Abstract
A kinetic model based on first principles, for β2-microglobulin, is presented to obtain precise parameter estimates for individual patient. To reduce the model complexity, the number of model parameters was reduced using a priori identifiability analysis. The model validity was confirmed with the clinical data of ten renal patients on post-dilution hemodiafiltration. The model fit resulted in toxin distribution volume (V d) of 14.22 ± 0.75 L, plasma fraction in extracellular compartment (f P) of 0.39 ± 0.03, and inter-compartmental clearance of 44 ± 4.1 mL min−1. Parameter estimates suggest that V d and f P are much higher in hemodialysis patients than in normal subjects. The developed model predicts larger removed toxin mass than that predicted by the two-pool model. On the application front, the developed model was employed to explain the effect of intra-dialytic exercise on toxin removal. The presented simulations suggest that intra-dialytic exercise not only increases the blood flow to low flow region, but also decreases the inter-compartmental resistance. Combined, they lead to increased toxin removal during dialysis and reduced post-dialysis rebound. The developed model can assist in suggesting the improved dialysis dose based on β2-microglobulin, and also lead to quantitative inclusion of intra-dialytic exercise in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemodialysis is a life saving treatment for more than one million chronic renal patients worldwide. More than 90 accumulated uremic toxins should be removed during dialysis.30 Since it is impossible to measure all toxins, urea has been used as their representative in defining the dialysis adequacy. However, urea is a small, water-soluble toxin; and many other uremic toxins are much larger in size. Recently, β2-microglobulin (β2M) has become widely accepted marker for larger molecules,1 and higher pre-dialysis serum concentrations of β2M have been associated with mortality and morbidity.2,16 β2M is found on the surface of nucleated cells. After being shed from cell surface, it is excreted by glomerular filtration. In subjects with renal insufficiency, it accumulates in the interstitium and plasma, and deposits as amyloid fibrils in osteoarticular structures.6,7 These deposits can lead to amyloidosis, carpal tunnel syndrome, or formation of bone cysts, which are causes of morbidity in long-term hemodialysis subjects.15 Vanholder et al. have recommended that monitoring the serum level of β2M alone might be sufficient for the evaluation of dialysis adequacy.25
Though considerable interest has been shown for intra-dialytic removal of β2M, urea is still considered to be the standard marker of dialysis adequacy in clinical practice. Incorporating β2M in the prescription of adequate dialysis requires comprehensive mathematical models that can describe β2M kinetics and motivate clinicians to accept its clinical use. Such mathematical models can also help in realizing the goal of optimal individualized treatment. So far, the two-pool model has been used to describe the kinetics of urea, creatinine,31 β2M,26 and other toxins23 in patients on renal replacement therapy. However, the practical application of two-pool models has been limited. Alternatively, regional blood flow (RBF) models can also explain urea kinetics.20 The RBF model describes solute kinetics in terms of an unequal distribution of blood flow to different body organs, and appears to be a better alternative to the two-pool model. The reasons are, RBF model is closely related to physiology and explains certain aspects of kinetics which the two-pool model cannot, such as cardiopulmonary recirculation,8 and the effect of intra-dialytic exercise.21 Recently, Schneditz et al. proposed a diffusion-adjusted regional blood flow (DA-RBF) model,19 which encompasses the characteristics of both the two-pool model and the simple RBF model, and brings it much closer to physiology.
Even after the improvements in the RBF model,19 this model has not been much valued by the research community. Probable reasons include the large number of parameters in the DA-RBF model,12 which are difficult to estimate from limited patient data, and clinicians’ reluctance to shift to a more complex model. Nevertheless, these reasons alone do not undermine the relevance of DA-RBF model in routine clinical setting. In the past, the DA-RBF model has been employed to simulate the kinetics of small molecules like urea and creatinine only.19 The applicability of DA-RBF model for larger molecules marker, such as β2M, and its relevance for renal subjects, is yet to be evaluated.
In this work, we have developed a reduced parameter DA-RBF model for β2M. To overcome the bottleneck of large number of parameters, a priori identifiability analysis was implemented, which elucidates the identifiable model parameters.28 Subsequently, using the data of ten stable patients, we calibrated the reduced parameter model for each patient and compared the estimated model parameters with those obtained from the two-pool model.26 The developed DA-RBF model was then used to estimate the removed toxin mass during hemodiafiltration (HDF) and to explain the effect of intra-dialytic exercise.
Model Description
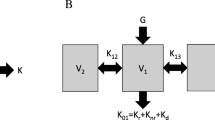
The kinetics of β2M differs from that of small molecules (e.g., urea, creatinine) in that the former is distributed in extracellular (plasma + interstitium) compartment only,13 while the latter are distributed in both extracellular compartment (EC) and intracellular compartment (IC) (Fig. 1). Considering this, the existing DA-RBF model19 was modified in this study. Based on specific perfusion (ratio of blood flow rate and organ fluid volume), the body organs can be divided into two major regions, namely, high flow region (HFR) and low flow region (LFR),19 (Fig. 2). HFR comprises small organs like heart, brain, kidney, and liver with specific perfusion greater than 0.2 min−1. They sum up to 20% of total body fluid volume, but are highly perfused and almost 85% of cardiac output goes to them. The remaining organs are part of LFR which mainly comprises the large body organs like skin, muscles, and bones. They sum up to 80% of total body volume and perfused by only 15% of total cardiac output.12,29 Heart pumps blood to various body organs; HFR and LFR plasma flow (Q hp and Q lp) moves the toxin between plasma compartments and dialyzer. Both HFR and LFR behave as two-compartmental structure where mass transfer between compartments is controlled by inter-compartmental mass transfer coefficient (K ip). It can be observed that this model structure comprises 2 two-pool compartments in parallel, or it can be called as parallel-cum-series representation of physiology (Fig. 2). In the following, all model equations are described.
Diffusion-adjusted regional blood flow model (parallel-cum-series representation of physiology) for explaining β2-microglobulin kinetics. Toxin transfer is due to diffusion across capillary endothelium, and blood/plasma circulation causes convective transport. Q h/Q hp, Q l/Q lp, and Q b/Q bp are blood/plasma flows to HFR, LFR, and dialyzer, respectively. Q cr and Q ar are cardiopulmonary and access recirculation, respectively. Shaded compartments represent contact with blood (A—arterial node and V—venous node)
Mass Balance During Dialysis
Toxin exchange between compartments depends on the concentration difference (diffusive flux), and fluid movement due to ultrafiltration (convective flux). Additionally, constant toxin generation and constant non-renal clearance (K NR) also contributes to toxin accumulation. For example, toxin accumulation in HFR plasma compartment depends on the following factors: (i) diffusive flux from interstitium to plasma, (ii) toxin transfer from interstitium to plasma with ultrafiltered fluid, (iii) toxin transfer from plasma to systemic circulation with ultrafiltered fluid, (iv) convective removal with systemic circulation, (v) non-renal clearance from plasma compartment, and (vi) constant toxin generation. All these factors appear in the same order in the right hand side of Eq. (1). Toxin balance equations for other compartments are written in the similar manner.
HFR plasma mass balance:
HFR interstitium mass balance:
LFR plasma mass balance:
LFR interstitium mass balance:
Calculation of Arterial Toxin Concentration (C art)
During dialysis, blood is taken from the arterial port (C art) and rendered to dialyzer; after purification, it is infused back through the venous port. Blood sampling is limited to this arterial blood, which is not purely from HFR or LFR; therefore, an expression relating arterial concentration to HFR and LFR plasma concentration is needed. According to Fig. 2, plasma mass balance across dialyzer (in lumen side) can be described by:
Fluid (blood/plasma) balance across arterial node (‘A’ in Fig. 2):
Plasma mass balance across venous node (‘V’ in Fig. 2):
Rearrangement of (8) using (5) and (7) gives:
Volume Balance During Dialysis
During dialysis fluid is removed from both IC and EC (interstitium + plasma). Fraction ‘e’ of total ultrafiltered fluid comes from EC and rest from IC. The fluid removal from EC is further divided into two components, (i) removal from interstitial compartment and (ii) removal from plasma compartment, based on plasma volume fraction in EC (f P). It is assumed that fluid removal from any compartment will be in proportion of that compartment fluid volume.22,26
HFR plasma volume depletion:
HFR interstitial volume depletion:
LFR plasma volume depletion:
LFR interstitial volume depletion:
Summing up all the volume balance equations (Eqs. 10–13) will give the total fluid removal from EC.
Auxiliary Equations
Sum of HFR and LFR volume fraction must be equal to 1. Therefore,
A part of cardiac output goes to dialyzer (Q b), and rest goes in systemic circulation (Fig. 2). In blood, β2M is found in plasma only, so, only a portion of systemic circulation participates in mass transfer which is called as systemic plasma (Q s).
This systemic plasma is sub-divided into two parts, one going to HFR and other to LFR, thus plasma flow fraction to HFR and LFR can be written as,
Sum of blood flow fraction to HFR and LFR must be equal to 1. Therefore,
Model Equations During Inter-dialysis Period
During inter-dialysis period, all the equations are modified by setting dialyzer clearance (K D), ultrafiltration rate (Q uf), and blood flow to dialyzer (Q b) to zero. It is assumed that distribution of fluid intake will be in proportion of compartmental fluid volume, i.e., part of fluid intake, proportional to intracellular fluid volume will move to IC. Sample equation describing the HFR plasma compartment is shown below; toxin accumulation in HFR plasma compartment depends on (i) diffusive flux from interstitium to plasma, (ii) toxin transfer from plasma to systemic circulation, (iii) non-renal clearance from plasma compartment, (iv) constant toxin generation, and (v) convective flux from plasma to interstitium with fluid intake. Similar equations hold for mass and volume balances in other compartments as well.
HFR plasma mass balance:
HFR plasma volume balance:
Materials and Methods
Patients and Data Used
Patient data were obtained from a previously published study of 10 patients (8 men and 2 women) treated with post-dilution HDF.26 Blood and dialysate flow rates were kept constant at 280 and 500 mL min−1, respectively. Total filtration volume was set at 18 L, and net fluid removal was set according to patients’ clinical needs. Treatment time was 240 min for all patients. Blood samples from arterial line were collected at the beginning of dialysis and subsequently at 60, 120, and 240 min during the session. Immediately after HDF, a sample was collected 20 s later; subsequent samples were collected at 5, 10, 30, 60, 90, 120, and 240 min for capturing the post-dialysis rebound. To calculate dialyzer clearance (K D), a venous blood sample at dialyzer exit point was also collected at the 60th min. Blood sample just before the next treatment session was collected for each patient to calculate toxin generation rate (\( G_{{\beta_{2} {\text{M}}}} \)).
Parameter Reduction
The developed model comprised seven unknown parameters, namely, inter-compartmental clearance (K ip), toxin distribution volume (V d), cardiac output (CO), HFR flow fraction (f h), HFR volume fraction (k h ), EC volume fraction (e), and plasma volume fraction in EC (f P). It is not possible to estimate all the parameters with precision from limited patient data, and one should replace weakly identifiable parameters with constants. To reduce the number of parameters, a priori identifiability analysis28 was employed for the developed model. A priori identifiability analysis helps to determine the subset of potentially identifiable parameters, and is based on the calculation of parametric sensitivities (Eq. 20). Large sensitivity value indicates the strong influence of that parameter on measured output state, or the parameter can be better estimated from available data.
Scaled sensitivities were calculated at each sample time point to form the scaled sensitivity matrix, which was evaluated for obtained parameter estimates.28
Absolute sum of elements in all columns of matrix Z provides the basis to identify the significant parameters. Larger column sum suggests that corresponding sensitivities are large, i.e., change in state (C art) with respect to change in the parameter is significant; so, the corresponding parameter is very important. After selecting the most significant parameter, scaled sensitivity matrix is deflated (i.e., removing inter-dependence among parametric sensitivities to evaluate the “net influence” of each parameter) using the column corresponding to the largest sum, and second important parameter is then obtained (see the Supplementary Material for calculation details). This process is iterative and continued till all the parameters are ranked according to their column sum.28
Estimation of Removed Toxin Mass
The developed model was used to estimate the removed toxin mass during HDF. This calculation can be performed using initial (at t = 0 min) and final toxin concentration after dialysis (at t = 240 min) and obtained estimates of toxin distribution volume,
However, above formulation will overestimate the removed toxin mass due to post-dialysis rebound. Thus, adjusting for post-dialysis rebound will result in,
where, C 0, C 240, and C 480, represent pre-dialysis toxin concentration (at t = 0), concentration at the end of dialysis (at t = 240 min), and concentration after 240 min of dialysis (at 480th min from the beginning of dialysis), respectively. We have further calculated the effect of change in each parameter on removed toxin mass. The details are included in Supplementary Material.
Simulating Effect of Exercise
To demonstrate the clinical application of developed model, the effect of intra-dialytic exercise on toxin rebound was simulated. Exercise was given to in silico patients at t = 150 min, and sustained till the end of HDF session (t = 240 min). It is hypothesized that intra-dialytic exercise increases both CO and K ip, and to compare their individual effect on toxin concentration, increase in both factors was studied independently. (i) The model was simulated for CO = 6 L min−1 (without exercise) and CO = 12 L min−1 (with intra-dialytic exercise,21) keeping K ip constant. To simulate the toxin kinetics during exercise, we kept the HFR blood flow same as in no exercise condition,21 thus rest of the increased cardiac output will perfuse LFR. (ii) To observe the effect of increase in K ip, a small increase of 15% was studied (keeping CO constant at 6 L min−1). In all the cases, the post-dialysis rebound was calculated as follows13:
Results
It can be observed that developed DA-RBF model suffers from the problem of having too many parameters; therefore a priori identifiability analysis was used to find the precisely identifiable parameters in the system. We found that the effect of change in CO, f h, e, and k h on arterial toxin concentration (C art) was very small in comparison to the effect of change in V d, f P, and K ip. Quantitatively, for Patient 1, absolute sum of columns corresponding to V d, f P, K ip, e, k h, CO, and f h are 5.30, 1.33, 0.45, 0.005, 0.001, 0.0008, and, 0.0001, respectively. It is evident from these results that column sum for e, k h, CO, and f h are much lower than that of V d, f P, and K ip. Similar results were observed for other patients too. These results reveal that arterial toxin concentration (C art) is little sensitive to change in e, k h, CO, and f h. Hence, we concluded that these were less sensitive parameters in the developed model, and cannot be estimated with precision from the present patient data. Thus, they were replaced by constants based on literature evidences (Table 1). Only V d, f P, and K ip were considered as unknown model parameters.
The estimated post-dialysis toxin distribution volume (V d) was 14.22 ± 0.75 L (equivalent to 20.3 ± 1.3% of end-dialysis body weight), plasma fraction in EC (f P) was 0.39 ± 0.03, and inter-compartmental mass transfer coefficient (K ip) was 44 ± 4.1 mL min−1. Estimated values of model parameters for each patient are listed in Table 2. For comparison, parameter estimates from two-compartment model approach are also presented in Table 2.26 Measured toxin concentrations and model fit is shown in Fig. 3, while toxin concentration in each compartment is illustrated in Fig. 4 (for Patients 1 and 10). Obtained estimates of V d were bigger than those obtained from the two-pool model (10.2 ± 0.6 L). Also, the obtained estimates for f P were bigger than f P of 0.25 in normal subjects. Toxin generation rate was 0.131 ± 0.007 mg min−1, which was calculated using estimated V d and toxin concentration measured at the beginning of next dialysis session. The estimates of removed toxin mass using the developed model were 196.31 ± 19.7 mg, which are higher than 141.45 ± 14.17 mg, obtained by the existing two pool model (Table 3). All these values are presented as mean ± SEM (standard error of mean).
The developed model was used to study the effect of intra-dialytic exercise (see “Simulating Effect of Exercise” section). Individual effect of factors, i.e., increase in CO (keeping K ip constant) and increase in K ip (keeping CO constant) was studied. Difference in percentage rebound was calculated for CO = 6 and 12 L min−1; similar calculations were performed between nominal K ip and 15% increased K ip. Quantitative results corresponding to both scenarios are presented in Table 4. Figure 5 illustrates the effect of increase in CO.
Discussion
Developing β2M removal strategies require an understanding of its kinetics.1 Various kinetic models have been proposed to describe β2M kinetics during hemodialysis or HDF.11,13,22,26 The single pool model is too simple to account for post-dialysis rebound. The two-pool model is widely used by the research community, but its clinical relevance is not much (as explained earlier). To overcome this limitation, the present study sets out to develop a reduced parameter DA-RBF model for β2M, and evaluate its validity with the real patient data. We have customized the existing DA-RBF model to satisfy the requirements of β2M (distributed in extracellular space). To make it more realistic, we have modified the assumption made in the two-pool model studies,26 which is discussed in the following. Previous studies have assumed the removal of accumulated fluid from EC alone,22,26 which is infeasible, as fluid removal from blood compartment will induce the fluid movement from interstitial compartment and then from IC. Hence, net fluid removal is considered from both EC and IC. Further, it is assumed that fluid removal from any compartment will be in proportion of that compartmental fluid volume.11 Hence, fluid removal from EC will be in proportion of volume fraction of EC (e), which was later assumed to be a constant value based on identifiability analysis. We have assumed that about 33% of fluid is removed from EC and the remaining 67% comes from IC.4,5,11 Considering this information, EC volume fraction (e) was replaced by factor of 1/3 in the equations for rate of volume depletion.
The reduced DA-RBF model adopts similar assumptions as in the two-pool study.26 In the absence of any literature evidence, membrane sieving coefficient was considered as one, inferred from the study by Harper et al., where they revealed in vivo that uremia enhances the membrane permeability.9 However, as dialysis progresses, uremia decreases; hence, membrane permeability and K ip should continuously decrease. Nevertheless, we considered sieving coefficient and K ip as constant in our study, which is the assumption in all previously developed kinetic models for urea, creatinine, and β2M. This may not be valid in reality. Experimental studies are required to find out how the sieving coefficient and K ip changes with the decrease in uremia. Secondly, even though the RBF model can account for cardiopulmonary (Q cr) and access recirculation (Q ar), they are neglected in the current study due to the absence of relevant individual patient data, and to make a valid comparison between the outputs of the developed model and that of the existing two-pool model.26 Q cr and Q ar dilute the inlet blood to dialyzer and reduce the toxin concentration. As a result of this reduced concentration, toxin removal or access clearance reduces, or post-dialysis toxin concentration increases. This leads to reduction in measure of dialysis adequacy (K d t/V d). As dialyzer clearance (K d) and time of dialysis (t) are independent of recirculation, only increase in distribution volume (V d) can explain the decreased adequacy index. Hence, inclusion of Q cr and Q ar will result in larger estimates of toxin distribution volume.3,18
Toxin generation was assumed to be in both interstitium and plasma compartments, and it was calculated after estimating toxin distribution volume. Toxin concentration at t = 480 min (with t = 0 min denoting start of dialysis), and pre-dialysis toxin concentration measured before next dialysis session were used for this purpose (assuming constant toxin generation rate). The calculated generation rate (0.131 ± 0.007 mg min−1) is similar to the results obtained for the same patient group using the two-pool study (0.136 ± 0.008 mg min−1),26 and in other hemodialysis subjects using the two-pool modeling approach (0.132 ± 0.006 mg min−1).14 Estimated inter-compartmental clearance (K ip) between interstitium and plasma compartment was 44 ± 4.1 mL min−1, which is much smaller than reported value of 82 ± 7 mL min−1 in the two-compartment study.26 In this study, measured dialyzer clearance (K D) of β2M was 73 ± 2 mL min−1. Despite this high K D, mass transfer is limited by smaller K ip, which is evident from the obtained parameter estimates for individual patient, i.e., toxin removal is primarily controlled by membrane resistance. The smaller value of K ip than K D explains, why convection-based dialysis (HDF) does not result into significantly improved toxin removal, and are limited to toxin removal from blood compartment only.
The toxin distribution volume for β2M obtained from fitting the model to experimental data (14.22 ± 0.75 L or 20.3 ± 1.3% of end-dialysis body weight) is greater than that estimated by anthropometric formulae given by Watson et al.,27 (12.62 ± 0.57 L or 17.6 ± 0.4% of end-dialysis body weight). The difference could be because anthropometric formulae were derived for normal subjects, and not for renal patients, who always have excess fluid. On the other hand, significant difference was observed between the results obtained here and from the two-pool study for V d (10.2 ± 0.6 L).26 This can be attributed to the improved physiological representation in the form of DA-RBF model, and our assumption that about 33% of ultrafiltered volume comes from EC and rest comes from IC. The higher estimated value of V d in the DA-RBF model results in a bigger estimates of removed toxin mass when compared with that obtained by the existing two-pool model26 (Table 3). In clinical scenario, one can invalidate one of the two models (the two pool model and developed DA-RBF model) by measuring the toxin concentration in spent dialysate. The correct (validated) model can be used to decide the dialysis dose. The parameter estimates for plasma fraction in EC are 0.39 ± 0.03, which is greater than plasma fraction found in normal subjects. It can be understood that this excess fluid contributes to blood volume; thus obtained parameter estimates also explain the reason for most renal patients being hypertensive. Specifically, f P for Patient 8 (0.54) was much higher than rest of the patients, probably because there is no fluid removal for this patient during dialysis (pre- and post-dialysis weight were same). The model fit for two example patients is shown in Fig. 3. Additionally, Fig. 4 gives the concentration profiles in various compartments. There is consistent difference between concentrations of C hp and C lp from the onset of dialysis, as the unequal distribution of cardiac output creates a concentration difference between HFR and LFR. Nevertheless, one can observe that HFR and LFR plasma concentration equilibrate immediately after dialysis due to systemic circulation (Fig. 4). This explains the cause of sharp rebound after dialysis. One can also observe that major contribution to rebound is concentration difference between interstitium and plasma compartment. In summary, the developed model gives better insight into toxin distribution in various compartments. The developed model can be employed for precise estimation of toxin distribution volume, i.e., extracellular fluid volume, which is one of the greatest challenges to practicing nephrologists.10 This may further help in accurate dialysis dose prediction for hemodialysis subjects based on modified dialysis adequacy index considering β2M (\( Kt/V_{{\beta_{2} {\text{M}}}} \)) as a global marker of toxin milieu.
Subsequently, the developed model was employed to explain the effect of intra-dialytic exercise. Intra-dialytic exercise, given to lower extremities which constitute LFR, increases the cardiac output. Smye et al. employed the simple RBF model and demonstrated via simulations that increase in cardiac output or increased perfusion of the skeletal muscles results in reduced post-dialysis rebound.21 Positive outcomes of intra-dialytic exercise have been observed experimentally as well.17,24 It has been suggested in the past that as a result of intra-dialytic exercise, a large fraction of increased CO reaches the LFR, where large fraction of toxin is present in comparison to HFR.8 This increased perfusion of LFR results in higher toxin removal through convection, and thus reduced post-dialysis rebound. To observe the same, the effect of increased CO (6 L min−1 (normal condition) to 12 L min−1 (during exercise),21) on arterial plasma concentration is studied for all 10 patients. Quantitatively, the 100% increase of CO results in only ~1% decrease in post-dialysis toxin concentration at t = 480 min. Similar results were observed for rest of the patients too (Table 4). Figure 5 shows the effect of 100% increase in cardiac output due to intra-dialytic exercise. This leads to the question—how does intra-dialytic exercise lead to reduced rebound when the effect of increased cardiac output is not significant?
To explain this, it is hypothesized that exercise to lower extremities leads to increase in both CO and K ip. Exercise to lower extremity causes increased blood flow to LFR, which results in dilated capillaries/membranes or increased surface area,17 so that the excess blood flow can be accommodated. This dilation will further lead to larger membrane pore size. These two reasons, namely, increased surface area of capillaries and increased membrane pore size, result in increased inter-compartmental mass transfer coefficient (K ip), and hence more toxin transfer to vascular compartment. This transferred toxin will be swept away by increased blood flow. This was verified by simulating the individual patient model for a hypothetical increase of 15% in K ip (keeping CO = 6 L min−1). Corresponding results are presented in Table 4. To substantiate this hypothesis, further clinical studies need to be carried out so as to segregate the effect of CO and K ip on arterial toxin plasma concentration, and formulating a relationship between the effect of exercise and toxin removal. This clinical study can also help in quantifying the effect of exercise on inter-compartmental clearance. We have also simulated the stand-alone HDF and HDF with exercise regimen. For simulating exercise effect, we varied both CO and K ip, as both change concomitantly. CO was increased to 12 L min−1,21 and K ip was increased between 10 and 70% of its nominal value. As anticipated, HDF with exercise protocol results in reduced toxin rebound than the standalone HDF (see the Supplementary Material). This indicates the intra-dialytic exercise can further improve the outcomes of HDF, which can be clinically relevant in long term.
In conclusion, the reduced parameter DA-RBF results in improved understanding of β2-microglobulin kinetics. Based on a priori identifiability analysis, it is found that cardiac output (CO), flow fraction to HFR (f h), fractional volume of EC (e), and fractional volume of HFR (k h) are less important model parameters, while toxin distribution volume (V d), plasma fraction in EC (f P), and inter-compartmental clearance (K ip) are more important parameters. Hence the number of estimable parameters is reduced from seven to three. We found that the estimated V d is greater than the estimates obtained by two-compartment models, which can be understood by the fact that patients on maintenance hemodialysis are fluid overloaded, and our assumption of fluid removal from both EC and IC. Estimates of f P suggested that more of this excess fluid stores in plasma compartment, which explains the reason for renal patients being hypertensive. The developed model resulted in higher estimates of removed toxin mass than that obtained by the two-pool model. This information can be used to validate the existing models and select the best representative of physiology. To demonstrate the clinical application of developed model, the effect of intra-dialytic exercise was examined. Based on the simulation results, it is hypothesized that intra-dialytic exercise not only increases the cardiac output but also increases the inter-compartmental mass transfer coefficient; both combined together results in lower post-dialysis rebound. After confirming the intra-dialytic exercise effect in clinical trials, the developed model can be beneficially utilized for systematic introduction of intra-dialytic exercise and synergizing its effect with dialysis to obtain improved patient outcomes.
Abbreviations
- α :
-
Fluid intake rate during inter-dialysis period (L min−1)
- C art :
-
Arterial toxin concentration (measured concentration) (mg L−1)
- C mn :
-
Toxin concentration in mth region, nth compartment (mg L−1) m \( \in \) [h,l], n \( \in \) [p,i]
- CO:
-
Cardiac output (L min−1)
- e :
-
Fluid volume fraction of extracellular space
- f m :
-
Blood flow fraction to mth region
- f P :
-
Fluid volume fraction of plasma compartment in extracellular space
- \( G_{{\beta_{2} {\text{M}}}} \) :
-
β2-Microglobulin generation rate (mg min−1)
- HCT:
-
Hematocrit
- K D :
-
Dialyzer clearance (mL min−1)
- K ip :
-
Inter-compartmental mass transfer coefficient (mL min−1)
- k m :
-
Fluid volume fraction of mth region
- K NR :
-
Non-renal clearance (mL min−1)
- Q b/Q bp :
-
Blood/plasma flow to dialyzer (L min−1)
- Q h/Q l :
-
Systemic blood flow to high/low flow region (L min−1)
- Q hp/Q lp :
-
Systemic plasma flow to high/low flow region (L min−1)
- Q s :
-
Systemic plasma flow (L min−1)
- Q uf :
-
Constant ultrafiltration rate (L min−1)
- V d :
-
Toxin distribution volume (L)
- V mn :
-
Fluid volume in mth region, nth compartment (L)
- Z :
-
Scaled sensitivity matrix
- h/l:
-
High/Low flow region
- p/i:
-
Plasma/Interstitium compartment
References
Canaud, B., M. Morena, J. P. Cristol, and D. Krieter. β2-Microglobulin, a uremic toxin with a double meaning. Kidney Int. 69:1297–1299, 2006.
Cheung, A. K., M. V. Rocco, G. Yan, J. K. Leypoldt, N. W. Levin, T. Greene, L. Agodoa, J. Bailey, G. J. Beck, W. Clark, A. S. Levey, D. B. Ornt, G. Schulman, S. Schwab, B. Teehan, and G. Eknoyan. Serum β2-microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J. Am. Soc. Nephrol. 17:546–555, 2006.
Daugirdas, J., D. Schneditz, and D. Leehey. Effect of access recirculation on the modeled urea distribution volume. Am. J. Kidney Dis. 27:512–518, 1996.
Debowska, M., B. Lindholm, and J. Waniewski. Adequacy indices for dialysis in acute renal failure: kinetic modeling. Artif. Organs 34:412–419, 2010.
Debowska, M., J. Waniewski, and B. Lindholm. Bimodal dialysis: theoretical and computational investigations of adequacy indices for combined use of peritoneal dialysis and hemodialysis. ASAIO J. 53:566–575, 2007.
Drüeke, T. B., and Z. A. Massy. Progress in uremis toxin research: β2-microglobulin. Semin. Dial. 22:378–380, 2009.
Farrell, J., and B. Bastani. Beta 2-microglobulin amyloidosis in chronic dialysis patients: a case report and review of the literature. J. Am. Soc. Nephrol. 8:509–514, 1997.
George, T. O., A. Priester-Coary, G. Dunea, D. Schneditz, N. Tarif, and J. T. Daugirdas. Cardiac output and urea kinetics in dialysis patients: evidence supporting the regional blood flow model. Kidney Int. 50:1273–1277, 1996.
Harper, S. J., C. R. V. Tomson, and D. O. Bates. Human uremic plasma increases microvascular permeability to water and proteins in vivo. Kidney Int. 61:1416–1422, 2002.
Ishibe, S., and A. J. Peixoto. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: implications in clinical practice. Semin. Dial. 17:37–43, 2004.
Kanamori, T., and K. Sakai. An estimate of β2-microglobulin deposition rate in uremic patients on hemodialysis using a mathematical kinetic model. Kidney Int. 47:1453–1457, 1995.
Korohoda, P. Flow based two-compartment models—a comparative computational study. World Congress on Medical Physics and Biomedical Engineering. Berlin, Heidelberg, Munich, Germany: Springer, pp. 838–841, 2009.
Leypoldt, J. K., A. K. Cheung, and R. B. Deeter. Rebound kinetics of β2-microglobulin after hemodialysis. Kidney Int. 56:1571–1577, 1999.
Maeda, K., T. Shinzato, T. Ota, H. Kobayakawa, I. Takai, Y. Fujita, and H. Morita. Beta-2 microglobulin generation rate and clearance rate in maintenance hemodialysis patients. Nephron 56:118–125, 1990.
Miyata, T., M. Jadoul, K. Kurokawa, and C. Van Ypersele de Strihou. Beta-2 microglobulin in renal disease. J. Am. Soc. Nephrol. 9:1723–1735, 1998.
Okuno, S., E. Ishimura, K. Kohno, Y. Fujino-Katoh, Y. Maeno, T. Yamakawa, M. Inaba, and Y. Nishizawa. Serum β2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 24:571–577, 2009.
Parsons, T. L., E. B. Toffelmire, and C. E. King-Van Vlack. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch. Phys. Med. Rehabil. 87:680–687, 2006.
Schneditz, D., A. M. Kaufman, H. D. Polaschegg, N. W. Levin, and J. T. Daugirdas. Cardiopulmonary recirculation during hemodialysis. Kidney Int. 42:1450–1456, 1992.
Schneditz, D., D. Platzer, and J. T. Daugirdas. A diffusion-adjusted regional blood flow model to predict solute kinetics during haemodialysis. Nephrol. Dial. Transplant. 24:2218–2224, 2009.
Schneditz, D., J. C. Van Stone, and J. T. Daugirdas. A regional blood circulation alternative to in-series two compartment urea kinetic modeling. ASAIO J. 39:M573–M577, 1993.
Smye, S., E. Lindley, and E. Will. Simulating the effect of exercise on urea clearance in hemodialysis. J. Am. Soc. Nephrol. 9:128–132, 1998.
Stiller, S., X. Q. Xu, N. Gruner, J. Vienken, and H. Mann. Validation of a two-pool model for the kinetics of β2-microglobulin. Int. J. Artif. Organs 25:411–420, 2002.
Sunny, E., T. An, S. Rita De, M. Bart, D. Peter Paul De, V. Pascal, and V. Raymond. Complex compartmental behavior of small water-soluble uremic retention solutes: evaluation by direct measurements in plasma and erythrocytes. Am. J. Kidney Dis. 50:279–288, 2007.
Vaithilingam, I., K. R. Polkinghorne, R. C. Atkins, and P. G. Kerr. Time and exercise improve phosphate removal in hemodialysis patients. Am. J. Kidney Dis. 43:85–89, 2004.
Vanholder, R., S. Eloot, and W. Van Biesen. Do we need new indicators of dialysis adequacy based on middle-molecule removal? Nat. Clin. Pract. Nephrol. 4:174–175, 2008.
Ward, R. A., T. Greene, B. Hartmann, and W. Samtleben. Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 69:1431–1437, 2006.
Watson, P., I. Watson, and R. Batt. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am. J. Clin. Nutr. 33:27–39, 1980.
Yao, K. Z., B. M. Shaw, B. Kou, K. B. McAuley, and D. W. Bacon. Modeling ethylene/butene copolymerization with multi-site catalysts: parameter estimability and experimental design. Polym. React. Eng. 11:563–588, 2003.
Yashiro, M., H. Watanabe, and E. Muso. Simulation of post-dialysis urea rebound using regional flow model. Clin. Exp. Nephrol. 8:139–145, 2004.
Yavuz, A., C. Tetta, F. F. Ersoy, V. D’intini, R. Ratanarat, M. D. Cal, M. Bonello, V. Bordoni, G. Salvatori, E. Andrikos, G. Yakupoglu, N. W. Levin, and C. Ronco. Uremic toxins: a new focus on an old subject. Semin. Dial. 18:203–211, 2005.
Ziolko, M., J. A. Pietrzyk, and J. Grabska-Chrzastowska. Accuracy of hemodialysis modeling. Kidney Int. 57:1152–1163, 2000.
Acknowledgments
We thank Dr. Richard A. Ward (University of Louisville, USA) for providing the de-identified patient data for testing the developed model. We also acknowledge Dr. Titus Lau and Dr. Kheng Boon Lim (National University Hospital, Singapore) for their valuable comments and suggestions.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Gerald Saidel oversaw the review of this article.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10439-012-0547-y.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maheshwari, V., Samavedham, L. & Rangaiah, G.P. A Regional Blood Flow Model for β2-Microglobulin Kinetics and for Simulating Intra-dialytic Exercise Effect. Ann Biomed Eng 39, 2879–2890 (2011). https://doi.org/10.1007/s10439-011-0383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0383-5