Abstract
Understanding how vascular wall endothelial cells (ECs), smooth muscle cells (SMCs), and fibroblasts (FBs) sense and transduce the stimuli of hemodynamic forces (shear stress, cyclic strain, and hydrostatic pressure) into intracellular biochemical signals is critical to prevent vascular disease development and progression. ECs lining the vessel lumen directly sense alterations in blood flow shear stress and then communicate with medial SMCs and adventitial FBs to regulate vessel function and disease. Shear stress mechanotransduction in ECs has been extensively studied and reviewed. In the case of endothelial damage, blood flow shear stress may directly act on the superficial layer of SMCs and transmural interstitial flow may be elevated on medial SMCs and adventitial FBs. Therefore, it is also important to investigate direct shear effects on vascular SMCs as well as FBs. The work published in the last two decades has shown that shear stress and interstitial flow have significant influences on vascular SMCs and FBs. This review summarizes work that considered direct shear effects on SMCs and FBs and provides the first comprehensive overview of the underlying mechanisms that modulate SMC secretion, alignment, contraction, proliferation, apoptosis, differentiation, and migration in response to 2-dimensional (2D) laminar, pulsatile, and oscillating flow shear stresses and 3D interstitial flow. A mechanistic model of flow sensing by SMCs is also provided to elucidate possible mechanotransduction pathways through surface glycocalyx, integrins, membrane receptors, ion channels, and primary cilia. Understanding flow-mediated mechanotransduction in SMCs and FBs and the interplay with ECs should be helpful in exploring strategies to prevent flow-initiated atherosclerosis and neointima formation and has implications in vascular tissue engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Smooth Muscle Cells and Fibroblasts in Vascular Remodeling and Disease
The major functions of vascular smooth muscle cells (SMCs) in the medial layer of the arterial wall are to maintain and regulate blood vessel tone, blood pressure, and blood flow distribution.62 Within adult blood vessels, SMCs have an extremely low proliferation rate and synthetic activity, and express a unique repertoire of contractile proteins required for cell contractile function.63 However, SMCs possess remarkable plasticity that allows rather profound and reversible changes in phenotype between a contractile state and a synthetic state in response to alterations in local environmental cues that plays a crucial role in vascular repair and remodeling.49,63,72,79 Furthermore, SMCs may contribute to vascular lesion formation, including neointima formation and atherosclerosis by activation and migration from the media into the intima under abnormal environmental conditions.20,49,63
In vivo work has shown that adventitial fibroblasts (FBs) and their activated counterpart, myofibroblasts (MFBs), also contribute to neointima formation following vascular injury.42,86 In response to vascular injury, in a manner similar to medial SMCs, adventitial FBs can be rapidly activated and undergo dramatic changes in phenotype, proliferation, and migration, that contribute to neointima formation.80,94
Neointima formation is often induced in regions where the endothelium has been damaged by vascular procedures such as angioplasty or at the anastomoses of vascular grafts. In addition, atherosclerosis occurs at sites where the blood flow is disturbed.51,53,81,102 Both conditions involve multiple processes including endothelial dysfunction, inflammation, vascular SMC and FB proliferation and migration and matrix alteration.14 Figure 1 shows the contribution of vascular SMCs and FBs to vascular lesion formation in response to vascular injury.
A model for SMC and FB contributions to neointima formation in response to injury. During vascular injury, contractile SMCs switch their phenotype to a synthetic state and migrate into the intima; adventitial FBs are activated and become MFBs and migrate into the intima across the media; in the intima, both SMCs and MFBs proliferate and secrete new ECM, forming neointima. IEL: internal elastic lamina; EEL: external elastic lamina (modified from Sartore et al.80)
SMCs and FBs Are Exposed to Fluid Flow and Shear Stress After Vascular Injury
Vascular SMCs normally reside in a 3-dimensional (3D) environment composed of ECM components mainly collagen and elastic fibers. SMCs are not normally exposed directly to the shear stresses of flowing blood in the vascular system, because the endothelial cell (EC) layer which lines all blood vessels provides the contacting surface for blood flow and the underlying SMCs are shielded. However, in cases of endothelial injury and denudation, the superficial layer of SMCs is exposed directly to blood flow shear stresses at similar levels that ECs experience in intact blood vessel.
A more subtle mechanism by which the medial SMCs are exposed to fluid shear stress is through transmural (interstitial) flow driven by the transvascular pressure differential (arterial pressure–tissue pressure) (Fig. 2). Because this transmural flow is typically very small (superficial velocity of the order 10−5 to 10−6 cm/s), its possible mechanical effects on SMCs remained unrecognized until Tarbell’s group estimated that maximum interstitial shear stresses on SMCs could be of order ~1 dyn/cm2.99–101,109 Their theory, based on cylindrical cells suspended in a Darcy media, led to the following estimate for the interstitial flow shear stress (τ):
where μ is the viscosity of interstitial fluid, U is the superficial flow velocity, and K p is the Darcy permeability of the tissues or porous media.
Interstitial flow and shear stress on SMCs and FBs after vascular injury. ECs directly contact blood flow. SMCs and FBs in the intact artery are not exposed to luminal blood flow shear stress, but are exposed to a very low physiological transmural interstitial flow. SMCs near the fenestral pores (FPs) experience higher transmural flow velocity and shear stress than those that are far away from the FPs. After endothelial denudation, the superficial SMCs may be exposed to blood flow, and the medial SMCs and adventitial FBs are exposed to elevated interstitial flow. The thickness of the dashed pink lines indicates the intensity of interstitial flow velocity. IEL: internal elastic lamina; EEL: external elastic lamina; FP: fenestral pore
However, SMCs near the fenestral pores may experience much higher transmural interstitial flow shear stresses due to the funneling effect of the pores.99 Furthermore, the interstitial flow shear stress on adventitial FBs should be lower than on SMCs, since the permeability of “loose” adventitia is higher than that of “dense” media.85 In addition, the adventitia of a large artery has its own microvessels (i.e., vasa vasorum and lymphatics). The pressure in the host artery lumen is higher than the pressure within these microvessels,76 resulting in a convective interstitial flow from the artery lumen towards the adventitia.52,76 The interstitial flow pattern within this loose adventitial layer may be very complicated, but the superficial flow velocity is expected to be rather small, resulting in a low flow velocity in the adventitial interstitium and a smaller shear stress on the adventitial FBs than on the medial SMCs.
As shown in Fig. 2, transmural interstitial flow and shear stress on SMCs and FBs are elevated after chemical or mechanical injury to endothelium and inflammation- or hypertension-induced enhancement of vascular permeability.52,77,102 For example, when the endothelium is denuded, the hydraulic conductance increases 2.5-fold in rabbit aortas6 and 1.75-fold in rat aortas,90 which leads to a proportionate increase in interstitial flow shear stress. During wound healing or vascular lesion formation, transmural interstitial flow on SMCs and FBs decreases. A recent study showed that early after endothelial denudation, medial SMCs are rapidly activated and dedifferentiated, while when intimal thickening appears, the majority of medial SMCs are no longer activated.49 Clearly, the activation profile of SMCs is concomitant with the change in interstitial flow intensity.
ECs sense primarily the blood flow shear stress on their luminal surface. Alterations in hemodynamic environment can be directly sensed by ECs which then communicate to the underlying SMCs and adventitial FBs through paracrine chemical signals to regulate vascular function and disease.12,21,27,28,58,105 In an analogous manner, after vascular injury blood flow shear stress and transmural interstitial flow have direct mechanical influences on SMCs and FBs modulating their vasoactive molecule release, contraction, proliferation, phenotype, and migration and thus play direct roles in vascular function, vascular remodeling, and vascular lesion formation.77,85 Most studies of direct shear effects on SMCs have been conducted in vitro, since it is difficult to study direct shear effects on vascular SMCs in vivo due to their location in the vessel wall. The biological effects of fluid flow shear stress on SMCs have been addressed in a number of studies in 2-dimensions (2D) for two decades. The effects of more subtle 3D interstitial flow on SMCs have not gained much attention until recently. This review summarizes these 2D and 3D studies in the following sections and closes with a section on conclusions and future directions.
Shear Stress Stimulates Cytokines and Signaling Molecules in SMCs
Cytokines and vasoactive mediators such as growth factors, nitric oxide (NO), prostaglandin, and other molecules can be secreted by both ECs and SMCs and play critical roles in vessel function and disease. Bodin et al.7 showed that, unlike vascular ECs, SMCs did not increase release of adenosine triphosphate (ATP) in response to increased flow. However, shear stress (3–25 dyn/cm2) promotes both platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF or FGF-2) release from SMCs.73,96,97 The increased secretion of bFGF may be involved in shear stress-induced angiotensin converting enzyme (ACE) expression.22 In 2D, shear stress (1 dyn/cm2) can induce a significant up-regulation of prostaglandin (PGE2 and PGI2) production.3 Interstitial flow can also induce prostaglandin production in SMCs in 3D, however, the production rate is much lower than observed in 2D.110 Papadaki et al.66 presented data indicating that high shear stress down regulates human protease activated receptor-1 (PAR-1) expression, whereas low shear stress up regulates it, consistent with the known variations in human PAR-1 expression in vascular injury and atherosclerosis. In contrast, they showed that tissue plasminogen activator (tPA) expression increases in areas of high shear stress and decreases in areas of low shear stress, where thrombus is likely to form.66 Shear stress can induce NO production via upregulation of neuronal or inducible nitric oxide synthase (NOS),23,67 and NO may crosstalk with prostacyclin.61 Contradictory to these studies, Wagner et al.108 observed no induction of inducible NOS in response to fluid shear stress.
Fluid shear stress may perturb the Na+/H+ exchanger leading to an increase in pH in SMCs, which is opposite to that in ECs at the same levels of shear stress.91 Turbulent flow can promote Na+ and cholesterol uptake in SMCs.78 Laminar shear stress also increases Ca2+ influx.82 In addition, laminar shear stress promotes translocation of TRPM7, a member of the transient receptor potential (TRP) family of cation channels, to the plasma membrane of A7R5 aortic SMCs, resulting in increased channel activity and increased influx of Ca2+ and Mg2+.24,57 In contrast to ECs, vascular SMCs have high levels of TRPM7-like current and TRPM7 protein that can be significantly activated by flow.24,57
In vivo, the release of cytokines and alterations in signaling molecules cannot only affect SMC functions via an autocrine pathway, but may also influence other cell types such as ECs and FBs through a paracrine pathway. The ability of shear stress to regulate cytokines, vasoactive mediators, and other signaling molecules in vascular SMCs indicates that shear effects on SMCs may play important roles in maintaining vascular homeostasis and modulating vascular pathologies.
Flow Induces SMC Alignment and Contraction
Fluid Flow Induces SMC Alignment
Vascular SMCs and ECs are arranged in distinct patterns in the artery wall. Different types of mechanical stimuli have been shown to regulate vascular cell morphology. Fluid shear stress induces EC orientation and elongation parallel with flow.8,40 However, Lee et al.38 demonstrated that laminar shear stress (20 dyn/cm2 for 48 h) induced perpendicular alignment of SMCs to flow, which varied with the magnitude of and exposure time to shear stress. The alignment of SMCs was also dependent on Ca2+ and cytoskeleton-based mechanisms.38 Rice et al.74 showed that ~10 dyn/cm2 of shear stress induced SMC aligned ~45° to the flow direction after 24 h. After exposure to pulsatile strain and shear stress, SMCs seeded onto 3D polymer scaffolds were aligned circumferentially, similar to that of native vascular SMCs.33 Interstitial flow induces dermal FB alignment perpendicular to flow in 3D collagen gels.55 In vivo studies show that when applying non-uniform blood flow shear stress on ECs in a vascular polymer implant, SMCs in the implant align perpendicular to luminal flow (parallel to transmural flow) and migrate toward the lumen, while uniform shear stress does not significantly affect SMC alignment and migration.47,48
The circumferential orientation of SMCs is important for blood vessels to resist the hoop stresses induced by the blood pressure. Changes in orientation may thus cause blood vessel dysfunction. A better understanding and control of SMC alignment under flow has implications for vascular tissue engineering.
Fluid Flow Induces SMC Contraction in 2D and 3D
The major function of vascular SMCs is contraction. Therefore, it is important to investigate whether fluid shear stress plays any role in myogenic response and flow-mediated vasomotion. Civelek et al.9 presented the first direct evidence showing that SMCs in a contractile phenotype will indeed contract when exposed to fluid shear stress in 2D. The contractile phenotype of SMCs was induced by removal of serum from normal growth medium. The shear-induced contraction response is regulated by a Rho kinase-mediated myosin light chain phosphatase (MLCP) pathway and independent of Ca2+.9 This is consistent with studies of the myogenic response (reduction in vessel diameter after an increase in pressure) in vivo that show a Ca2+-independent contraction that is independent of vessel stretch31 and is regulated by transvascular interstitial flow.35 Ainslie et al.1 further showed that the SMC surface glycocalyx components heparan sulfate and chondroitin sulfate may serve as sensors to regulate shear stress-induced contraction.
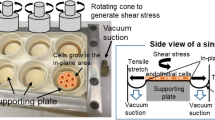
In unpublished experiments, we suspended serum-starved contractile rat aortic SMCs in 3D collagen type I gels, and after cell spreading, we treated the cells with either serum or KCl. We observed that both serum and KCl significantly induced collagen gel contraction (Fig. 3a). Using confocal microscopy, we found that SMCs were significantly contracted after exposure to serum for 30 min (Fig. 3b). Furthermore, when we applied interstitial flow (flow medium was DMEM without serum) to the SMCs in collagen gels, the cells contracted significantly after exposure to interstitial flow for 60 min (Fig. 3c). KCl induces SMC contraction via Ca2+-dependent mechanism,9 while both serum and 2D shear stress-induced SMC contraction are independent of Ca2+.2,9 It would be interesting to determine whether 3D interstitial flow-induced SMC contraction in collagen gels is also independent of Ca2+ and Rho-dependent. Our studies suggest that both laminar shear stress (2D) and interstitial flow (3D) can induce SMC contraction. These studies provide a new view of the mechanism of myogenic control of blood flow that regulates flow distribution in response to blood pressure changes. These studies also indicate that, in vascular injury, elevated shear stress on contractile SMCs may be able to induce vasoconstriction that limits bleeding.
Serum, KCl, and interstitial flow induce SMC contraction in 3D collagen gels. (a) Addition of FBS or KCl-induced collagen gel contraction. 200 μL of serum-starved SMCs and collagen mixture (cell density: 1 × 106 cells/mL; collagen concentration: 1.25 mg/mL) were loaded in 12-well cell culture inserts and incubated for 16 h. Then 250 μL of DMEM, fetal bovine serum (FBS, 10%) or KCl (110 mM) were added to the gels for 1 h. (b) Serum (10% FBS)-induced SMC contraction in collagen gels and cell shape tended to be less elongated at 30 min compared to 0 min; the average length to width ratio of the cells decreased to 38 ± 5% (20 cells in the focal plane were randomly picked for the measurement from each image; p < 0.001) (images were taken by a Leica SP2 confocal microscope with 10× lens and zoomed in four times; these two images are not from the same field). (c) Interstitial flow (10 cmH2O) induces SMC contraction in collagen gels; the average length of the cells shortened to 72 ± 7% (same 8 cells in the focal plane were measured in the two images; p < 0.001) compared to their initial average length (these two images were taken with 63× lens from the same field). Individual cell shortening is apparent in these fields
Fluid Shear Stress Affects SMC Proliferation and Survival
It has been shown that laminar shear stress activates signaling pathways that control EC to arrest in G0 or G1 phase, while disturbed flow accelerates EC turnover and low flow induces EC apoptosis.8,27 In 2D in vitro studies, laminar shear stress also reduces SMC proliferation,15,34,58,65,95,96,98,106 and inhibition of SMC proliferation may be mediated by transforming growth factor-beta 1 (TGF-β1).106 In vivo studies of vascular SMC growth rates after balloon catheter injury have demonstrated an inverse correlation between growth rates and shear stress,37 supporting the in vitro observations. Laminar shear stress can also induce SMC apoptosis by inhibiting Akt activity,16 increasing expression of SMC tissue factor pathway inhibitor-2 (TFPI-2),15 or via an autocrine Fas/FasL pathway.4
Other 2D studies, however, have shown that pulsatile or oscillatory shear stress can promote SMC proliferation.5,26,78,88 The increased SMC proliferation is regulated by shear stress-induced activation of Akt and ERK1/2.5,26 Another recent study revealed that laminar shear stress can also induce expression of transcription factor early growth response-1 (Egr-1) in SMCs.56 This induction is controlled by shear-induced c-Jun activation via an ERK1/2- and JNK-dependent and p38-independent mechanism.56
Fluid Flow Modulates SMC Phenotype
Effects of shear stress on vascular EC differentiation have been well reviewed.75 Shear stress can also modulate vascular SMC phenotype. It has been shown that shear stress induced in an orbital shaker reduces expression of SMC contractile markers (α-actin and calponin) and stimulates expression of synthetic phenotype markers (vimentin and β-actin).5 Other studies indicate that laminar shear stress can also decrease the levels of SMC markers (α-actin, calponin, SM-MHC, and SM22),83,111 while increasing expression of EC markers (PECAM-1, vWF, and VE-cadherin).111 The latter study suggests that shear stress might promote EC transdifferentiation from SMCs.111
Most recently, Shi et al.83 presented the first evidence that 3D interstitial flow also modulates expression of vascular SMC phenotypic markers. This study showed that interstitial flow (velocity: 0.5 μm/s; shear: ~0.05 dyn/cm2, 4.5 h) inhibits expression of SM-MHC, smoothelin, and calponin genes in 3D collagen, which is consistent with 2D laminar shear stress (average shear stress: 8 dyn/cm2; 15 h). However, in contrast to 2D laminar flow, interstitial flow enhances expression of α-actin and SM22 in 3D.83 The differential effects of laminar flow and interstitial flow may be due to the different initial phenotypic state of the SMCs, because SMCs cultured in 3D collagen display less spreading and less proliferation and express lower levels of α-actin.45,72,92 This study further demonstrates that modulation of SMC phenotype by both laminar flow and interstitial flow is dependent on mechanotransduction by heparan sulfate proteoglycan (HSPG)-mediated ERK1/2 activation.83 Interstitial flow also induces α-actin expression in both FBs and MFBs in 3D collagen.54,83
Other in vitro studies showed that when immature, dedifferentiated vascular SMCs were seeded into 3D polymer scaffolds and exposed to both pulsatile strain and shear stress at the same time, SMCs displayed significantly increased ECM production, proliferation, and SMC marker expression.33,60 An in vivo study also demonstrated that when rat mesenteric microvessels (<40 μm in diameter) are exposed to elevated pressure and wall strain for 5 to 10 days, the microvessels exhibit an enhanced coverage of mature and differentiated SMCs expressing SM-MHC and α-actin.107 Shear stress acting on ECs can induce synthetic-to-contractile phenotypic modulation of SMCs both in vitro and in vivo.59,105
Taken together, the studies in this section have suggested that, during vascular injury, interstitial flow and shear stress may have significant effects on vascular SMC phenotypic modulation and thus contribute to vascular remodeling or lesion formation. However, shear stress appears to induce endothelial differentiation from embryonic stem cells113 and bone marrow mesenchymal stem cells,13 while stretch induces stem cell differentiation towards SMC.89 Hydrostatic pressure promotes higher expression of SMC markers in stem cells than shear stress does,36 and elevated pressure and wall strain enhance SMC coverage of microvessels.107 These results indicate that mechanical strain and pressure may play more important roles than shear stress in vascular SMC development and arteriogenesis, while shear stress may be more important in vascular SMC pathology and disease.
Fluid Flow Regulates SMC and FB Migration
2D Shear Stress Affects SMC and FB Migration
Migration of vascular SMCs and FBs from the media and the adventitia play key roles in neointima formation, atherosclerosis, and restenosis. SMCs have displayed reduced migratory activity in response to elevated blood flow in a balloon catheter injury model in vivo.37 SMCs have also demonstrated inhibition of migration in response to laminar shear stress (12 dyn/cm2) in vitro via downregulation of matrix metalloproteinases (MMPs) and PDGF receptor-β.64 Garanich et al.19 observed that laminar shear stress (average ~15 dyn/cm2) suppressed SMC migration via NO-mediated downregulation of MMP-2 activity. Other studies, however, showed that pulsatile flow shear stress increases SMC migration in vitro.29,71 An in vivo vascular implant study indicated that vortex blood flow acting on ECs significantly induces SMCs migration via activation of ERK1/2 and myosin light chain kinase (MLCK).21 Garanich et al.18 revealed that shear stress could also inhibit MFB migration and promote FB migration. The enhancement of FB migration in response to shear stress suggests a pathophysiologic condition in which FBs are exposed to enhanced interstitial flow shear stress during arterial injury which stimulates their migration to the intima and accelerates lesion formation.
3D Interstitial Flow Promotes SMC and FB Motility
Fluid flow in the tissue interstitium is very low due to the resistance of ECM fibrils and cells.41 It has been shown, however, that such low flow can significantly affect cell physiology and function.11,30,32,83–85,87,110 Wang and Tarbell110 reported the first 3D interstitial flow effects on SMCs in vitro. They showed that there are dramatic differences in the cytokine release rates between cells in 2D and 3D models reinforcing the importance of studying vascular SMC and FB behavior in response to interstitial flow using realistic 3D in vitro models.
Recently, to investigate interstitial flow influences on SMC and FB motility, Shi and Tarbell established a 3D interstitial flow-cell migration assay using a modified Boyden chamber system.84,85,87 In these studies, the flow period (up to 6 h) was separated from the migration period (48 h) to minimize possible flow-induced autologous chemotaxis effects on cell migration.17,85,87 Using this 3D system, they have generated the following primary findings: (1) interstitial flow can promote rat vascular SMC, FB, and MFB motility in collagen gels by upregulation of rat interstitial collagenase (MMP-13)85; (2) high intensity interstitial flow suppresses vascular cell motility due to a combination of effects including enhanced expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and cell apoptosis and necrosis85; (3) flow-induced upregulation of MMP-13 is mediated by activation of ERK1/2 mitogen-activated protein kinase (MAPK) and the downstream transcription factor, activating protein-1 (AP-1), specifically c-Jun84; (4) interstitial flow also induces p38 MAPK activation, but it seems that p38 MAPK does not play a major role in flow-induced MMP expression and cell motility,84 supported by a recent report56; (5) furthermore, cell surface glycocalyx HSPGs sense interstitial flow, mediating activation of focal adhesion kinase (FAK) and ERK1/2 signaling axis87; and (6) finally, a conceptual mechanotransduction model was proposed in which cell surface glycocalyx, with cooperation of integrin-mediated cell–matrix adhesions and cytoskeleton rigidity, sense interstitial flow and activate the FAK-ERK axis, leading to upregulation of MMP-13 and cell motility in 3D.87 This is the first study to describe a flow-induced mechanotransduction mechanism in 3D. It should be a good starting point for understanding flow-related mechanobiology in vascular remodeling and disease, stem cell differentiation and has implications in tissue engineering.
In 2D flow studies, laminar shear stress enhances FB migration, but inhibits MFB and SMC migration using Matrigel, gelatin, or fibronectin as substrate materials.18,19,64 Other studies show that 2D pulsatile flow shear stress, however, increases SMC migration in vitro.29,71 In 3D flow studies, it has been demonstrated that interstitial flow can increase MMP activity and promote motility of FBs, MFBs, and SMCs in collagen gels.85 Differences in flow pattern, shear stress level, matrix material, and system dimension (2D vs. 3D) undoubtedly contributed to these differences. In addition, in the more physiological 3D system, the distinct cell–matrix adhesions,10 and other elements such as matrix structure, surface glycocalyx, and tethering may give rise to amplified mechanosignaling.43,68,87 The underlying mechanotransduction mechanisms remain to be further investigated.
Limitations of Current 3D Studies
The 3D in vitro studies described above do have limitations related to the permeability of the matrix. The permeability of tissue is very important as it controls mass transport to cells by diffusion and convection, and shear stress on cells. The permeability depends strongly on the matrix material concentration. The Darcy permeability (K p) is about 10−8 to 10−12 cm2 for 2.5–45 mg/mL collagen gels,55,70,85,110 which is at least two orders higher than in the layers of the rabbit aortic wall (10−14 cm2).41 The interstitial flow velocities used in the 3D studies were greater than 0.5 μm/s, which is also substantially higher than the transmural flow velocity in the normal aorta (0.01–0.1 μm/s).104,109 However, the estimated shear stresses on suspended cells (0.05–1.0 dyn/cm2) are in the expected range for the aorta.85 Therefore, how the vascular SMCs and FBs respond to flow in a 3D model with similar shear stress but more physiological permeability, matrix structure, and flow velocity remain interesting, but challenging, to explore. The major components of the interstitial matrix of the media and adventitia are collagen I and III, produced by vascular SMCs and FBs.69,72 However, elastic fibers and proteoglycans are also abundantly presented in the interstitial matrix.69,72,93 Therefore, it would be of great interest to determine the responses of vascular SMCs and FBs to interstitial flow in a mixed ECM model. This could include investigations of vascular wall cell responses to interstitial flow using a 3D model cocultured with ECs or using an intact or injured vessel that will faithfully recapitulate the in vivo environment.
Flow-Initiated Mechanotransduction Pathways in SMCs
To understand the mechanisms of vascular disease development, it is of great importance to unravel the mechanisms by which vascular wall cells sense and transduce the stimuli of hemodynamic forces (shear stress, cyclic strain, and hydrostatic pressure) into intracellular biochemical signals.27,39,46 Studies of shear effects on SMCs reviewed above suggest that cell membrane-related receptors, ion channels, cell surface glycocalyx, as well as integrins are mechanosensors for shear forces, while NO, Ca2+, kinases, MAPKs are signaling messengers to shear-sensitive genes that regulate SMC responses including synthesis, secretion, proliferation, apoptosis, differentiation, and migration. Fluid flow may also regulate SMC contraction via glycocalyx-mediated Rho-MLCP pathway activation. In addition, vascular SMC surfaces also contain primary cilia, which may act as mechanosensors regulating Ca2+ influx and SMC migration in response to flow or cell–ECM interaction.50 The displacement of primary cilia and glycocalyx by flow may affect mechanosensitive membrane-related receptors, ion channels, integrins, or other structures. These shear-induced mechanotransduction pathways in vascular SMCs are briefly summarized in Fig. 4.
A generic model of fluid flow mechanotransduction modulation of vascular SMC gene expression and function. Vascular SMCs sense flow stimulation via cell membrane-related receptors, ion channels, glycocalyx, integrins, as well as primary cilia, activating NO, Ca2+, kinases, and MAPKs signaling messengers to shear-sensitive genes. Fluid flow mechanotransduction regulates SMC responses including synthesis, secretion, proliferation, apoptosis, differentiation, and migration. Fluid flow can stimulate SMC contraction via glycocalyx-mediated Rho-MLCP pathway activation. The glycocalyx-mediated mechanotransduction may interact with other mechanosensitive structures such as membrane receptors, ion channels, and integrins. Together, fluid flow mechanotransduction regulates vascular function and disease
In this model, cell surface glycocalyx HSPGs play a dominant role in sensing 2D laminar shear stress to control SMC contraction in vitro 1 and in sensing 3D interstitial flow to modulate SMC phenotype83 and motility.87 Cell contraction and migration requires integrin-mediated focal adhesion disassembly. Therefore, in 2D, shear forces may be sensed by the glycocalyx on the apical surface of the cell and then transmitted to integrins on the basolateral side via the cytoskeleton.103,112 In 3D, on the other hand, both integrin-mediated cell matrix adhesions and glycocalyx cover the entire cell surface, which may amplify flow-mediated mechanotransduction of interstitial flow with its relatively small shear stress.83–85,87 It remains interesting to determine whether the primary cilia also sense interstitial flow. In addition, shear flow-sensitive signal transduction pathways may also be shared by stretch and pressure.46 In vivo, SMCs may be exposed to different hemodynamic forces (shear, stretch, and pressure) at the same time, thus these hemodynamic forces may act in concert to regulate mechanosensitive signaling pathways controlling vascular function and disease.25
Conclusions and Future Directions
Although direct shear effects on vascular SMCs and FBs have not been studied as extensively as on ECs and the mechanotransduction mechanisms by which vascular SMCs and FBs sense fluid flow require further investigation, the accumulating data demonstrate that the direct effects of shear stress and interstitial flow on SMCs and FBs can trigger cell signaling pathways leading to altered pathophysiological consequences associated with vascular remodeling and disease. We conclude that after endothelial damage, the alterations in fluid flow can be directly sensed by SMCs and FBs and trigger many mechanotransduction pathways that in turn induce profound changes in cell properties and functions including secretion, proliferation, motility, and contractility. Fluid flow modulation of SMC and FB phenotype from a quiescent state to a more activated state eventually contributes to vascular remodeling and disease.
Unlike ECs lining the luminal surface of the blood vessel wall, SMCs and FBs reside in a 3D ECM and are not normally exposed to blood flow, but to the more subtle interstitial flow. The orientation, morphology, and cell–cell and cell–matrix contacts are quite different between SMCs/FBs and ECs. The structures utilized by SMCs and ECs to sense fluid flow are also not the same, although there are similarities. For example, ECs can sense fluid shear through a cell–cell junctional complex containing VE-cadherin and PECAM-1,27,44 which is not present in SMCs. However, both ECs and SMCs have a glycocalyx which participates in mechanotransduction, and both utilize integrins to bind to their ECM. In addition, the phenotypes of SMCs are highly plastic compared to those of ECs. These differences and others manifest themselves in distinct fluid flow responses between 2D (ECs) and 3D (SMCs/FBs) cellular environments.
After vascular endothelial damage, the shear flows experienced by SMCs and FBs are predominantly associated with 3D interstitial flow. 3D culture better represents SMC and FB in vivo physiology than 2D. Therefore, it is more physiologically relevant to investigate SMC and FB function and behavior (e.g., contraction, proliferation, differentiation, and migration) in response to interstitial flow in more sophisticated 3D models. In addition, sheared ECs have significant influences on SMC biology and function. Yet most co-culture studies have used EC-SMC direct-contact co-culture models that omit the effects of interstitial flow. Therefore, cultured SMCs in 3D matrix and co-cultured ECs on the surface of the 3D matrix would more faithfully mimic in vivo physiological conditions, which may provide better views of the interplay between ECs and SMCs under laminar flow and interstitial flow. Furthermore, besides fluid flow, mechanical stretch and pressure also affect SMCs and FBs. Therefore, better understanding of flow-induced mechanotransduction in SMCs and FBs coupled with other hemodynamic forces may provide clues for treating vascular diseases including atherosclerosis, neointima formation, and restenosis, and have implications in vascular tissue engineering.
References
Ainslie, K. M., J. S. Garanich, R. O. Dull, and J. M. Tarbell. Vascular smooth muscle cell glycocalyx influences shear stress-mediated contractile response. J. Appl. Physiol. 98:242–249, 2005.
Ainslie, K., Z. D. Shi, J. S. Garanich, and J. M. Tarbell. Rat aortic smooth muscle cells contract in response to serum and its components in a calcium independent manner. Ann. Biomed. Eng. 32:1667–1675, 2004.
Alshihabi, S. N., Y. S. Chang, J. A. Frangos, and J. M. Tarbell. Shear stress-induced release of pge2 and pgi2 by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 224:808–814, 1996.
Apenberg, S., M. A. Freyberg, and P. Friedl. Shear stress induces apoptosis in vascular smooth muscle cells via an autocrine fas/fasl pathway. Biochem. Biophys. Res. Commun. 310:355–359, 2003.
Asada, H., J. Paszkowiak, D. Teso, K. Alvi, A. Thorisson, J. C. Frattini, F. A. Kudo, B. E. Sumpio, and A. Dardik. Sustained orbital shear stress stimulates smooth muscle cell proliferation via the extracellular signal-regulated protein kinase 1/2 pathway. J. Vasc. Surg. 42:772–780, 2005.
Baldwin, A. L., L. M. Wilson, I. Gradus-Pizlo, R. Wilensky, and K. March. Effect of atherosclerosis on transmural convection an arterial ultrastructure. Implications for local intravascular drug delivery. Arterioscler. Thromb. Vasc. Biol. 17:3365–3375, 1997.
Bodin, P., D. Bailey, and G. Burnstock. Increased flow-induced atp release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 103:1203–1205, 1991.
Chiu, J. J., S. Usami, and S. Chien. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann. Med. 41:19–28, 2009.
Civelek, M., K. Ainslie, J. S. Garanich, and J. M. Tarbell. Smooth muscle cells contract in response to fluid flow via a Ca2+-independent signaling mechanism. J. Appl. Physiol. 93:1907–1917, 2002.
Cukierman, E., R. Pankov, D. R. Stevens, and K. M. Yamada. Taking cell–matrix adhesions to the third dimension. Science 294:1708–1712, 2001.
Dan, L., C. K. Chua, and K. F. Leong. Fibroblast response to interstitial flow: a state-of-the-art review. Biotechnol. Bioeng. 107:1–10, 2010.
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75:519–560, 1995.
Dong, J. D., Y. Q. Gu, C. M. Li, C. R. Wang, Z. G. Feng, R. X. Qiu, B. Chen, J. X. Li, S. W. Zhang, Z. G. Wang, and J. Zhang. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol. Sin. 30:530–536, 2009.
Dzau, V. J., R. C. Braun-Dullaeus, and D. G. Sedding. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 8:1249–1256, 2002.
Ekstrand, J., A. Razuvaev, L. Folkersen, J. Roy, and U. Hedin. Tissue factor pathway inhibitor-2 is induced by fluid shear stress in vascular smooth muscle cells and affects cell proliferation and survival. J. Vasc. Surg. 52:167–175, 2010.
Fitzgerald, T. N., B. R. Shepherd, H. Asada, D. Teso, A. Muto, T. Fancher, J. M. Pimiento, S. P. Maloney, and A. Dardik. Laminar shear stress stimulates vascular smooth muscle cell apoptosis via the akt pathway. J. Cell. Physiol. 216:389–395, 2008.
Fleury, M. E., K. C. Boardman, and M. A. Swartz. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys. J. 91:113–121, 2006.
Garanich, J. S., R. A. Mathura, Z. D. Shi, and J. M. Tarbell. Effects of fluid shear stress on adventitial fibroblast migration: implications for flow-mediated mechanisms of arterialization and intimal hyperplasia. Am. J. Physiol. Heart Circ. Physiol. 292:H3128–H3135, 2007.
Garanich, J. S., M. Pahakis, and J. M. Tarbell. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am. J. Physiol. Heart Circ. Physiol. 288:H2244–H2252, 2005.
Gerthoffer, W. T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 100:607–621, 2007.
Goldman, J., L. Zhong, and S. Q. Liu. Negative regulation of vascular smooth muscle cell migration by blood shear stress. Am. J. Physiol. Heart Circ. Physiol. 292:H928–H938, 2007.
Gosgnach, W., M. Challah, F. Coulet, J. B. Michel, and T. Battle. Shear stress induces angiotensin converting enzyme expression in cultured smooth muscle cells: possible involvement of bfgf. Cardiovasc. Res. 45:486–492, 2000.
Gosgnach, W., D. Messika-Zeitoun, W. Gonzalez, M. Philipe, and J. B. Michel. Shear stress induces inos expression in cultured smooth muscle cells: role of oxidative stress. Am. J. Physiol. Cell Physiol. 279:C1880–C1888, 2000.
Gurney, A. M. Going with the flow: smooth muscle trpm7 channels and the vascular response to blood flow. Circ. Res. 98:163–164, 2006.
Haga, J. H., Y. S. Li, and S. Chien. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J. Biomech. 40:947–960, 2007.
Haga, M., A. Yamashita, J. Paszkowiak, B. E. Sumpio, and A. Dardik. Oscillatory shear stress increases smooth muscle cell proliferation and akt phosphorylation. J. Vasc. Surg. 37:1277–1284, 2003.
Hahn, C., and M. A. Schwartz. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10:53–62, 2009.
Hastings, N. E., M. B. Simmers, O. G. McDonald, B. R. Wamhoff, and B. R. Blackman. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am. J. Physiol. Cell Physiol. 293:C1824–C1833, 2007.
Hendrickson, R. J., S. S. Okada, P. A. Cahill, E. Yankah, J. V. Sitzmann, and E. M. Redmond. Ethanol inhibits basal and flow-induced vascular smooth muscle cell migration in vitro. J. Surg. Res. 84:64–70, 1999.
Hernandez Vera, R., E. Genove, L. Alvarez, S. Borros, R. Kamm, D. Lauffenburger, and C. E. Semino. Interstitial fluid flow intensity modulates endothelial sprouting in restricted src-activated cell clusters during capillary morphogenesis. Tissue Eng. A 15:175–185, 2009.
Hill, M. A., H. Zou, M. J. Davis, S. J. Potocnik, and S. Price. Transient increases in diameter and [Ca(2+)](i) are not obligatory for myogenic constriction. Am. J. Physiol. Heart Circ. Physiol. 278:H345–H352, 2000.
Hosseinkhani, H., Y. Inatsugu, Y. Hiraoka, S. Inoue, and Y. Tabata. Perfusion culture enhances osteogenic differentiation of rat mesenchymal stem cells in collagen sponge reinforced with poly(glycolic acid) fiber. Tissue Eng. 11:1476–1488, 2005.
Jeong, S. I., J. H. Kwon, J. I. Lim, S. W. Cho, Y. Jung, W. J. Sung, S. H. Kim, Y. H. Kim, Y. M. Lee, B. S. Kim, C. Y. Choi, and S. J. Kim. Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic plcl scaffolds. Biomaterials 26:1405–1411, 2005.
Kang, H., Y. Fan, and X. Deng. Vascular smooth muscle cell glycocalyx modulates shear-induced proliferation, migration, and no production responses. Am. J. Physiol. Heart Circ. Physiol. 300:H76–H83, 2011.
Kim, M. H., N. R. Harris, D. H. Korzick, and J. M. Tarbell. Control of the arteriolar myogenic response by transvascular fluid filtration. Microvasc. Res. 68:30–37, 2004.
Kobayashi, N., T. Yasu, H. Ueba, M. Sata, S. Hashimoto, M. Kuroki, M. Saito, and M. Kawakami. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp. Hematol. 32:1238–1245, 2004.
Kohler, T. R., and A. Jawien. Flow affects development of intimal hyperplasia after arterial injury in rats. Arterioscler. Thromb. 12:963–971, 1992.
Lee, A. A., D. A. Graham, S. Dela Cruz, A. Ratcliffe, and W. J. Karlon. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J. Biomech. Eng. 124:37–43, 2002.
Lehoux, S., Y. Castier, and A. Tedgui. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 259:381–392, 2006.
Levesque, M. J., and R. M. Nerem. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 107:341–347, 1985.
Levick, J. R. Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72:409–437, 1987.
Li, G., S. J. Chen, S. Oparil, Y. F. Chen, and J. A. Thompson. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation 101:1362–1365, 2000.
Li, S., J. L. Guan, and S. Chien. Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 7:105–150, 2005.
Li, Y. S., J. H. Haga, and S. Chien. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38:1949–1971, 2005.
Li, S., J. Lao, B. P. Chen, Y. S. Li, Y. Zhao, J. Chu, K. D. Chen, T. C. Tsou, K. Peck, and S. Chien. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 17:97–99, 2003.
Li, C., and Q. Xu. Mechanical stress-initiated signal transduction in vascular smooth muscle cells in vitro and in vivo. Cell Signal. 19:881–891, 2007.
Liu, S. Q., D. Tang, C. Tieche, and P. K. Alkema. Pattern formation of vascular smooth muscle cells subject to nonuniform fluid shear stress: mediation by gradient of cell density. Am. J. Physiol. Heart Circ. Physiol. 285:H1072–H1080, 2003.
Liu, S. Q., C. Tieche, D. Tang, and P. Alkema. Pattern formation of vascular smooth muscle cells subject to nonuniform fluid shear stress: role of pdgf-beta receptor and src. Am. J. Physiol. Heart Circ. Physiol. 285:H1081–H1090, 2003.
Louis, H., P. Lacolley, A. Kakou, V. Cattan, D. Daret, M. Safar, J. Bonnet, and J. M. Daniel Lamaziere. Early activation of internal medial smooth muscle cells in the rabbit aorta after mechanical injury: relationship with intimal thickening and pharmacological applications. Clin. Exp. Pharmacol. Physiol. 33:131–138, 2006.
Lu, C. J., H. Du, J. Wu, D. A. Jansen, K. L. Jordan, N. Xu, G. C. Sieck, and Q. Qian. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press. Res. 31:171–184, 2008.
MacLeod, D. C., B. H. Strauss, M. de Jong, J. Escaned, V. A. Umans, R. J. van Suylen, A. Verkerk, P. J. de Feyter, and P. W. Serruys. Proliferation and extracellular matrix synthesis of smooth muscle cells cultured from human coronary atherosclerotic and restenotic lesions. J. Am. Coll. Cardiol. 23:59–65, 1994.
Michel, J. B., O. Thaunat, X. Houard, O. Meilhac, G. Caligiuri, and A. Nicoletti. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler. Thromb. Vasc. Biol. 27:1259–1268, 2007.
Newby, A. C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 85:1–31, 2005.
Ng, C. P., B. Hinz, and M. A. Swartz. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 118:4731–4739, 2005.
Ng, C. P., and M. A. Swartz. Fibroblast alignment under interstitial fluid flow using a novel 3-d tissue culture model. Am. J. Physiol. Heart Circ. Physiol. 284:H1771–H1777, 2003.
Ni, J., A. Waldman, and L. M. Khachigian. C-jun regulates shear- and injury-inducible egr-1 expression, vein graft stenosis after autologous end-to-side transplantation in rabbits, and intimal hyperplasia in human saphenous veins. J. Biol. Chem. 285:4038–4048, 2010.
Oancea, E., J. T. Wolfe, and D. E. Clapham. Functional trpm7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 98:245–253, 2006.
Ono, O., J. Ando, A. Kamiya, Y. Kuboki, and H. Yasuda. Flow effects on cultured vascular endothelial and smooth muscle cell functions. Cell Struct. Funct. 16:365–374, 1991.
Opitz, F., K. Schenke-Layland, T. U. Cohnert, and U. A. Stock. Phenotypical plasticity of vascular smooth muscle cells-effect of in vitro and in vivo shear stress for tissue engineering of blood vessels. Tissue Eng. 13:2505–2514, 2007.
Opitz, F., K. Schenke-Layland, W. Richter, D. P. Martin, I. Degenkolbe, T. Wahlers, and U. A. Stock. Tissue engineering of ovine aortic blood vessel substitutes using applied shear stress and enzymatically derived vascular smooth muscle cells. Ann. Biomed. Eng. 32:212–222, 2004.
Osanai, T., N. Akutsu, N. Fujita, T. Nakano, K. Takahashi, W. Guan, and K. Okumura. Cross talk between prostacyclin and nitric oxide under shear in smooth muscle cell: role in monocyte adhesion. Am. J. Physiol. Heart Circ. Physiol. 281:H177–H182, 2001.
Owens, G. K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75:487–517, 1995.
Owens, G. K., M. S. Kumar, and B. R. Wamhoff. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84:767–801, 2004.
Palumbo, R., C. Gaetano, G. Melillo, E. Toschi, A. Remuzzi, and M. C. Capogrossi. Shear stress downregulation of platelet-derived growth factor receptor-beta and matrix metalloprotease-2 is associated with inhibition of smooth muscle cell invasion and migration. Circulation 102:225–230, 2000.
Papadaki, M., L. V. McIntire, and S. G. Eskin. Effects of shear stress on the growth kinetics of human aortic smooth muscle cells in vitro. Biotechnol. Bioeng. 50:555–561, 1996.
Papadaki, M., J. Ruef, K. T. Nguyen, F. Li, C. Patterson, S. G. Eskin, L. V. McIntire, and M. S. Runge. Differential regulation of protease activated receptor-1 and tissue plasminogen activator expression by shear stress in vascular smooth muscle cells. Circ. Res. 83:1027–1034, 1998.
Papadaki, M., R. G. Tilton, S. G. Eskin, and L. V. McIntire. Nitric oxide production by cultured human aortic smooth muscle cells: stimulation by fluid flow. Am. J. Physiol. 274:H616–H626, 1998.
Pedersen, J. A., F. Boschetti, and M. A. Swartz. Effects of extracellular fiber architecture on cell membrane shear stress in a 3d fibrous matrix. J. Biomech. 40:1484–1492, 2007.
Prockop, D. J., and K. I. Kivirikko. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64:403–434, 1995.
Ramanujan, S., A. Pluen, T. D. McKee, E. B. Brown, Y. Boucher, and R. K. Jain. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 83:1650–1660, 2002.
Redmond, E. M., J. P. Cullen, P. A. Cahill, J. V. Sitzmann, S. Stefansson, D. A. Lawrence, and S. S. Okada. Endothelial cells inhibit flow-induced smooth muscle cell migration: role of plasminogen activator inhibitor-1. Circulation 103:597–603, 2001.
Rensen, S. S., P. A. Doevendans, and G. J. van Eys. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 15:100–108, 2007.
Rhoads, D. N., S. G. Eskin, and L. V. McIntire. Fluid flow releases fibroblast growth factor-2 from human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 20:416–421, 2000.
Rice, K. M., S. K. Kakarla, S. P. Mupparaju, S. Paturi, A. Katta, M. Wu, R. T. Harris, and E. R. Blough. Shear stress activates akt during vascular smooth muscle cell reorientation. Biotechnol. Appl. Biochem. 55:85–90, 2010.
Riha, G. M., P. H. Lin, A. B. Lumsden, Q. Yao, and C. Chen. Roles of hemodynamic forces in vascular cell differentiation. Ann. Biomed. Eng. 33:772–779, 2005.
Ritman, E. L., and A. Lerman. The dynamic vasa vasorum. Cardiovasc. Res. 75:649–658, 2007.
Rizzo, V. Enhanced interstitial flow as a contributing factor in neointima formation: (shear) stressing vascular wall cell types other than the endothelium. Am. J. Physiol. Heart Circ. Physiol. 297:H1196–H1197, 2009.
Rosati, C., and R. Garay. Flow-dependent stimulation of sodium and cholesterol uptake and cell growth in cultured vascular smooth muscle. J. Hypertens. 9:1029–1033, 1991.
Rzucidlo, E. M., K. A. Martin, and R. J. Powell. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 45(Suppl A):A25–A32, 2007.
Sartore, S., A. Chiavegato, E. Faggin, R. Franch, M. Puato, S. Ausoni, and P. Pauletto. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ. Res. 89:1111–1121, 2001.
Schwartz, R. S., W. D. Edwards, K. C. Huber, L. C. Antoniades, K. R. Bailey, A. R. Camrud, M. A. Jorgenson, and D. R. Holmes, Jr. Coronary restenosis: prospects for solution and new perspectives from a porcine model. Mayo Clin. Proc. 68:54–62, 1993.
Sharma, R., C. E. Yellowley, M. Civelek, K. Ainslie, L. Hodgson, J. M. Tarbell, and H. J. Donahue. Intracellular calcium changes in rat aortic smooth muscle cells in response to fluid flow. Ann. Biomed. Eng. 30:371–378, 2002.
Shi, Z. D., G. Abraham, and J. M. Tarbell. Shear stress modulation of smooth muscle cell marker genes in 2-d and 3-d depends on mechanotransduction by heparan sulfate proteoglycans and erk1/2. PLoS One 5:e12196, 2010.
Shi, Z. D., X. Y. Ji, D. E. Berardi, H. Qazi, and J. M. Tarbell. Interstitial flow induces mmp-1 expression and vascular smc migration in collagen i gels via an erk1/2-dependent and c-jun-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 298:H127–H135, 2010.
Shi, Z. D., X. Y. Ji, H. Qazi, and J. M. Tarbell. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-d collagen i via upregulation of mmp-1. Am. J. Physiol. Heart Circ. Physiol. 297:H1225–H1234, 2009.
Shi, Y., J. E. O’Brien, A. Fard, J. D. Mannion, D. Wang, and A. Zalewski. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94:1655–1664, 1996.
Shi, Z. D., H. Wang, and J. M. Tarbell. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating mmp-13 expression and cell motility via fak-erk in 3d collagen. PLoS One 6:e15956, 2011.
Shigematsu, K., H. Yasuhara, H. Shigematsu, and T. Muto. Direct and indirect effects of pulsatile shear stress on the smooth muscle cell. Int. Angiol. 19:39–46, 2000.
Shimizu, N., K. Yamamoto, S. Obi, S. Kumagaya, T. Masumura, Y. Shimano, K. Naruse, J. K. Yamashita, T. Igarashi, and J. Ando. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating pdgf receptor beta. J. Appl. Physiol. 104:766–772, 2008.
Shou, Y., K. M. Jan, and D. S. Rumschitzki. Transport in rat vessel walls. I. Hydraulic conductivities of the aorta, pulmonary artery, and inferior vena cava with intact and denuded endothelia. Am. J. Physiol. Heart Circ. Physiol. 291:H2758–H2771, 2006.
Stamatas, G. N., C. W. Patrick, Jr., and L. V. McIntire. Intracellular ph changes in human aortic smooth muscle cells in response to fluid shear stress. Tissue Eng. 3:391–403, 1997.
Stegemann, J. P., H. Hong, and R. M. Nerem. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J. Appl. Physiol. 98:2321–2327, 2005.
Stegemann, J. P., and R. M. Nerem. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp. Cell Res. 283:146–155, 2003.
Stenmark, K. R., N. Davie, M. Frid, E. Gerasimovskaya, and M. Das. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 21:134–145, 2006.
Sterpetti, A. V., A. Cucina, L. S. D’Angelo, B. Cardillo, and A. Cavallaro. Response of arterial smooth muscle cells to laminar flow. J. Cardiovasc. Surg. (Torino) 33:619–624, 1992.
Sterpetti, A. V., A. Cucina, L. S. D’Angelo, B. Cardillo, and A. Cavallaro. Shear stress modulates the proliferation rate, protein synthesis, and mitogenic activity of arterial smooth muscle cells. Surgery 113:691–699, 1993.
Sterpetti, A. V., A. Cucina, A. Fragale, S. Lepidi, A. Cavallaro, and L. Santoro-D’Angelo. Shear stress influences the release of platelet derived growth factor and basic fibroblast growth factor by arterial smooth muscle cells. Eur. J. Vasc. Surg. 8:138–142, 1994.
Sterpetti, A. V., A. Cucina, L. Santoro, B. Cardillo, and A. Cavallaro. Modulation of arterial smooth muscle cell growth by haemodynamic forces. Eur. J. Vasc. Surg. 6:16–20, 1992.
Tada, S., and J. M. Tarbell. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 278:H1589–H1597, 2000.
Tada, S., and J. M. Tarbell. Fenestral pore size in the internal elastic lamina affects transmural flow distribution in the artery wall. Ann. Biomed. Eng. 29:456–466, 2001.
Tada, S., and J. M. Tarbell. Flow through internal elastic lamina affects shear stress on smooth muscle cells (3d simulations). Am. J. Physiol. Heart Circ. Physiol. 282:H576–H584, 2002.
Tarbell, J. M. Mass transport in arteries and the localization of atherosclerosis. Annu. Rev. Biomed. Eng. 5:79–118, 2003.
Tarbell, J. M., and M. Y. Pahakis. Mechanotransduction and the glycocalyx. J. Intern. Med. 259:339–350, 2006.
Tedgui, A., and M. J. Lever. Filtration through damaged and undamaged rabbit thoracic aorta. Am. J. Physiol. 247:H784–H791, 1984.
Tsai, M. C., L. Chen, J. Zhou, Z. Tang, T. F. Hsu, Y. Wang, Y. T. Shih, H. H. Peng, N. Wang, Y. Guan, S. Chien, and J. J. Chiu. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ. Res. 105:471–480, 2009.
Ueba, H., M. Kawakami, and T. Yaginuma. Shear stress as an inhibitor of vascular smooth muscle cell proliferation. Role of transforming growth factor-beta 1 and tissue-type plasminogen activator. Arterioscler. Thromb. Vasc. Biol. 17:1512–1516, 1997.
Van Gieson, E. J., W. L. Murfee, T. C. Skalak, and R. J. Price. Enhanced smooth muscle cell coverage of microvessels exposed to increased hemodynamic stresses in vivo. Circ. Res. 92:929–936, 2003.
Wagner, C. T., W. Durante, N. Christodoulides, J. D. Hellums, and A. I. Schafer. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J. Clin. Invest. 100:589–596, 1997.
Wang, D. M., and J. M. Tarbell. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J. Biomech. Eng. 117:358–363, 1995.
Wang, S., and J. M. Tarbell. Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model. Arterioscler. Thromb. Vasc. Biol. 20:2220–2225, 2000.
Wang, H., S. Yan, H. Chai, G. M. Riha, M. Li, Q. Yao, and C. Chen. Shear stress induces endothelial transdifferentiation from mouse smooth muscle cells. Biochem. Biophys. Res. Commun. 346:860–865, 2006.
Weinbaum, S., J. M. Tarbell, and E. R. Damiano. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 9:121–167, 2007.
Yamamoto, K., T. Sokabe, T. Watabe, K. Miyazono, J. K. Yamashita, S. Obi, N. Ohura, A. Matsushita, A. Kamiya, and J. Ando. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 288:H1915–H1924, 2005.
Acknowledgments
This work was partially supported by National Institutes of Health grants RO1 HL 094889 (to JMT).
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Aleksander S. Popel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Shi, ZD., Tarbell, J.M. Fluid Flow Mechanotransduction in Vascular Smooth Muscle Cells and Fibroblasts. Ann Biomed Eng 39, 1608–1619 (2011). https://doi.org/10.1007/s10439-011-0309-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0309-2