369–375, 1977.
Shepherd, B. R., H. Y. Chen, C. M. Smith, G. Gruionu, S. K. Williams, and J. B. Hoying. Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler. Thromb. Vasc. Biol. 24:898–904, 2004.
Street, J., M. Bao, L. Deguzman, S. Bunting, F. V. Peale, Jr., N. Ferrara, H. Steinmetz, J. Hoeffel, J. L. Cleland, A. Daugherty, N. Van Bruggen, H. P. Redmond, R. A. Carano, and E. H. Filvaroff. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 99:9656–9661, 2002.
Street, J., and B. Lenehan. Vascular endothelial growth factor regulates osteoblast survival—evidence for an autocrine feedback mechanism. J. Orthop. Surg. Res. 4:19, 2009.
Tamimi, F., P. Comeau, D. Le Nihouannen, Y. L. Zhang, D. C. Bassett, S. Khalili, U. Gbureck, S. D. Tran, S. Komarova, and J. E. Barralet. Perfluorodecalin and bone regeneration. Eur. Cell Mater. 25:22–36, 2013.
Tang, Y. L., Q. Zhao, Y. C. Zhang, L. Cheng, M. Liu, J. Shi, Y. Z. Yang, C. Pan, J. Ge, and M. I. Phillips. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul. Pept. 117:3–10, 2004.
Tarkka, T., A. Sipola, T. Jamsa, Y. Soini, S. Yla-Herttuala, J. Tuukkanen, and T. Hautala. Adenoviral VEGF-a gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J. Gene Med. 5:560–566, 2003.
Turner, C. H. Three rules for bone adaptation to mechanical stimuli. Bone 23:399–407, 1998.
Uhrig, B. A., J. D. Boerckel, N. J. Willett, M. T. Li, N. Huebsch, and R. E. Guldberg. Recovery from Hind Limb Ischemia Enhances rhBMP-2-mediated segmental bone defect repair in a rat composite injury model. Bone 55:410–417, 2013.
Utvåg, S. E., K. B. Iversen, O. Grundnes, and O. Reikerås. Poor muscle coverage delays fracture healing in rats. Acta Orthopaedica 73:471–474, 2002.
Vacanti, C. A., L. J. Bonassar, M. P. Vacanti, and J. Shufflebarger. Replacement of an avulsed phalanx with tissue-engineered bone. N. Engl. J. Med. 344:1511–1514, 2001.
Volkmer, E., I. Drosse, S. Otto, A. Stangelmayer, M. Stengele, B. C. Kallukalam, W. Mutschler, and M. Schieker. Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone. Tissue Eng. Part A 14:1331–1340, 2008.
Wallace, A. L., E. R. Draper, R. K. Strachan, I. D. Mccarthy, and S. P. Hughes. The vascular response to fracture micromovement. Clin. Orthop. Relat. Res. 281–290, 1994. doi:10.1097/00003086-199404000-00044.
Wang, L., H. Fan, Z. Y. Zhang, A. J. Lou, G. X. Pei, S. Jiang, T. W. Mu, J. J. Qin, S. Y. Chen, and D. Jin. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized beta-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31:9452–9461, 2010.
Wang, H., X. Li, E. Tomin, S. B. Doty, J. M. Lane, D. H. Carney, and J. T. Ryaby. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J. Orthop. Res. 23:671–679, 2005.
Willett, N. J., M. T. Li, B. A. Uhrig, J. D. Boerckel, N. Huebsch, T. L. Lundgren, G. L. Warren, and R. E. Guldberg. Attenuated human bone morphogenetic protein-2-mediated bone regeneration in a rat model of composite bone and muscle injury. Tissue Eng. Part C 19:316–325, 2013.
Wu, K. H., X. M. Mo, Z.-C. Han, and B. Zhou. Stem cell engraftment and survival in the ischemic heart. Ann. Thorac. Surg. 92:1917–1925, 2011.
Yang, Y. Q., Y. Y. Tan, R. Wong, A. Wenden, L. K. Zhang, and A. B. Rabie. The role of vascular endothelial growth factor in ossification. Int. J. Oral. Sci. 4:64–68, 2012.
Zimmermann, G., and A. Moghaddam. Allograft bone matrix versus synthetic bone graft substitutes. Injury 42(Suppl 2):S16–S21, 2011.
Zisch, A. H., M. P. Lutolf, and J. A. Hubbell. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 12:295–310, 2003.
Acknowledgments
Supported by funding from National Institutes of Health (NIH R01 AR051336) and Armed Forces Institute of Regenerative Medicine (AFIRM W81XWH-08-2-0032).
Conflicts of Interest
Authors report no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Gang Bao oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Krishnan, L., Willett, N.J. & Guldberg, R.E. Vascularization Strategies for Bone Regeneration. Ann Biomed Eng 42, 432–444 (2014). https://doi.org/10.1007/s10439-014-0969-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-0969-9