Abstract
Survival of functional tissue constructs of clinically relevant size depends on the formation of an organized and uniformly distributed network of blood vessels and capillaries. The lack of such vasculature leads to spatio-temporal gradients in oxygen, nutrients and accumulation of waste products inside engineered tissue constructs resulting in negative biological events at the core of the scaffold. Unavailability of a well-defined vasculature also results in ineffective integration of scaffolds to the host vasculature upon implantation. Arguably, one of the greatest challenges in engineering clinically relevant bone substitutes, therefore, has been the development of vascularized bone scaffolds. Various approaches ranging from peptide and growth factor functionalized biomaterials to hyper-porous scaffolds have been proposed to address this problem with reasonable success. An emerging alternative to address this challenge has been the fabrication of pre-vascularized scaffolds by taking advantage of biomanufacturing techniques, such as soft- and photo-lithography or 3D bioprinting, and cell-based approaches, where functional capillaries are engineered in cell-laden scaffolds prior to implantation. These strategies seek to engineer pre-vascularized tissues in vitro, allowing for improved anastomosis with the host vasculature upon implantation, while also improving cell viability and tissue development in vitro. This book chapter provides an overview of recent methods to engineer pre-vascularized scaffolds for bone regeneration. We first review the development of functional blood capillaries in bony structures and discuss controlled delivery of growth factors, co-culture systems, and on-chip studies to engineer vascularized cell-laden biomaterials. Lastly, we review recent studies using microfabrication techniques and 3D printing to engineer pre-vascularized scaffolds for bone tissue engineering.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascularization
- Bone regeneration
- Bone scaffolds
- Angiogenesis
- Microfabrication
- Bioprinting
- Tissue engineering
1 Introduction
Treatment of bone related diseases and injuries results in a significant financial burden for health care systems of countries worldwide. In the United States alone, an estimate of approximately $796.3 billion is spent yearly for the treatment of bone related diseases (Reports of the Surgeon General 2004). This expenditure is expected to increases drastically with the increasing population age. For instance, the number of hip fractures in the world is predicted to increase from 1.7 million in 1990 to a staggering 6.3 million by 2050 (Johnell 1997; Praemer et al. 1992; Cheung 2005). Autologous bone grafts, allogeneic scaffolds or synthetic and avascular materials, including polymers and metals, are the current materials of choice for treatment of bone loss or repair (Goldberg and Stevenson 1987). Despite the use of various biomaterials and different treatment strategies, only approximately 30 % of patients treated with bone replacements regain function without requiring further surgical intervention (Woolf and Pfleger 2003). The current gold standard of treatment for bone regeneration is the use of autologous bone grafts, which is surgically removed from healthy structures in a patient’s body and then re-implanted in the compromised areas (Reports of the Surgeon General 2004). However, these techniques have largely been recognized to face serious limitations, such as the need for invasive surgical procedures, difficult post-operative care, high cost, and unpredictable outcomes (Goldberg and Stevenson 1987). Tissue engineering has long held great promises as improved treatment options for these conditions. However, successful outcomes using tissue engineering approaches for bone regeneration remain far from ideal in the clinical setting. Hence, the development of improved strategies to regenerate bone remains a major clinical need.
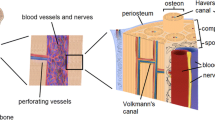
Long bones are made of a hard external cortical layer constituting approximately 80 % of the total bone mass and a highly porous internal trabecular layer (Curray 2006; Clarke 2008) (Fig. 5.1). The external layer is highly dense with a mechanical strength of 130–190 MPa and a porosity of only about 20 % (Zimmermann et al. 2011; Koester et al. 2008; Karageorgiou and Kaplan 2005). On the other hand, trabecular bone has a strength of only about 10 % of the cortical layer (Hernandez and Keaveny 2006). In spite of their structural differences, both layers in the bone structure rely on a vast and intricate vasculature for their homeostasis (Kanczler and Oreffo 2008; Brandi and Collin-Osdoby 2006). In mature bone, the existing vasculature plays an important role in providing the necessary signaling factors, hormones and metabolites needed to recruit circulating cells, such as haematopoietic cells to the bone marrow (Brandi and Collin-Osdoby 2006; Schmid et al. 1997). Moreover, both in engineered tissue constructs and in the body, cells must be sufficiently close to oxygen and nutrient supplies (Jain et al. 2005; Laschke et al. 2009; Sasagawa et al. 2010) to prevent the formation of necrotic spots (Radisic et al. 2004). The structural arrangement of osteons, the functional units of cortical bone (Wegst et al. 2015), provides the necessary physical space for the blood vessels in the highly dense cortical layer by means of central haversian canals (Portal-Nunez et al. 2012; Risau 1997). Hence, approaches for engineering bone need to be customized to comprehensively facilitate biomechanical integrity, remodeling and metabolic activity as regulated by an existing vasculature.
There are a number of strategies that have been studied towards the formation of microvascular networks in tissue engineering constructs. A large body of work on bone regeneration has been devoted to understanding the formation of microvascular beds that appear after osteoinductive and osteoconductive scaffolds are implanted in the body (Mercado-Pagan et al. 2015; Liu et al. 2013, 2015; Zhang et al. 2012). These strategies rely on host-capillary invasion mediated by angiogenesis and vasculogenesis on tissue constructs post-implantation. Unfortunately, the slow rate of host-capillary migration (Clark and Clark 1939) in the implanted scaffolds limits the usefulness of these methods to engineer fully vascularized bone. An exciting alterative to overcome these limitations has been the fabrication of biomimetic microvascular networks in-vitro and prior to implantation.
The process of engineering vascularized tissues in-vitro generally relies either on (1) cell-based strategies or (2) controlled manufacturing of microchannel networks in biomaterials: (1) Cell-based approaches involve primarily endothelial cells, which form self-organized capillary beds embedded within engineered tissue constructs (Black et al. 1998; Chen et al. 2012; Chiu et al. 2012; Elbjeirami and West 2006; Leslie-Barbick et al. 2009, 2011a, b; Peters et al. 2002). (2) Biomanufacturing approaches for engineering vascularized tissues use precise micro-scale fabrication methods, such as soft- and photo-lithography or 3D printing, to create microchannel networks that function as templates for subsequent population of endothelial cells (Mercado-Pagan et al. 2015; Liu et al. 2015, 2013; Chiu et al. 2012; Bae et al. 2012; Baranski et al. 2013; Bertassoni et al. 2014a; Lee et al. 2014; Miller et al. 2012; Obregon et al. 2015), thus forming vascular networks within engineered tissue constructs.
In this book chapter we provide an overview of the mechanisms involved in bone vascularization and angiogenesis. We discuss existing and emerging strategies to engineer pre-vascularized tissues, including cellular- and biofabrication-based approaches, particularly in the scope of bone and load bearing tissue engineering. We argue that a thorough understanding of the mechanisms underlying the natural formation of vascular tissues in the body, combined with an overview of existing methods and challenges related to fabrication of pre-vascularized scaffolds, may provide the fundamental knowledge required to develop effective approaches to engineer vascularized tissues.
2 Vasculogenesis and Angiogenesis
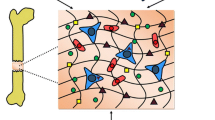
In order to better understand the existing strategies to engineer vascularized bone and mineralized tissues, it is relevant to discuss the processes that guide and control formation of blood vessels and capillaries in the human body. Blood vessels are formed through two key and often complementing processes: vasculogenesis and angiogenesis (Risau 1997; Folkman and Shing 1992; Vailhe et al. 2001) (Fig. 5.2). Vasculogenesis is the origination of new blood vessels from angioblastic progenitor cells (APCs) or endothelial progenitor cells (EPCs). APCs are recruited during embryonic and fetal growth, whereas EPCs are seen in post-natal vasculogenesis comprising wound and fracture healing, myocardial infarction, ischemia, atherosclerosis and tumor growth (Folkman and Shing 1992; Real et al. 2008; Invernici et al. 2008). Conversely, angiogenesis utilizes sprouting or intussusception to generate microvessels from already existing blood vessels and capillaries (Real et al. 2008). Driven by pro-angiogenic factors, sprouting is characterized by matrix remodeling to allow for the endothelial cells to branch out and proliferate. Subsequently, the migrating cells re-assemble to produce lumen and eventually mature into a functional endothelium (Folkman and Shing 1992; Invernici et al. 2008; Ribatti and Crivellato 2012). These systemic sequence of events is divided under two distinct phases of sprouting angiogenesis: growth and stabilization (Ribatti and Crivellato 2012).
Different steps involved in vasculogenesis and angiogenesis, and influence of different growth factors in formation of new vessels. (a) Sprouting angiogenesis. (b) Vasculogenesis. (c) Intussusception. (d) Selection of tip cell (Reproduced from Carmeliet and Jain with permission. Copyright 2011 Nature publishing group)
In the initial growth phase, vasodilation of existing vessels causes an increase in permeability and degradation of the basement membrane, enabling the migration of endothelial cells towards the tissue mass, expressing angiogenic growth factors, and inducing proliferation and tubulogenesis. The migration of endothelial cells is mainly facilitated by the VEGF receptor 2 (VEGFR2), which aligns the cells with respect to a gradient in the growth factor, VEGF A (Lamalice et al. 2007). During the stabilization phase, endothelial cells cease to proliferate and a basement membrane starts to form around the newly derived capillary (Bergers and Song 2005). Further, this phase is completed by the recruitment of pericytes around the neocapillary (Bergers and Song 2005). Alternatively, intussusception is the phenomenon of vascular network expansion through transcapillary pillars, which ultimately bridges opposite capillary walls. The formation of transcapillary bridges is followed by endothelium reorganization, invasion of myofibroblasts and pericytes leading to vascular network remodeling. Intussusception occurs at a relatively rapid pace and with lower proliferation compared to sprouting (LeBlanc et al. 2012).
During bone formation, more specifically, signaling molecules secreted by the bone endothelium help to recruit circulating cells to coordinate skeletal development (Brandi and Collin-Osdoby 2006). During this process, two well-defined and distinct set of events that are orchestrated by the bone vasculature take place, namely endochondral and intramembranous ossification (Kanczler and Oreffo 2008; Hu et al. 2013). Endochondral ossification occurs in regions occupied by cartilage, where the transition from cartilage to bone depends on the level of vascularization on the particular site of ossification (Gerber and Ferrara 2000). During embryonic bone growth, blood vessels originate from the perochondrium and invaginate into the cartilage by the development of immature vascular networks that enter cartilage canals already formed in the expanding cortical bone (Burkus et al. 1993). After birth, canals formed for blood vessels develop together with maturation of endothelium monolayers that invade cartilage growth plates (Burkus et al. 1993). Lastly, during adulthood, angiogenesis becomes a more dynamic process, which is either up- or down-regulated depending on the remodeling needs of a particular site due to aging, disease, or injury. Intramembranous ossification, on the other hand, is characterized by growth of microcapillary networks into the mesenchymal zone, triggering the recruitment and differentiation MSCs into mature osteoblasts. During this process osteoblasts secrete bone matrix surrounding blood vessels forming the trabeculae. As the trabeculae increase in size and density, they coalesce forming grooves around existing blood capillaries. This interconnected and poorly organized immature bone structure is later replaced by a more organized, stronger, lamellar bone, which emerges as a consequence of continuous deposition of bone matrix from the surface of the haversian canals inwards, towards existing blood vessels (Rodan and Raisz 1996).
3 Cellular Approaches to Engineer Vascular Networks
Cellular approaches to engineer vascularized tissues are characterized by the spontaneous organization of cells to form architecturally and functionally relevant vascular networks, by secreting their own extra cellular matrix and remodeling the microenvironment without external intervention (Bae et al. 2012). A variety of pre-vascularization approaches have exploited the inherent propensity of endothelial cells to form capillary-like structures under specific conditions.
A number of studies focused on understanding the relationship of osteoprogenitor and endothelial cells have shed light onto the importance of co-culturing different cell types to mimic the formation of vascularized bone. Several other studies have demonstrated that primitive microvascular structures are formed when endothelial cells are cultured under specific conditions inside hydrogels that mimic the extracellular matrix (ECM) 3D microenvironment, particularly in the presence of growth factors such as VEGF (Folkman and Haudenschild 1980). An early study by Wang et al. for instance, demonstrated that the presence of endothelial cells near osteoblast-like cells enhance the alkaline phosphatase activity of the bone-derived cells (Wang et al. 1997). It has also been well documented that endothelial cells cultured with smooth muscle progenitor cells can form primitive microvascular networks that anastomose with the host vasculature upon implantation (Black et al. 1998; Sato et al. 1987; Berthod et al. 2006; Supp et al. 2002; Vernon and Sage 1999; Kubota et al. 1988; Montesano et al. 1983).
A key study attempting to control the formation of vascularized bone by taking advantage of the cross-communication between endothelial and bone progenitor cells was published by Tsigkou et al. In this work, the authors showed that human mesenchymal stem cells (hMSCs) co-cultured with endothelial cells (ECs) in a fibronectin-containing collagen gels functioned as a source of perivascular cells, hence promoting the formation of a mature and stable vasculature both in vitro and in vivo over time (Tsigkou et al. 2010). The endothelial cells formed tube-like structures and stable networks 4–7 days after implantation, whereas anastomosis occurred after 11 days and mineralization was present after 4 weeks. The authors demonstrated that hMSCs were essential for stable vasculature development, primarily due to their potential to differentiate towards a perivascular lineage expressing smooth muscle cell (SMC) phenotype. Interestingly, a delayed differentiation of hMSCs towards the perivascular phenotype, due to the lack of TGF-β in the media, formed more stable and extensive microvascular networks.
In a similar report, Ying et al. encapsulated human blood-derived endothelial colony-forming cells (ECFCs) and bone marrow derived mesenchymal stem cells (MSCs) in a methacrylated gelatin hydrogel (GelMA) (Chen et al. 2012). The presence of MSCs not only induced the self-assembly of ECFCs into tubular structures, but also improved their survival rate. However, this effect was not observed in a mono-culture system. The self-assembly induced the vacuoles of ECFCs to coalesce and form capillary like structures with a lumen, also expressing intercellular CD-31 markers. At the same time, the study demonstrated that MSCs provided coverage of the capillary like structures, expressing α-smooth muscle actin (α-SMA) and presenting pericyte-like phenotype. It is noteworthy that this effect was highly dependent on the crosslinking degree and the stiffness of the hydrogel, which could be easily controlled by polymerization with UV light, or the degree of methacrylation of the polymer. Once implanted, these capillary structures also showed a strong presence of erythrocytes within their lumen only after 6 days. A more recent and clinically relevant approach utilized human pluripotent stem cells (hPSCs) to induce co-differentiation of this cell population into early vascular cells that could mature into a bilayer of endothelial cells and pericytes. Interestingly, the hPSCs-derived primitive networks that formed inside 3D collagen gels went on to self organize and form more stable networks that survived implantation, integrated with the host vasculature, and established functional blood flow over time (Kusuma et al. 2013).
3.1 Growth Factor Delivery
In vivo formation of a vascular plexus is widely known to be controlled by the association of a cascade of factors and kinetics, spatially and temporally designed to achieve angiogenesis and tubulogenesis. Thus, controlled use of pro-angiogenic growth factors (Vailhe et al. 2001; Jansen et al. 2005; Simmons et al. 2004), like vascular endothelial growth factors (VEGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF), in cell-loaded scaffolds has been extensively studied (Bae et al. 2012; Jansen et al. 2005; Simmons et al. 2004; Nguyen et al. 2012). A number of comprehensive reviews have been published on this topic, and we encourage the reader to refer to the cited literature for a more throughout understanding of the complexity of existing methods for growth factor delivery in bone vascularization. The delivery of growth factors using biomaterials for vascular tissue engineering has been studied using primarily one of the tow methods: physical entrapment or chemical immobilization. A few noteworthy examples of these strategies will be described below.
3.1.1 Physical Entrapment of Growth Factors
Physical entrapment of growth factors consists of embedding specific biomolecules, generally in polymeric matrices that allow for controlled diffusion into the surrounding matrices. Such process is usually performed prior to inducing gelation or solidification of the delivery vehicle (Mehta et al. 2012), provided that it is not harmful for the biological activity of the growth factor. Hydrogels are commonly used for these applications due to their controllable porosity, mesh size, degradation rates, and other physical properties relevant for controlled diffusion (Slaughter et al. 2009; Annabi et al. 2014). Additionally, growth factor release can be controlled by regulating the rate of swelling of the gel matrix, the mechanism of degradation, the interactions holding the polymer together, and other factors.
Several studies have shown that determining the optimal rate of release of VEGF can be critical for ensuring the therapeutic effect of the growth factor in inducing angiogenesis and vasculogenesis (Street et al. 2002). For this reason, an important challenge in the field of vascularized bone regeneration has been to deliver angiogenic growth factors, such as VEGF and PDGF, in combination with osteoinductive growth factors, such as BMPs in a controlled fashion. Polymeric micro- and nano-particles exhibiting different physical characteristics and embedded with different growth factors have been extensively researched to address this challenge. Richardson et al. (2001), for instance, studied the effects of the combination of both VEGF and PDGF in a degradable PEG-based hydrogel scaffold. Growth factors were embedded either by mixing lyophilized VEGF directly with poly(lactide-co-glycolide) pre-polymer before constructing the scaffolds, or by encapsulating PDGF in poly(lactide-co-glycolide) microparticles that were subsequently embedded in the VEGF-containing scaffolds. Results showed that release of these two factors alone did not allow for a stable and mature formation of vascular networks, while their combination had a more successful outcome. This results not only confirmed the role of spatial and temporally controlled biological organization in vascular formation, but also provided a powerful tool for therapeutic strategies. Controlled release of other factors involved in bone formation, such as fibroblast growth factor-2 and insulin growth factor-1 have also been demonstrated in calvaria defect and tibial fracture models (Kimoto et al. 1998; Oest et al. 2007). In summary, the delivery of growth factors in micro and nanoparticles by physical entrapment allows for a more controlled delivery and more adequate approach to mimic the gradual cascade of biological events occurring during formation of vascularized bone (Ennett et al. 2006; Santo et al. 2009; Chen et al. 2009; Patel et al. 2008; Yilgor et al. 2010; Oldham et al. 2000), then simple bolus injection of growth factors in the site of regeneration.
In a different approach, Jeong et al. developed a “living” microvascular stamp engineered by encapsulating fibroblasts, which endogenously express angiogenic factors, into a three-dimensio-nally fabricated permeable poly(ethylene glycol) diacrylate/methacrylic alginate (PEGDA-MA) hydrogel via stereo lithography (SLA). The permeable hydrogel allowed for sustainable release of growth factors at pre-defined locations, after the “living” stamps were implanted onto a chick embryo chorioallantoic membrane. This approach illustrates how endogenous growth factors can also be used for therapeutic applications using simple physical entrapment methods, not only in vitro but also in vivo (Jeong et al. 2012).
3.1.2 Chemically Immobilized Growth Factors
Chemically immobilized growth factors have been proposed to better mimic the dynamic mechanisms of binding and selective delivery of factors that is operated in the body (Jeon et al. 2007; Lutolf and Hubbell 2005). By chemically immobilizing growth factors to the delivery vehicle, the actual factor may also be released only upon degradation of the vehicle itself, thus providing another mechanism of controlled delivery that is not dependent upon diffusion, rather on controlled enzymatic degradation or hydrolysis (Lutolf et al. 2003).
Barbick and colleagues, in a series of papers, focused their attention in the optimization of vasculogenesis and tubular formation in PEG-based hydrogels modified with a VEGF mimetic (QK) peptide (Leslie-Barbick et al. 2009, 2011a, b). PEG-based hydrogels can undergo chemical modification to retain growth factors, adhesive ligands and degradable peptides in their matrix (Leslie-Barbick et al. 2009, 2011a, b). In a specific study, a laser scanning lithograph (LSL) technique was used to precisely control crosslinking of a polymer coupled with the VEGF mimetic growth factor at specific regions in a PEG-based hydrogel. Results showed that VEGF in the hydrogels enhanced the angiogenic processes and tubular formation in vitro, especially in the LSL pre-patterned areas after 2 days. In addition, covalent bonding and immobilization of these factors and ligands allowed the spatial distribution and local controlling on the positioning in the entire construct (Leslie-Barbick et al. 2009, 2011a, b).
Alginate hydrogels functionalized with BMPs and VEGF have also been shown to allow for sustained released of growth factors for segmental bone repair (Oest et al. 2007). Immobilizing specific matrix components, such as proteins or growth factors, or even only RGD adhesive biding sites (Alsberg et al. 2003; Comisar et al. 2007; Ratner 1996; Rowley et al. 1999), to synthetic biomaterials is arguably the most effective method to more closely mimic the natural ECM in controllably delivering molecules to improve vascularization in bone regeneration.
3.2 On-Chip Vascularization Studies
Although we are unaware of existing reports specifically studying bone vascularization using lab-on-a-chip methods, a number of recent publications have provided important information about vascularization of tissue-engineered constructs using on-chip approaches. A key publication on the integration of microfluidics and bone tissue engineering that requires attention, and which represented the first bone-on-a-chip model, was focused on the engineering of bone marrow, rather than bone vascularization per se (Torisawa et al. 2014). In this work, the authors first engineered new bone in vivo by implanting a bone inducing biomaterial scaffold in a subcutaneous mouse model. The engineered tissue was then retrieved surgically and cultured under continuous perfusion with culture medium in a microfluidic device. The bone marrow on-a-chip showed preserved hematopoietic stem and progenitor cell function for at least 1 week in the device, also forming an improved platform to study bone marrow toxicity under physiologically relevant culture conditions (Torisawa et al. 2014).
In another recent study of vascularization on-a-chip, Cuchiara et al. reported the co-culture of HUVECs and mesenchymal progenitors (10T1/2) cells in a hybrid matrix of collagen and fibronectin in a hybrid microfluidic device. The authors showed stable pre-vascular network formation with the 10T1/2 cells expressing a pericyte phenotype (Cuchiara et al. 2012). This co-culture system was used to create perfusable structures by integrating a microfabricated cell-laden hydrogel with a microfluidic platform (Cuchiara et al. 2012). The hydrogel containing HUVECs and 10T1/2 cells was sandwiched between two separate channels, where one channel was perfused with culture medium and the other with PBS, creating a gradient in the nutrient concentration over the thickness of the hydrogel. This enabled the cells to proliferate, degrade the matrix and remodel along the gradient to produce perfusable vascular channels.
In another attempt to replicate and study the physiologic conditions for angiogenesis and vasculogenesis on-a-chip, Nguyen et al. and Kim et al. developed very similar methods to generate perfusable 3D microvessels in vitro. Both papers utilized relatively simple microfluidic chips to generate spatially controlled gradients of angiogenic growth factors and co-cultures of endothelial cells with stromal fibroblasts, pericytes or cancer cells. These reports not only elucidated the steps involved in microvessel formation and maturation in ECM derived 3D matrices (Kim et al. 2012, 2013, 2015), but also provided clear visualization of angiogenic sprouting originating from a larger engineered parent vessel, forming stable vascular networks that migrated towards the gradient of growth factors on chip (Nguyen et al. 2013). The advantages of on-chip models for studying angiogenesis and vasculogenesis are countless (Bischel et al. 2013; Zheng et al. 2012), and the field of vascularized bone tissue engineering can benefit tremendously from this set of techniques.
4 Biofabrication Approaches to Engineer Pre-vascularized Scaffolds
Although several advances have been made towards achieving vascularization in bone scaffolds, growth factor delivery and cell-based approaches generally lack control over the arrangement of the engineered vascular networks in 3D space, thus leading to inefficient distribution of oxygen, nutrients and waste products in 3D tissue constructs (Baranski et al. 2013; Chen et al. 2003). Vascular systems are inherently highly organized and hierarchically structured networks to enable efficient oxygenation of every cell in the body. Hence, coupling microenvironmental cues with microfabrication methods is an exciting alternative to engineer functional vascular networks.
An ideal fabrication platform should allow for high fidelity in architecture at microscopic scales, predictable oxygen and pressure distribution patterns, while being amenable to a wide range of biomaterials. It should also possess a fast, automated, and reproducible process. On the cellular level, the fabrication system parameters should entail high cellular viability and functionality. Multiple fabrication methodologies such as soft lithography, photopatterning and rapid-prototyping are currently being studied due to their ability to fabricate inter-connected and organized pre-vascular networks. Moreover, computational approaches to predict oxygen consumption and pressure distribution in a given scaffold are also investigated to predict the requirements of different constructs and provide guidance on the actual fabrication processes. The following sections will provide an overview of the various fabrication strategies to engineer pre-vascularized tissue constructs by taking advantage of various biofabrication methods.
4.1 Lithography and Microfabrication
The previously discussed techniques (i.e. growth factor delivery, cell-based approaches, etc.) have been extensively used to improve the process of vascularization for bone tissue engineering. However, these techniques usually result in the formation of randomly organized networks. For these reasons alternative methods for controlled fabrication of microvascular channels with precise and pre-defined locations have been developed.
Photolithography, for instance, is a microfabrication process derived from the semiconductor industry for generating geometrically defined micro-scale structures. Using a laser-patterned photomask for restricting UV light exposure to specific regions on pre-polymer solutions containing a photoinitiator, micropatterns can be created in a range of photocrosslinkable polymers that are compatible with cell viability (Chen et al. 2003). Soft lithography, on the other hand, comprises of various micro-molding or micro-contact printing approaches, which essentially use a stamp-based technique to create the desired microscale structures (Qin et al. 2010).
Several studies have adopted these methods with natural or synthetic hydrogels to enhance ECs alignment and promote angiogenesis. For instance, West and colleagues (Leslie-Barbick et al. 2009, 2011b) used microfabrication techniques on a PEG-diacrylate (PEGDA) hydrogel to improve the vascularization process. The surface of the patterned hydrogels was modified by using cell-adhesive ligand sites, such as RGD and growth factors, in order to enhance ECs function and morphogenesis. The patterning induced formation of cord-like structures along the prefabricated regions. Nikkhah et al. investigated organization and alignment of ECs using microfabricated methacrylate gelatin (GelMA) hydrogels. In this work, it was shown that HUVECs alignment was enhanced in patterned hydrogels (50 mm width) as compared to unpatterned substrates (Nikkhah et al. 2012). In a similar study, Raghavan et al. fabricated constructs using a micromolding method to create cell-laden collagen gels with prefabricated microchannels. In principle, a polydimethylsiloxane (PDMS) micromold with the desired channel dimensions was first manufactured and then filled with an endothelial cell-laden collagen hydrogel. A tubular organization with lumens was then observed after 24–48 h and the authors also demonstrated that the concentration of the collagen and channel width influenced the tubule diameter (Raghavan et al. 2010).
In another recent study, polydimethylsiloxane (PDMS) was micro-patterned using soft-lithography and the effect of textures on human bone marrow derived cells (hBMDCs) was analyzed under osteogenic conditions. The textured PDMS consisted of 45 μm wide curved channels with a height of 11 μm, which were separated by ridges of 5 μm. Although the expression of osteogenic phenotype was similar in both textured and non-textured substrates, the patterned PDMS triggered a denser cellular arrangement. Also, the micro-pattern induced a better alignment and high cellular aspect ratio (Mata et al. 2002). Hence, soft lithography provides a cheap screening platform for testing multiple topographic settings, the results of which could be easily applied to fabricate scaffolds with predefined patterns to guide vascular formation.
Micro-molding approaches were also utilized for directing controlled tubulogenesis. Baranski et al. utilized PDMS microchannels to self-assemble HUVECs embedded in a collagen matrix (Fig. 5.3). The cells in the microstructures rapidly reassembled within 4 h through cytoskeletal-induced contraction to generate dense tubular endothelial structures. These tubular cords were subsequently embedded in fibrin matrix and implanted in vivo. After 28 days of implantation, the vascularized fibrin bodies integrated with the host tissue and pericyte cells supported the HUVEC cords to form perfusable capillaries (Baranski et al. 2013). Mapili et al. conducted a similar study regarding the design of precise spatial patterning using a stereolithography technique (SL). PEGDMA hydrogel was dispensed and UV crosslinked using a pulsed Nd: YAG laser (10 mJ/pulse) in order to fabricate scaffolds with controlled pore size and microstrucutre (Mapili et al. 2005). Further functionalization with RGD was then provided in order to study OP-9 cells attachment on the patterned substrates. Results showed no attachment in the RGD-free structures, whereas DAPI staining revealed the opposite on the functionalized scaffolds. This technique revealed an innovative and inexpensive way to fabricate precise geometrical features and point-by-point crosslinking of the scaffold along with biological and physical factors. However, this methodology suffers of limitations such as the lack of feasibility using cells encapsulation because of slow process of fabrication.
Microfabricated pre-vascularized tissue constructs. (a) Collagen type I microfabricated in a microfluidic device (b) cultured with endothelial cells and pericytes for an in-vitro study of angiogenesis and thrombosis (Reproduced from Zheng et al. (2012) with permission. Copyright 2012. The National Academy of Science). (c) Micromolding of a cell-collagen gel in a fibrin matrix to engineer pre-vascularized tissues in-vitro and (d) in-vivo (Reproduced from Baranski et al. (2013) with permission. Copyright 2012 The National Academy of Science)
Another recent study conducted via soft lithography (Zheng et al. 2012) used for microfabrication of type I collagen to fabricate microchannels, which were then seeded with HUVECs and cultured to achieve endothelialized lumen (Zheng et al. 2012). Results showed the spontaneous formation of tubular constructs and angiogenic sprouting when perivascular cells and SMCs were encapsulated in the matrix (Fig. 5.3). Similarly, Wray et al. (Wray et al. 2013) developed a new technology to create microchannels in a porous silk-based matrix using soft lithography. Results showed that endothelial cells reached confluence and proliferation in the highly porous areas. Moreover, co-culturing of hMVECs in the microchannels and of hMSCs embedded in the hydrogel revealed that morphogenesis and lumen formation was more active near the channel regions. These methods are certainly the strong candidates for generating more complex tissues and organs scaffolds in bone tissue engineering applications and a potent tool for future therapeutic methodologies.
4.2 3D Printing
The automated deposition of biomaterials embedded with cells to fabricate three-dimensionally defined patterns giving rise to macroscopic engineered tissue construct is called 3D bioprinting (Bertassoni et al. 2014a, b; Obregon et al. 2015; Tasoglu and Demirci 2013). With high reproducibility, bioprinting offers unique and significant advantages over other traditional fabrication techniques that are restricted to planar substrates, such as lithography-based fabrication. Bioprinting generates versatile patterns by exploiting Computer Aided Design (CAD) and modeling and can be employed to fabricate a wide range of materials (Obregon et al. 2015; Derby 2012). However, rapid prototyping strategies for vascularization have, thus far, been mainly utilized to develop simple sacrificial template based approaches to engineer vascularized tissues. Still, this strategy represents one of the most effective methods for vascularizing engineered tissue constructs.
3D sacrificial template approaches basically involve printing of a primary well-defined 3D template structure through rapid prototyping, which functions as a mold for a secondary biomaterial with or without cells. Upon crosslinking of the secondary matrix, the partially embedded primary sacrificial template is then removed by physical, chemical or thermal routes, thus generating a 3D scaffold with hollow interconnected microchannels. In one of the first studies using a sacrificial template method, Miller et al. developed a carbohydrate glass template system, where the hygroscopic behavior of the printed materials allowed for its dissolution by the application of normal cell culture medium (Miller et al. 2012). Further, to avoid the osmotic shock to the cells during the dissolution of the template, the 3D printed carbohydrate glass lattice was coated with poly(d-lactide-co-glycolide) (PDLGA). To circumvent such release of potentially toxic and cellular phenotype modifying byproducts, Bertassoni et al. printed agarose templates to engineer hollow cylindrical channels in pre-osteoblast cell-laden tissue constructs (Bertassoni et al. 2014a) (Fig. 5.4). The flexibility and poor molecular interaction of the 3D printed agarose gel with the secondary biomaterials (four types of photocrosslinkable gels were tested) contributed to the easy removal of the template simply by using a slight vacuum. Of particular interest, Bertassoni et al. demonstrated that the presence of the integrated vascular channels allowed for significantly higher viability and expression of alkaline phosphatase by the pre-osteoblast cells, thus suggesting improved differentiation towards the osteogenic lineage. When seeded with endothelial cells, the hollow channels acted as a provision for organized vasculature by allowing for sprouting of the cells lining the lumen in the surrounding matrix (Bertassoni et al. 2014a).
(a, b) 3D printed microvascular network templates with various morphologies inside GelMA hydrogels and (c, d) after perfusion with a fluorescent dye. (e) Confocal image of endothelial cells forming a confluent monolayer inside 3D printed microchannels (Reproduced from Bertassoni et al. (2014a) with permission. Copyright 2014 The Royal Society of Chemistry)
3D bioprinting was also used by Lee and colleagues (2014) in an attempt to build up a scaffold using collagen as matrix. A mixture of gelatin and HUVECs was dispensed on a collagen layer and then covered by a layer-by-layer deposition of collagen. The channel were then formed by liquefaction of the gelatin at 37 °C, allowing the endothelial cells to attach to the inner layer of the channel. Results revealed that the method has a great potential in tissue engineering since a well-defined vasculature was created without the necessity of printing cells in the gelatin hydrogel.
5 Final Remarks
For a long time, engineering vascularized tissues was arguably the main roadblock preventing tissue engineering from widespread clinical application. As we summarize in this chapter, recent developments towards engineering of vascularized tissues have changed this scenario significantly. It may be argued that engineering vascularized tissues is no longer an unachievable goal. Nevertheless, understanding the methods to develop functional and clinically relevant vascularized tissues remains a great challenge in bone tissue engineering. There are a variety of methods that promote vascularization in tissue constructs. Cell-based approaches perhaps offer a more biologically relevant strategy, since they take advantage of the ability of cells to self-communicate, secrete paracrine factors and control formation of a stable vasculature in vitro. However, difficulties to control spatially organized vascular channels in macroscale and thick cell-laden constructs remains a major problem. Delivery of growth factors, via physical entrapment or chemical immobilization has also offered a myriad of answers to improve our understanding of vascular formation in engineered bone. Still, the complex cascade of multiple factors naturally occurring in the human body is yet to be artificially mimicked in vitro or in vivo. More recently, biofabrication methods, such as lithography techniques and 3D printing have provided novel and exciting avenues towards fabrication of microchannels that form artificially engineered vascular capillaries and vessels. Although these methods do not mimic the complex and dynamic steps involved in the vasculogenesis of mature and stable capillaries composed of heterotypic cells and growth factors, they allow for better controlled and on-demand fabrication of vascular networks that improve oxygen and nutrient diffusion, as well as waste removal in cell-laden tissue constructs. Perhaps the integration of these different methods, combined with novel strategies for tissue fabrication and control of cellular function in future strategies will enable true fabrication of fully pre-vascularized, biomimetic, load-bearing bone scaffolds prior to implantation.
References
Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ (2003) Regulating bone formation via controlled scaffold degradation. J Dent Res 82(11):903–908
Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C et al (2014) 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater 26(1):85–123
Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA et al (2012) Building vascular networks. Sci Transl Med 4(160):160ps23
Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD et al (2013) Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A 110(19):7586–7591
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7(4):452–464
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL et al (2014a) Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14(13):2202–2211
Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA et al (2014b) Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6(2):024105
Berthod F, Germain L, Tremblay N, Auger FA (2006) Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol 207(2):491–498
Bischel LL, Young EW, Mader BR, Beebe DJ (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34(5):1471–1477
Black AF, Berthod F, L’Heureux N, Germain L, Auger FA (1998) In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J Off Publ Fed Am Soc Exp Biol 12(13):1331–1340
Brandi ML, Collin-Osdoby P (2006) Vascular biology and the skeleton. J Bone Miner Res Off J Am Soc Bone Miner Res 21(2):183–192
Burkus JK, Ganey TM, Ogden JA (1993) Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. Yale J Biol Med 66(3):193–202
Chen C, Hirdes D, Folch A (2003) Gray-scale photolithography using microfluidic photomasks. Proc Natl Acad Sci U S A 100(4):1499–1504
Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF (2009) In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials 30(28):5215–5224
Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM et al (2012) Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater 22(10):2027–2039
Cheung C (2005) The future of bone healing. Clin Podiatr Med Surg 22(4):631–641 viii
Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M (2012) Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A 109(50):E3414–E3423
Clark ER, Clark LB (1939) Microscopic observations on the growth of blood capillaries in the living mammal. Dev Dyn 64(2):251–301
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol CJASN 3(Suppl 3):S131–S139
Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ (2007) Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials 28(30):4409–4417
Cuchiara MP, Gould DJ, McHale MK, Dickinson ME, West JL (2012) Integration of self-assembled microvascular networks with microfabricated PEG-based hydrogels. Adv Funct Mater 22(21):4511–4518
Curray JD (2006) Bones: structure and mechanics. Princeton University Press, Princeton, p 456
Derby B (2012) Printing and prototyping of tissues and scaffolds. Science 338(6109):921–926
Elbjeirami WM, West JL (2006) Angiogenesis-like activity of endothelial cells co-cultured with VEGF-producing smooth muscle cells. Tissue Eng 12(2):381–390
Ennett AB, Kaigler D, Mooney DJ (2006) Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A 79(1):176–184
Folkman J, Haudenschild C (1980) Angiogenesis in vitro. Nature 288(5791):551–556
Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267(16):10931–10934
Gerber HP, Ferrara N (2000) Angiogenesis and bone growth. Trends Cardiovasc Med 10(5):223–228
Goldberg VM, Stevenson S (1987) Natural history of autografts and allografts. Clin Orthop Relat Res 225:7–16
Hernandez CJ, Keaveny TM (2006) A biomechanical perspective on bone quality. Bone 39(6):1173–1181
Hu X, Zhang P, Xu Z, Chen H, Xie X (2013) GPNMB enhances bone regeneration by promoting angiogenesis and osteogenesis: potential role for tissue engineering bone. J Cell Biochem 114(12):2729–2737
Invernici G, Madeddu P, Emanueli C, Parati EA, Alessandri G (2008) Human fetal aorta-derived vascular progenitor cells: identification and potential application in ischemic diseases. Cytotechnology 58(1):43–47
Jain RK, Au P, Tam J, Duda DG, Fukumura D (2005) Engineering vascularized tissue. Nat Biotechnol 23(7):821–823
Jansen JA, Vehof JW, Ruhe PQ, Kroeze-Deutman H, Kuboki Y, Takita H et al (2005) Growth factor-loaded scaffolds for bone engineering. J Control Release 101(1–3):127–136
Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS (2007) Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly (L-lactic-co-glycolic acid) scaffold. Biomaterials 28(17):2763–2771
Jeong JH, Chan V, Cha C, Zorlutuna P, Dyck C, Hsia KJ et al (2012) “Living” microvascular stamp for patterning of functional neovessels; orchestrated control of matrix property and geometry. Adv Mater 24(1):58–63, 1
Johnell O (1997) The socioeconomic burden of fractures: today and in the 21st century. Am J Med 103(2A):20S–25S; discussion 5S–6S
Kanczler JM, Oreffo RO (2008) Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15:100–114
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26(27):5474–5491
Kim C, Chung S, Yuchun L, Kim MC, Chan JK, Asada HH et al (2012) In vitro angiogenesis assay for the study of cell-encapsulation therapy. Lab Chip 12(16):2942–2950
Kim S, Lee H, Chung M, Jeon NL (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13(8):1489–1500
Kim C, Kasuya J, Jeon J, Chung S, Kamm RD (2015) A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 15(1):301–310
Kimoto T, Hosokawa R, Kubo T, Maeda M, Sano A, Akagawa Y (1998) Continuous administration of basic fibroblast growth factor (FGF-2) accelerates bone induction on rat calvaria–an application of a new drug delivery system. J Dent Res 77(12):1965–1969
Koester KJ, Ager JW 3rd, Ritchie RO (2008) The true toughness of human cortical bone measured with realistically short cracks. Nat Mater 7(8):672–677
Kubota Y, Kleinman HK, Martin GR, Lawley TJ (1988) Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107(4):1589–1598
Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S (2013) Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A 110(31):12601–12606
Lamalice L, Le Boeuf F, Huot J (2007) Endothelial cell migration during angiogenesis. Circ Res 100(6):782–794
Laschke MW, Vollmar B, Menger MD (2009) Inosculation: connecting the life-sustaining pipelines. Tissue Eng Part B Rev 15(4):455–465
LeBlanc AJ, Krishnan L, Sullivan CJ, Williams SK, Hoying JB (2012) Microvascular repair: post-angiogenesis vascular dynamics. Microcirculation 19(8):676–695
Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS et al (2014) Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35(28):8092–8102
Leslie-Barbick JE, Moon JJ, West JL (2009) Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly (ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed 20(12):1763–1779
Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL (2011a) The promotion of microvasculature formation in poly (ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials 32(25):5782–5789
Leslie-Barbick JE, Shen C, Chen C, West JL (2011b) Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng Part A 17(1–2):221–229
Liu Y, Lim J, Teoh SH (2013) Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv 31(5):688–705
Liu Y, Chan JK, Teoh SH (2015) Review of vascularised bone tissue-engineering strategies with a focus on co-culture systems. J Tissue Eng Regen Med 9(2):85–105
Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23(1):47–55
Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R et al (2003) Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 21(5):513–518
Mapili G, Lu Y, Chen S, Roy K (2005) Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J Biomed Mater Res B Appl Biomater 75(2):414–424
Mata A, Boehm C, Fleischman AJ, Muschler G, Roy S (2002) Analysis of connective tissue progenitor cell behavior on polydimethylsiloxane smooth and channel micro-textures. Biomed Microdevices 4(4):267–275
Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ (2012) Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev 64(12):1257–1276
Mercado-Pagan AE, Stahl AM, Shanjani Y, Yang Y (2015) Vascularization in bone tissue engineering constructs. Ann Biomed Eng 43(3):718–729
Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM et al (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11(9):768–774
Montesano R, Orci L, Vassalli P (1983) In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol 97(5 Pt 1):1648–1652
Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S et al (2012) Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev 18(5):363–382
Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA et al (2013) Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A 110(17):6712–6717
Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K et al (2012) Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 33(35):9009–9018
Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE (2015) Three dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J Dental Res 94(9, suppl no. 2):1435S–152S
Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE (2007) Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res 25(7):941–950
Oldham JB, Lu L, Zhu X, Porter BD, Hefferan TE, Larson DR et al (2000) Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng 122(3):289–292
Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG (2008) Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43(5):931–940
Peters MC, Polverini PJ, Mooney DJ (2002) Engineering vascular networks in porous polymer matrices. J Biomed Mater Res 60(4):668–678
Portal-Nunez S, Lozano D, Esbrit P (2012) Role of angiogenesis on bone formation. Histol Histopathol 27(5):559–566
Praemer A, Furner S, Rice D (1992) Musculoskeletal conditions in the United States. American Academy of Orthopaedic Surgeons, Park Ridge
Qin D, Xia Y, Whitesides GM (2010) Soft lithography for micro- and nanoscale patterning. Nat Protoc 5(3):491–502
Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE et al (2004) Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol 286(2):H507–H516
Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS (2010) Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A 16(7):2255–2263
Ratner BD (1996) The engineering of biomaterials exhibiting recognition and specificity. J Mol Recog JMR 9(5–6):617–625
Real C, Caiado F, Dias S (2008) Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc Hematol Disord Drug Targets 8(3):185–193
Reports of the Surgeon General (2004) Bone health and osteoporosis: a report of the surgeon general, Rockville
Ribatti D, Crivellato E (2012) “Sprouting angiogenesis”, a reappraisal. Dev Biol 372(2):157–165
Richardson TP, Peters MC, Ennett AB, Mooney DJ (2001) Polymeric system for dual growth factor delivery. Nat Biotechnol 19(11):1029–1034
Risau W (1997) Mechanisms of angiogenesis. Nature 386(6626):671–674
Rodan GA, Raisz LG, Bilezikian JP (1996) Principles of bone biology. Academic, San Diego, pp 3–24
Rowley JA, Madlambayan G, Mooney DJ (1999) Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20(1):45–53
Santo VE, Frias AM, Carida M, Cancedda R, Gomes ME, Mano JF et al (2009) Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules 10(6):1392–1401
Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y et al (2010) Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31(7):1646–1654
Sato N, Sawasaki Y, Senoo A, Fuse Y, Hirano Y, Goto T (1987) Development of capillary networks from rat microvascular fragments in vitro: the role of myofibroblastic cells. Microvasc Res 33(2):194–210
Schmid J, Wallkamm B, Hammerle CH, Gogolewski S, Lang NP (1997) The significance of angiogenesis in guided bone regeneration. A case report of a rabbit experiment. Clin Oral Implants Res 8(3):244–248
Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ (2004) Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 35(2):562–569
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21(32–33):3307–3329
Street J, Bao M, de Guzman L, Bunting S, Peale FV Jr, Ferrara N et al (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A 99(15):9656–9661
Supp DM, Wilson-Landy K, Boyce ST (2002) Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J 16(8):797–804
Tasoglu S, Demirci U (2013) Bioprinting for stem cell research. Trends Biotechnol 31(1):10–19
Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T et al (2014) Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods 11(6):663–669
Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E et al (2010) Engineered vascularized bone grafts. Proc Natl Acad Sci U S A 107(8):3311–3316
Vailhe B, Vittet D, Feige JJ (2001) In vitro models of vasculogenesis and angiogenesis. Lab Investig J Tech Methods Pathol 81(4):439–452
Vernon RB, Sage EH (1999) A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res 57(2):118–133
Wang DS, Miura M, Demura H, Sato K (1997) Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology 138(7):2953–2962
Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO (2015) Bioinspired structural materials. Nat Mater 14(1):23–36
Woolf AD, Pfleger B (2003) Bulletin of the world health organization. Contract No.: 9
Wray LS, Tsioris K, Gi ES, Omenetto FG, Kaplan DL (2013) Slowly degradable porous silk microfabricated scaffolds for vascularized tissue formation. Adv Funct Mater 23(27):3404–3412
Yilgor P, Hasirci N, Hasirci V (2010) Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J Biomed Mater Res A 93(2):528–536
Zhang R, Gao Z, Geng W, Yan X, Chen F, Liu Y (2012) Engineering vascularized bone graft with osteogenic and angiogenic lineage differentiated bone marrow mesenchymal stem cells. Artif Organs 36(12):1036–1046
Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A et al (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109(24):9342–9347
Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P et al (2011) Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A 108(35):14416–14421
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Barabaschi, G.D.G., Manoharan, V., Li, Q., Bertassoni, L.E. (2015). Engineering Pre-vascularized Scaffolds for Bone Regeneration. In: Bertassoni, L., Coelho, P. (eds) Engineering Mineralized and Load Bearing Tissues. Advances in Experimental Medicine and Biology, vol 881. Springer, Cham. https://doi.org/10.1007/978-3-319-22345-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-22345-2_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22344-5
Online ISBN: 978-3-319-22345-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)