Abstract
The endothelium consists of a single layer of vascular endothelial cells (ECs) and serves as a selective barrier between the blood and arteries. ECs are constantly exposed to blood flow- and pulsatile blood pressure-induced hemodynamic forces. The cells are able to convert these mechanical stimuli into biochemical signals and then transmit the signals into the cell interior to affect cellular functions. These mechanical stimuli are detected by multiple mechanosensors in ECs that activate signaling pathways through their associated adaptor proteins, eventually leading to the maintenance of vascular homeostasis or the development of the pathogenesis of vascular disorders. These mechanosensors are distributed in different parts of the ECs, including the cell membrane, cell-to-cell junctions, the cytoplasm, and the nucleus. This review attempts to bring together recent findings on these mechanosensors and presents a conceptual framework for understanding the regulation of endothelial mechanosensors in response to hemodynamic forces. With verification by in vitro and in vivo evidence, endothelial mechanosensors have been demonstrated to contribute to health and disease by regulating physiological and pathophysiological processes in response to mechanical stimuli.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The human body is constantly exposed to various types of mechanical forces , such as the stretching of skeletal muscle, the compression of cartilage and bone, and the hemodynamic forces on blood vessels (Butcher et al. 2009). As the monolayer is in direct contact with flowing blood, endothelial cells (ECs) are constantly exposed to blood flow- and pulsatile blood pressure-induced hemodynamic forces. These hemodynamic forces include shear stress and cyclic stretch . Fluid shear stress is the frictional force per unit area from flowing blood and acts on the ECs present on the luminal surface of the vessel (Chien 2007). Cyclic stretch arises due to the blood pressure and causes circumferential stretching of the vessel wall that affects both the ECs and the smooth muscle cells (SMCs) that surround the endothelium in the arteries. An increasing number of studies have indicated that hemodynamic forces regulate EC functions and vascular physiology and pathophysiology, thereby contributing to health and disease (Davies 2009; Hahn and Schwartz 2009; Li et al. 2005).
To accomplish these hemodynamic force -induced physiological and pathophysiological modulations, ECs must be able to initially sense these mechanical stimuli using mechanosensors. These mechanosensors then translate mechanical input into biochemical output that is transmitted into the interior of the cell, thereby initiating mechanoresponsive signaling pathways. These processes are known as mechanosensing and mechanotransduction (Fedorchak et al. 2014; Jaalouk and Lammerding 2009). Mechanosensors are defined as proteins or molecules (part of the cellular structure) that can receive the mechanical stimuli, translate these stimuli into a biochemical signal, and then transmit the signal into the interior of the cell. Generally, mechanosensing is initially dependent on the capacity of mechanical stimuli to induce conformational changes in the mechanosensor that lead to alterations of the association of the mechanosensor with its partner proteins or changes in the activity of these proteins.
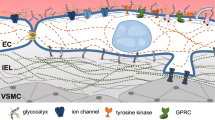
Many different cellular proteins have been proposed to function as mechanosensors of hemodynamic forces in ECs. These mechanosensors are primarily located at the plasma membrane and include ion channels, G proteins and G protein-coupled receptors (GPCRs ) (Gudi et al. 1996), endothelial glycocalyx (Florian et al. 2003), primary cilia (Pazour and Witman 2003), caveolae (Park et al. 2000; Yu et al. 2006), cell matrix receptors such as integrins (Tzima et al. 2001; Li et al. 1997), cell–cell adhesion junction proteins (Tzima et al. 2005), receptor tyrosine kinases (RTKs) (Jin et al. 2003; Shay-Salit et al. 2002), and bone morphogenetic protein receptors (BMPRs) (Zhou et al. 2012; 2013). The cytoskeleton and the nucleus were also reported to be mechanosensors in recent studies (Fedorchak et al. 2014; Helmke and Davies 2002; Tkachenko et al. 2013). In response to hemodynamic stimuli, these mechanosensors activate upstream signaling molecules through their associated adaptor proteins and mediate intracellular signaling through phosphorylation cascades, eventually leading to morphological and functional changes that maintain homeostasis. These changes include the regulation of gene expression, differentiation, proliferation, angiogenesis, and migration. Vascular cell dysfunction due to the impairment of these changes may lead to a pathophysiological state that contributes to the development of vascular disorders, such as atherosclerosis and hypertension (Hahn and Schwartz 2009).
This chapter provides an introduction to hemodynamic force-specific mechanosensors in ECs. We also provide in vitro and in vivo evidence of the importance of mechanosensors for the regulation of endothelial functions. In conclusion, we propose that these mechanosensors play initial and necessary roles in the hemodynamic force-modulated vascular biology and pathophysiology that contribute to health and disease states.
2 Membrane Molecules
2.1 Ion Channels
Although our understanding of shear stress-triggered endothelial signaling pathways has greatly increased over the past two decades, the mechanisms by which ECs sense shear stress remain largely unknown. Activation of shear stress-sensitive ion channels is among the fastest known endothelial responses to shear stress; hence, ion channels have been proposed to serve as endothelial mechanosensors. Ion channels are pore-forming membrane proteins whose functions include establishing a resting membrane potential and shaping action potentials and other electrical signals by gating the flow of ions across cell membranes (Doyle 2004). ECs express a bewildering variety of ion channels that promote the activation of ion fluxes. Among these ion fluxes, the transport of calcium (Ca2+) across the cell membrane is involved in the early cellular response to shear stress. Ca2+ has been proposed to play a key role in signal transduction events, and an increase in the Ca2+ level stimulates the Ca2+-dependent synthesis of vasodilators, such as nitric oxide (NO) and prostacyclin (Kuchan and Frangos 1994; Rubanyi et al. 1986; Falcone et al. 1993). In addition to Ca2+, the flows of potassium (K+), chloride (Cl−), and sodium (Na+) ions are rapidly elicited in ECs in response to shear stress (Nilius and Droogmans 2001). These shear stress-activated ion channels play a central role in the regulation of endothelial mechanoresponsive events (Gautam et al. 2006a).
2.1.1 Ca2+ Channel
In human ECs, the cation channel has been reported to be activated by shear stress, and Ca2+ is more permeable than other cations (Schwarz et al. 1992). In mammals, the gene encoding the Ca2+-permeable cation channel has been identified as a Drosophila homologue of the transient receptor potential (TRP) gene . Several members of the TRP superfamily are expressed in humans and other species (Nilius et al. 2003). The TRP protein consists of six transmembrane segments, all of which constitute cation channels. TRP channels can be grouped into 7 subfamilies, including TRPC (C for canonical), TRPM (M for melastatin), TRPV (V for vanilloid), TRPA (A for ankyrin), TRPP (P for polycystic kidney disease), TRPML (ML for mucolipin), and TRPN (N for no mechanoreceptor potential C) (Pedersen et al. 2005). Functional TRP channels are tetrameric complexes formed by the heteromerization of TRP subunits crossing different TRP subfamilies. A variety of TRPs have been identified and demonstrated to serve as mechanosensors in ECs in response to shear stress . TRPP1 and TRPP2 are also known as polycystin-1 and polycystin-2. The C-terminal domain of TRPP1 can interact with the C-terminal domain of TRPP2, and this complex serves as a mechanosensor to activate the Ca2+ influx induced by shear stress (Nauli et al. 2008). Both TRPP1 and TRPP2 are localized to endothelial primary cilia and are required for mechanotransduction (Nauli et al. 2003; AbouAlaiwi et al. 2009). Moreover, TRPP2 depletion results in the loss of shear stress-induced NO production in ECs. This finding suggests that TRPP1 serves as a mechanosensor to transduce the mechanical force to TRPP2, which allows the Ca2+ influx and leads to the activation of intracellular signaling pathways. TRPV4 (also named VR-OAC, VRL-2, OTRPC4, and TRP12) has moderately high Ca2+ permeability in ECs (Watanabe et al. 2002). Activation of TRPV4 by 4αPDD, the selective TRPV4 opener, increased Ca2+ entry into ECs and caused vasodilatation of the carotid artery in rats following intraluminal application of 4αPDD. Similar to 4αPDD, a shear stress of 3 dynes/cm2 elicited vasodilation that could be blocked by a TRPV channel blocker (ruthenium red), a Ca2+ chelator (BAPTA-AM), and a NO synthase blocker (Nω-nitro-l-arginine). These results suggest that TRPV4 is activated by shear stress, causing Ca2+ entry and triggering NO-dependent vasodilatation (Kohler et al. 2006). Indeed, shear stress-induced vasodilation was lost in TRPV4-knockout mice (Hartmannsgruber et al. 2007). Du et al. demonstrated that TRPV4, TRPC1, and TRPP2 formed a heteromeric channel in rat mesenteric artery endothelial cells and that this heteromeric channel could induce vascular relaxation (Du et al. 2014). Pore-dead mutants for each of the TRP isoforms reduced the shear stress-induced Ca2+ current. These results suggest that TRP channels can assemble into heteromeric complexes that induce Ca2+ influx, resulting in vasodilation in response to shear stress. In addition to the TRP channels, TRPC3 and TRPM7 are expressed in ECs and are activated by shear stress. TRPC3 is activated by agonist-induced activation of plasma membrane GPCRs , synthetic diacylglycerols, and depletion of intracellular Ca2+ stores in some cell types (Birnbaumer et al. 1996; Montell 2001; Trebak et al. 2003). Knockdown of TRPC3 reduced Ca2+ influx and vasodilation in response to shear stress (Liu et al. 2006). TRMP7 is widely expressed in vascular SMCs and ECs (Runnels et al. 2001). The effect of TRPM7, which is heterologously expressed in human embryonic kidney (HEK) 293 cells , can be augmented by shear stress (Numata et al. 2007). Laminar shear stress also increases the plasma membrane translocation of TRPM7 to amplify its current amplitude (Oancea et al. 2006). In addition to the TRP channels, the P2X receptors are membrane ion channels that allow extracellular Ca2+ to enter and activate intracellular signaling pathways to evoke a variety of cellular responses (North 2002). Several P2X subtypes (P2X1-7) have been identified. P2X4 is the most abundantly expressed subtype of the P2X receptor in vascular ECs and is the major contributor to the shear stress-induced Ca2+ influx (Glass and Burnstock 2001; Ray et al. 2002; Yamamoto et al. 2000a, b). P2X4-deficient mice exhibit abnormal Ca2+ influx and NO production and lose their vasodilation capacity in response to shear stress (Yamamoto et al. 2006).
2.1.2 K+ Channel
Inward-rectifying K+ (Kir) channels were the first type of shear stress-activated ion channel reported in ECs and were identified using whole-cell patch-clamp recordings (Olesen et al. 1988). The Kirs are represented by the Kir2.1 family cloned from bovine aortic ECs (BAECs ) (Forsyth et al. 1997). Overexpression of the Kir2.1 channels in either Xenopus oocytes or mammalian HEK293 cells results in a large shear stress-activated K+ current in these cells (Hoger et al. 2002). In addition to the Kir2.1 channels, ATP-activated Kir6.2 currents were increased in rat pulmonary microvascular ECs and bovine pulmonary artery cells subjected to a shear stress of 10 dynes/cm2 (Chatterjee et al. 2003). The role of Ca2+-activated potassium channels (IKCa) in the shear stress-induced K+ current in ECs has been studied. Application of a shear stress of 5 or 15 dynes/cm2 upregulated IKCa expression, resulting in the enhancement of the whole cell K+ current and increased membrane hyperpolarization (Brakemeier et al. 2003). Intraluminal administration of iberiotoxin, an inhibitor of high conductance KCa channels (BKCa), eliminated the shear stress-induced dilations of the arterial s (Sun et al. 2001).
2.1.3 Cl− Channels
Whole-cell patch-clamp recordings and measurements from fluorescent potentiometric dyes have demonstrated the presence of shear stress-sensitive outward-rectifying Cl− channels in ECs (Barakat et al. 1999). Activation of outward-rectifying Cl− channels by shear stress leads to membrane depolarization after membrane hyperpolarization due to the activation of shear stress-sensitive K+ channels. K+ and Cl− channels can both immediately sense shear stress. However, the net electrochemical driving forces acting on Cl− channels is larger than the forces acting on K+ channels, and the dynamics of Cl− current activation are slower than those of the K+ current. This finding suggests that shear stress-sensitive Cl− channels attain maximal activation slower than shear stress-sensitive K+ channels. Lieu et al. demonstrated that a shear stress-induced hyperpolarizing current was carried in part by K+, whereas the depolarizing current was carried in part by Cl− (Lieu et al. 2004). A laminar shear stress of 1–10 dyns/cm2 was able to active the hyperpolarizing current; however, the depolarizing current was less responsive to 1 dyns/cm2 compared to 10 dyns/cm2. These results indicate that shear stress-sensitive K+ channels can respond to a wider range of shear stress for activation than Cl− channels, which may be involved in sensing shear stress changes at a high shear stress of 10 dyns/cm2. In addition to the magnitude of the shear stress, K+ channels can sense both laminar and oscillatory flows, whereas Cl− channels are only activated by laminar shear stress (Lieu et al. 2004). Previous studies demonstrated that laminar shear stress induced anti-inflammatory gene expression, whereas the oscillatory flow was associated with atherosclerotic development. These results imply that the shear stress-induced activation of the Cl− channel may play an important role in the atheroprotective property of the endothelium (Gautam et al. 2006b). Human aortic ECs exposed to a stream of fluid through a pipette exhibit an increase in their intracellular calcium concentration ([Ca2+]i) that is caused by both the magnitude of the shear stress and the extracellular Ca2+ concentration (Nakao et al. 1999; Jow and Numann 1999). This finding suggests that the activation of the Cl− current sufficiently changes the cell membrane potential to modulate the Ca2+ influx. However, the detailed mechanism underlying the shear stress induction of the Cl− channel remains unclear. The volume-regulated anion current (VRAC) is responsible for the regulation of osmolarity in a cell. Although shear stress does not induce VRAC currents in BAECs , it potentiates these currents in the presence of an osmotic challeng e (Romanenko et al. 2002).
2.1.4 Na+ Channels
Shear stress-increased Na+ permeability has been reported in rat cardiac microvascular ECs (Moccia et al. 2000). Epithelial Na+ channels (ENaCs) consist of three basic subunits: α, β, and γ. The α subunit of ENaCs forms the pore structure that allows Na+ to permeate, whereas the β and γ subunits can interact with most of the ENaC regulators (Canessa et al. 1994). The α subunit of ENaCs is expressed in cultured human dermal microvascular ECs, human umbilical cord ECs (HUVEC ) , and rat ECs. Shear stress increased the open probability of ENaCs in ECs (Wang et al. 2009). There is evidence that shear stress-sensitive cation channels in HUVECs are permeable to not only Ca2+ but also Na+ (Schwarz et al. 1992).
2.2 G Proteins and GPCRs
G proteins are known as guanine nucleotide-binding proteins . The heterotrimeric G proteins, which consist of α, β, and γ subunits, transduce signals from activated GPCRs into intercellular signaling molecules. The α subunit is a GTPase switch protein that alternates between an active status with bound GTP and an inactive status with bound GDP (Neves et al. 2002). G proteins are activated by shear stress and act as mediators of shear stress-induced EC responses, such as Ras activation and NO production (Ohno et al. 1993; Kuchan et al. 1994; Gudi et al. 2003). The G proteins are the one of the earliest known shear stress-induced cellular responses. Gudi and Frangos showed that G proteins were rapidly activated within 1 s in ECs subjected to shear stress. By immunoprecipitating AAGTP-labeled proteins with polyclonal anti-Gα antibodies, the G-protein subunits Gαq/α11 and Gαi3/αo were identified as activated by shear stress in a shear dose-dependent manner (Gudi et al. 1996, 1998). This shear stress-induced activation of G proteins was modulated by a shear stress-induced increase in membrane fluidity that was independent of cytoskeleton and cytosolic components (Gudi et al. 1998). Using time-resolved fluorescence microscopy and GPCR conformation-sensitive fluorescence resonance energy transfer (FRET) analysis , bradykinin B2 GPCR (BKRK2 ) was demonstrated to induce the conformational changes that led to the activation by shear stress at physiological shear stress values (≅15 dynes/cm2). Conversely, a B2-selective antagonist blocked the shear stress-induced activation of BKRK2 (Chachisvilis et al. 2006). These specific features, including the cellular location, rapid activation, and force discrimination, strongly indicate that G proteins and GPCRs serve as primary mechanosensors of shear stress. Furthermore, recent studies demonstrated that BKRK2 , the G-protein subunits Gαq/11, and PECAM-1 form a mechanosensitive complex in ECs in response to shear stress (Otte et al. 2009; Yeh et al. 2008; dela Paz et al. 2014). In primary human ECs, the oscillatory flow induced the rapid dissociation of the Gαq/11–PECAM-1 complex within 30 s, and Gαq/11 localized in the perinuclear region within 150 min. In a mouse study, co-localization of G αq/11 and PECAM-1 at the cell–cell junction in the atheroprotective areas of the mouse aorta was observed by immunohistochemical staining. In contrast, G αq/11 was absent from the junctions in atheroprone areas (Otte et al. 2009). These results indicate that the Gαq/11–PECAM-1 complex may be involved in the regulation of atherosclerosis.
2.3 Endothelial Glycocalyx
The endothelial glycocalyx is a gel-like thin layer covering the luminal surface of ECs that interacts directly with hemodynamic forces (Fu and Tarbell 2013). The endothelial glycocalyx is 0.4–4 μm thick based on in vivo measurements; however, Ebong et al. used rapid freezing/freeze substitution transmission electron microscopy to show that the thickness of the endothelial glycocalyx in cultured ECs was 11 μm (Fu and Tarbell 2013; Ebong et al. 2011). These inconsistent results indicate that the integrity of the endothelial glycocalyx may be different following exposure to different factors, such as the different types of cells used in in vivo and in vitro studies or the measurement techniques employed. The endothelial glycocalyx can directly bind or selectively block some biomolecules to regulate the EC barrier function (Bernfield et al. 1999). Additionally, the endothelial glycocalyx has important functions in leukocyte recruitment and the inflammatory response (Mulivor and Lipowsky 2009). The endothelial glycocalyx is composed of proteoglycans, glycosaminoglycans (GAGs ) , glycoproteins, and plasma proteins (Weinbaum et al. 2007). The three major GAGs [heparan sulfate (HS ) , chondroitin sulfate (CS ), and hyaluronic acid (HA) ] are found in the endothelial glycocalyx. HS is the most abundant, accounting for 50–90 % of the total GAGs . The transmembrane syndecans, membrane-bound glypicans, and basement matrix-associated perlecans are the three major protein core families of the heparan sulfate proteoglycans (HSPGs) found on ECs. The series of studies by J. Tarbell indicated that the endothelial glycocalyx acted as a mechanosensor. The abolishment of the endothelial glycocalyx by heparinase III impaired the shear stress-induced organization of the cytoskeleton and NO production (Florian et al. 2003; Thi et al. 2004). Several studies demonstrated that following the inhibition of the endothelial glycocalyx by heparinase III, ECs not only lost the capacity to sense shear stress and to modulate cell motility but also exhibited an increased proliferation rate in response to shear stress (Yao et al. 2007; Moon et al. 2005).
HS and associated proteoglycans have been extensively studied and have demonstrated their capacity to function as signal transduction molecules. The syndecans, including syndecan-1, -2, and -4, have three GAG attachment sites close to their N-termini and distal to their apical surfaces. Their cytoplasmic tails associate with the cytoskeleton and a signaling protein, such as protein kinase C (PKC)-α or phosphatidylinositol-4,5-biphosphate (PIP2) (Florian et al. 2003; Weinbaum et al. 2007; Tarbell and Pahakis 2006). Koo et al. demonstrated that an atheroprotective flow increased the expression of the endothelial glycocalyx and syndecan-1 on the apical surface of ECs; however, this effect was decreased after EC exposure to the atheroprone flow. Silencing of syndecan-1 inhibited the shear stress-induced expression of the endothelial glycocalyx (Koo et al. 2013). Knockout of syndecan-1 abolished the shear stress-induced phosphorylation of Akt and paxillin and, in turn, the activation of integrin αvβ3 and gene expression, including Kruppel-like factors (KLFs)-2 , -4, and -5, endothelial nitric oxide synthase (eNOS), and angiopoietin-2 (Voyvodic et al. 2014). Deletion of syndecan-4 in ECs inhibited the shear stress-induced alignment with the flow direction. Syndecan-4 knockdown activated the pro-inflammatory response and decreased atheroprotective flow-induced anti-inflammatory gene expression (i.e., KLF-2 and KLF-4), resulting in a significant increase in atherosclerotic lesions in normally resistant locations, such as the thoracic region (Baeyens et al. 2014). These results suggest that the syndecans play key roles in endothelial mechanosensing and are involved in hemodynamic force-modulated vascular pathophysiology .
2.4 Caveolae
Caveolae are cholesterol- and glycosphingolipid-rich, 50- to 100-nm vesicular invaginations of the plasma membrane that are found in many types of vascular cells, including ECs, SMCs, fibroblasts, and macrophages. Caveolae serve as transmembrane signaling microdomains for the transport of large and small molecules, such as the transcytosis of macromolecules and potocytosis of ions and folate. The chief structural proteins of caveolae are caveolins (Sowa 2012; Okamoto et al. 1998; Hansen and Nichols 2010). Three distinct caveolins have been identified: caveolin-1 (Cav-1), caveolin-2 (Cav-2), and caveolin-3 (Cav-3). Cav-1 and Cav-2 are the most abundantly expressed caveolins in ECs and fibroblasts, whereas Cav-3 expression is muscle-specific (Parton 1996). Caveolin is a 21- to 24-kDa membrane protein that binds directly to cholesterol and forms a scaffold. Many classes of signaling molecules can assemble on this scaffold to generate preassembled signaling complexes, resulting in the concentration of these signal molecules within a distinct region of the plasma membrane (Okamoto et al. 1998). These caveolin-interacting signaling molecules include the G protein α-subunit, Ras, Src family tyrosine kinases, eNOS, receptor tyrosine kinases, and PKC proteins. Caveolae have been demonstrated to be involved in shear stress-induced endothelial activation (Park et al. 2000; Yu et al. 2006; Fujioka et al. 2000; Sun et al. 2002; Radel and Rizzo 2005). Using recombinant glutathione S-transferase fusion proteins containing the epitopes of anti-cav-1 caveolin antibodies, Park et al. demonstrated that the scaffolding/oligomerization domain of cav-1 was critical for the regulation of the mechanosensitive activation of the extracellular signal-regulated kinase (ERK ) (Park et al. 2000). Blocking Cav-1 resulted in the inhibition of the shear stress-induced activation of ERK . Shear stress induces an increase in Cav-1 density and the tyrosine phosphorylation of Cav-1 at the plasma membrane, along with its translocation from the Golgi to the plasma membrane (Fujioka et al. 2000; Sun et al. 2002). Moreover, the shear stress-induced translocation and tyrosine phosphorylation of Cav-1 is regulated by the shear stress-activated β1-integrin C-Src kinase (Csk) and the Src family kinases. Treatment with the β1-integrin-blocking antibody JB1A, a type 1 protein phosphatase, or Csk knockdown leads to the inhibition of the shear stress-induced Cav-1 phosphorylation in ECs. Immunoprecipitation and immunostaining revealed that Csk interacted with phosphorylated Cav-1 and integrins . These results suggest the detailed mechanism underlying the response of the β1-integrin-Cav-1-Csk complex to shear stress (Radel and Rizzo 2005). An in vivo study further supported the importance of Cav-1 in shear stress-induced arterial responses. Yu et al. demonstrated that the loss of Cav-1 impaired shear stress-induced vasodilation and eNOS activation and resulted in medial thickening. Cav-1 overexpression rescued these effects in Cav-1-knockout mice (Yu et al. 2006). This direct evidence indicates that Cav-1 may serve as a mechanosensor or mechanotransducer in the arterial response to shear stress .
2.5 RTKs
Shear stress rapidly activates several tyrosine kinases, including the Src family (Okuda et al. 1999), focal adhesion kinase (FAK ) (Li et al. 1997), proline-rich tyrosine kinase (PyK2) (Tai et al. 2002), and vascular endothelial growth factor receptor-2 (VEGFR-2, also known as Flk-1/KDR ) (Chen et al. 1999). VEGFR-2 can act as a mechanosensor in cell-to-cell junctions (Tzima et al. 2005; Shay-Salit et al. 2002; Osawa et al. 2002). Shear stress rapidly induces VEGFR-2 oligomerization, tyrosine phosphorylation, and association with different adaptor proteins, resulting in the transduction of signals and the activation of cellular functions. Several studies showed that shear stress transiently induced tyrosine phosphorylation of VEGFR-2 , VEGFR-2 -Shc association, or VEGFR-2 -casitas B-lineage lymphoma (CDl) association, leading to the activation of ERK , c-Jun N-terminal kinases (JNK), or IkB kinase (IKK ), respectively (Chen et al. 1999; Wang et al. 2004). This shear stress-induced activation of VEGFR-2 was not affected by treatment with an anti-VEGF antibody, suggesting that the shear stress-induced activation of VEGFR-2 was independent of its ligand. Jin et al. reported that shear stress induced the ligand-independent activation of VEGFR-2 and caused the recruitment of phosphatidylinositol 3-kinase (PI3K) and consequent Akt activation and NO production (Jin et al. 2003). Wang et al. showed that shear stress induced the activation of integrins to transactivate the association of VEGFR-2 and Cbl, leading to the tyrosine phosphorylation of Cbl and creating potential docking sites for downstream signaling molecules. These results indicate that integrin interacts with VEGFR-2 in response to shear stres s (Wang et al. 2002).
2.6 BMPRs
BMPRs are a family of transmembrane serine/threonine kinases, including the type I receptors BMPR1A and BMPR1B and the type II receptor BMPR2, both of which are required for Smad signal transduction and transcriptional activity (Miyazono et al. 2005). In the canonical bone morphogenetic protein (BMP) pathway, the type I and II BMPRs do not associate, and Smad remains in the cytoplasm in the absence of BMP binding. Upon BMP binding, the type I and II BMPRs associate into dimers, leading to the phosphorylation of BMPR type I by BMPR type II. This phosphorylation of BMPR type I results in the phosphorylation of downstream Smads and their subsequent translocation into the nucleus. Different combinations of type I and II BMPRs are expressed in ECs, and their responses to the binding of various BMPs have been studied. For example, BMP2/4 induce the activation of BMPR1A or BMPR1B and BMPR2, leading to Smad1/5/8 phosphorylation and the regulation of cell proliferation, inflammation, and angiogenesis (Dyer et al. 2014). Ankeny et al. showed that Smad1/5 were highly activated in the calcified fibrosa endothelia of human aortic valves, which experience disturbed flow (Ankeny et al. 2011). These results suggest that Smad molecules may be regulated by oscillatory flow and contribute to shear stress-associated vascular disorders. Using a combination of in vitro/in vivo studies and clinical specimens, Zhou et al. demonstrated that BMPRs served as mechanosensors and that BMPR-specific Smad1/5 acted as important mechanosensitive molecules for vascular EC cycle progression in response to oscillatory flow (Zhou et al. 2012, 2013). This oscillatory flow induced the activation of BMPRII through its intracytoplasmic kinase domain to induce the BMPR1B-integrin αvβ3 association, resulting in Smad 1/5 phosphorylation and Smad1/5-Runx2 association (Zhou et al. 2013). This oscillatory flow-induced sustained activation of BMPR-specific Smad1/5 was mediated by the Shc/FAK /ERK pathway . This activation led to the activation of Runx2, mTOR, and p70S6K and the subsequent upregulation of cyclin A and the downregulation of p21CIP1 and p27KIP1 and, hence, EC cell cycle progression (Fig. 2.1a). Pretreating these ECs with the BMP ligand inhibitor Noggin and BMP2/4-specific siRNAs could not abolish the oscillatory flow-induced activation of Smad1/5, indicating that the oscillatory flow-induced activation of BMPR-specific Smad1/5 was BMP ligand independent. The study of experimentally stenosed abdominal aortae in rats further substantiated that the activation of BMPR-specific Smad1/5 and cell cycle progression in vascular ECs in response to the oscillatory flow occurred in a manner similar to that observed in vitro. The force specificity of BMPR-specific Smad1/5 activation was induced in the EC layer of post-stenotic sites that experienced disturbed flow (Fig. 2.1b). However, Smad1/5 activation was not inhibited by intra-arterial injection of Noggin into the stenosed rat abdominal aortae. BrdU-positive cells were highly prevalent in the region of BMPR-specific Smad1/5 activation, and the percentage of BrdU-positive cells was reduced by a lentiviral Smad5-specific shRNA. These data suggest that BMPR-specific Smad1/5 may serve as promising hemodynamic-based targets for therapeutic intervention against EC dysfunction-associated vascular disorders, such as atherosclerosis.
Oscillatory flow induces EC cycle progression through the activation of BMPR-specific Smad1/5 in a BMP -independent manner. (a) Oscillatory flow induces BMPRII activation through its intracytoplasmic kinase domain to induce the BMPR1B-integrin αvβ3 association, resulting in Smad1/5 phosphorylation and its association with Runx2. Smad1/5 activation is mediated by the Shc/FAK /ERK pathway, leading to Runx2, mTOR, and p70S6K activation and the consequential upregulation of cyclin A and downregulation of p21CIP1 and p27KIP1 in ECs. (b) The panoramic examination of Smad1/5 phosphorylation levels in stenosed rat abdominal aortae from the upstream through midpoint to downstream of the constriction. Auto, autofluorescence of the vessel wall. Pictures modified from Zhou et al. (2012, 2013)
3 Cell-to-Cell Junctions
Endothelial junctions are formed by tight junctions, vascular endothelial cadherin (VE-cadherin), and their intracellular components (Dejana 2004). ECs also express the platelet endothelial cell adhesion molecule (PECAM ), which promotes hemophilic adhesion at sites of intercellular contact. The mechanosensory complex of cell-to-cell junctions is composed of PECAM-1 , VE-cadherin, and VEGFR-2 (Tzima et al. 2005). This mechanosensory complex is necessary for the activation of shear stress-induced signal pathways.
3.1 PECAM-1
PECAM-1 (also known as CD31 ) is a glycoprotein with six Ig-like loops. It is a transmembrane protein with a short cytoplasmic domain that is expressed by ECs, platelets, and leukocytes (Newman et al. 1990). In EC monolayers, PECAM-1 is located at intercellular junctions and utilizes hemophilic binding to neighboring ECs to form calcium-independent cell-to-cell adhesions (Ayalon et al. 1994). The cytoplasmic tail of PECAM-1 contains two tyrosine residues (Y663 and Y686), each of which is located in an immunoreceptor tyrosine-based inhibitory motif. These residues can be phosphorylated by Src family kinases, leading to binding to SH2 -domain-containing protein tyrosine phosphatase-2 (SHP-2) domains (Jackson et al. 1997). PECAM-1 has been demonstrated to serve as a mechanosensor in ECs exposed to shear stress (Tzima et al. 2005; Osawa et al. 2002; Chiu et al. 2008). Shear stress induces the rapid phosphorylation of PECAM-1 , resulting in SHP-2 binding, and mediates nuclear factor (NF)-kB and ERK /mitogen-activated protein kinase (MAPK) activation (Tzima et al. 2005; Osawa et al. 2002). Knockdown of PECAM-1 reduced the extent of lesions at the aortic arch and the aortic sinus, suggesting that PECAM-1 played an important role in the regulation of atherosclerotic progression (Goel et al. 2008; Stevens et al. 2008).
3.2 VE-Cadherin
VE-cadherin is an endothelial cell-specific main adhesive protein that is critical for the control of vascular permeability. VE-cadherin is mediated by transmembrane proteins that promote the homophilic interaction with neighboring ECs and form calcium-dependent cell-to-cell adhesion sites (Dejana et al. 1995). In the cytoplasmic domain, VE-cadherin forms complexes with α- and β-catenin, plakoglobin, vinculin, and cingulin that directly or indirectly bind to the actin cytoskeleton and signaling proteins, allowing the transfer of intracellular signals inside the ECs (Dejana 2004; Lampugnani et al. 1995). These cadherin complexes are actively involved in force-dependent junction remodeling. For example, vinculin has been demonstrated to not be necessary for cell-to-cell junction formation or maintenance but is needed for force-dependent junction remodeling to protect junctions from opening by leukocyte extravasation or angiogenic sprouting (Huveneers et al. 2012). Tolbert et al. demonstrated that Y1065 phosphorylation in vinculin regulated mechanical force-induced F-actin bundle formation (Tolbert et al. 2014). These results indicated that vinculin might serve as a mechanosensitive molecule in response to mechanical forces. Shear stress induced the alignment of ECs with the flow direction and altered the protein levels of VE-cadherin and its complexes; however, the partial disassembly of VE-cadherin was localized at EC junctions (Noria et al. 1999). These results imply that VE-cadherin controls the endothelial permeability barrier and is necessary for EC responses to shear stress. Although VE-cadherin is essential for the EC response to shear stress, it does not serve as a major mechanosensor for ECs in response to shear stress. PECAM-1 depletion abolished the shear stress-induced activation of PI3K -dependent events and Src family kinases. However, VE-cadherin depletion did not inhibit the activation of Src family kinases. A previous study showed that shear stress-induced activated VEGFR-2 formed a complex with VE-cadherin/β-catenin that was required for PI3K activation (Shay-Salit et al. 2002; Carmeliet et al. 1999). These results suggest that VE-cadherin primarily functions as an adapter molecule in association with PECAM-1, whereas VEGFR-2 plays a role in transducing shear stress-dependent signals into ECs.
4 Cell Matrix Receptor
4.1 Integrins
Integrins are transmembrane receptors that function in cell adhesion to extracellular matrix (ECM) proteins. Integrins play important roles in cell–cell adhesion and communication with the ECM . Integrins are composed of α and β subunits that form 24 distinct heterodimers from a combination of 18 α and 8 β subunits (Hynes 2002). Each subunit has a large extracellular domain, a transmembrane-spanning region, and a short cytoplasmic domain. The extracellular domain typically binds to an Arg-Gly-Asp (RGD) sequence that is present in various ECM ligands, such as collagen, fibronectin, laminin, and vitronectin. The cytoplasmic domains of both the α and β subunits are the sites of interaction with cytoskeletal and signaling proteins, including talin, α-actinin, focal adhesion kinase (FAK ), and c-Src. This important linkage modulates cell motility, cytoskeletal organization, signal transduction, and transcription via integrin activity (Hynes 1999). The unique structural features of integrins enable them to use bidirectional (outside-in and inside-out) signaling to integrate the intracellular and extracellular environments. In outside-in signaling, extracellular stimuli induce the intracellular signaling cascade via integrin activation. In inside-out signaling, intracellular signals trigger the cytoplasmic domains that induce the affinity of integrins for extracellular ligands (Schwartz and Ginsberg 2002; Takagi et al. 2002; Campbell and Humphries 2011). Evidence for integrins functioning as mechanosensors was provided by the finding that pulling integrins using RGD-coated microbeads or micropipettes resulted in cytoskeletal filament reorientation and nuclear distortion and redistribution along the axis of the applied forces (Maniotis et al. 1997). This evidence also provides a molecular connection between the integrin, cytoskeletal filaments, and nuclear scaffolds. Shear stress also induces FAK , MAPK, and IkB kinase (IKK) activation in a manner that can be attenuated by the anti-αvβ3 antibody LM609. These results show that the shear stress-induced activation of signaling molecules is integrin dependent (Li et al. 1997; Bhullar et al. 1998). The direct activation of integrin activity in ECs by shear stress was confirmed by immunostaining with the WOW-1 antibody, which specifically recognizes the activated integrin αvβ3. Shear stress rapidly activates the cluster of integrins into a high-affinity state and induces their association with the adaptor protein Shc (Tzima et al. 2001; Wang et al. 2002). FAK and Shc are major molecules that mediate the integrin-dependent activation of downstream MAPKs in response to shear stress (Shyy and Chien 1997, 2002). In the FAK -dependent pathway, FAK is autophosphorylated at Tyr397 and associates with the Src homology 2 (SH2) domain of c-Src, leading to paxillin and p130CAS phosphorylation by c-Src and the recruitment of various adaptor proteins as a result of ERK activation (Geiger et al. 2009). Cav-1 and the Fyn tyrosine kinase are critical molecules in the Shc-dependent pathway. Cav-1 constitutively associates with the Src family member Fyn and interacts with the integrin α subunit within the lipid bilayer. Following integrin activation, Fyn is activated and binds to Shc, leading to the phosphorylation of Shc at Tyr317 and the recruitment of the adaptor protein Grb2. This sequence of events leads to the activation of the Ras-ERK pathway (Hynes 2002; Geiger et al. 2009).
5 Cytoskeleton and Nucleus
5.1 Cytoskeleton
Maniotis et al. demonstrated that mechanical tugging on the integrin receptors caused reorientation of the cytoskeletal filaments, nuclear distortion, and nucleolar redistribution along the axis of the applied tension field. These effects were specific for integrins and were mediated by the direct linkage between the cytoskeleton and the nucleus (Maniotis et al. 1997). The cytoskeleton is composed of three major types of protein filaments: microfilaments, intermediate filaments, and microtubules. Microfilaments are actin polymers, which are the most abundant molecules in the cell and form a continuous dynamic connection between cellular structures. The assembly of actin filaments is controlled by the Rho family of small GTP-binding proteins, including Rho, Rac, and cdc42, which dynamically remodel themselves in response to mechanical stimuli (Goehring and Grill 2013). Intermediate filaments contain eight protofilaments wound around each other in a ropelike structure. Intermediate filaments include class I, II, III, IV, and V proteins. The class III protein vimentin regulates the focal contact size to stabilize cell–matrix adhesion and is displaced by shear stress (Tsuruta and Jones 2003; Helmke et al. 2000). Recent studies suggested that the class V protein lamin also contributes to the mechanosensing pathway to propagate mechanical forces to the nuclear surface (Fedorchak et al. 2014; Alam et al. 2014; Osmanagic-Myers et al. 2015). Microtubules are assembled by protofilaments surrounding a hollow center, and the microtubule organizing center (MTOC) mediates the nucleation of the tubulins into microtubules. Under stimulation by shear stress, the MTOC is located posterior to the nucleus and helps to orient EC polarity (Masuda and Fujiwara 1993; Tzima et al. 2003). The cilia and flagella are composed of microtubules, and primary cilia have been identified as an important mechanosensor (Pazour and Witman 2003). These filaments themselves are interconnected and are also linked to membrane proteins throughout the cell. The linkages between external cellular contacts, adhesion receptors, and the cytoskeleton serve as mechanotransmitters for bidirectional communication between the interior and exterior of the cell. Furthermore, the cytoskeleton is “hardwired” to propagate mechanical forces to the nuclear surface, leading to nuclear deformation and gene expression (Thomas et al. 2002; Ingber 1997, 2003a, b).
5.2 Primary Cilia
There are two types of cilia in eukaryotic cells: motile cilia and nonmotile cilia (primary cilia). Primary cilia function as sensory organelles in response to chemical and physical stimuli and regulate tissue morphogenesis (Pazour and Witman 2003). In human aortic ECs, primary cilia were first observed by electron microscopy (Bystrevskaya et al. 1992). Single endothelial primary cilia protrude 1–5 μm from the apical surface and consist of a 9+0 bundle core of microtubule doubles. They extend from the basal body of the cell, where they connect to the cytoskeleton microtubules in the cytoplasm (Egorova et al. 2012). Using immunostaining with acetyl-α-tubulin, Iomini et al. (2004) found that endothelial primary cilia were disassembled by laminar shear stress at 15 dynes/cm2. Nauli et al. (2008) demonstrated that cytosolic calcium and eNOS were induced in ECs by shear stress at 1.1 dynes/cm2. Knockdown of endothelial primary cilia resulted in an inability to transmit the shear stress stimulus into intracellular calcium signaling and NO synthesis. Additionally, the loss of primary cilia promoted a shear stress-induced endothelial-to-mesenchymal transition (EMT ) in ECs and a fibroblast-like phenotype (Egorova et al. 2011). The in vivo zebrafish model demonstrated that endothelial primary cilia could sense extraordinarily low shear stress in a polycystin-dependent manner and transduce the shear stress stimulus into intracellular calcium signaling (Goetz et al. 2014). These results demonstrated that endothelial primary cilia served as mechanosensors and modulated cellular functions. In vivo observations from C57BL/6 and apolipoprotein-E-deficient mice demonstrated that endothelial primary cilia were found in increased numbers in the area of atherosclerotic predilection where the shear was low and oscillatory, whereas endothelial primary cilia were absent in the areas of the atheroprotective region where the shear had a high and uniform direction (Van der Heiden et al. 2008). Thus, it is exciting to postulate that endothelial primary cilia may contribute to the formation of atherosclerosis .
5.3 Nucleus
The nucleus is encapsulated by the nuclear envelope, a double lipid membrane composed of the inner and outer nuclear membranes. Lamins are subjacent to the inner membrane of the nuclear envelope and tether the nucleus to the surrounding cytoskeleton via linker of nucleoskeleton and cytoskeleton (LINC) complexes. The LINC complexes are composed of SUN (Sad1p, UNC-84) domain proteins that span the inner membrane and KASH (Klarsicht/ANC-1/Syne Homology) domain proteins on the outer membrane (Crisp et al. 2006). The nucleus is connected to the rest of the cell through the LINC complexes. Mechanosensory complexes are known to transduce mechanical stimuli from the extracellular environment to the inside of the nucleus, where these stimuli are converted into biochemical signals and consequently result in gene expression and cellular functions. Whether the capacity of the nucleus to directly sense mechanical stimuli from the extracellular environment is dependent on the cellular geography and unique features of the mechanotransensing pathway requires further investigation. The nucleus was suggested to function as an intracellular mechanical force-bearing organelle in studies by Deguchi et al. and Dahl et al. (Deguchi et al. 2005; Dahl et al. 2005). Indeed, EC nuclei were significantly elongated after exposure to shear stress at 20 dynes/cm2 for 24 h. The micropipette aspiration technique on the isolated nuclei revealed that the elastic modulus of the shear stress-induced elongated nuclei was significantly higher compared to the control nuclei. These results suggested that the structure of the nuclei could be directly remodeled by the shear stress. Tkachenko et al. demonstrated that shear stress rapidly displaced the EC nuclei forward to downstream of the flow direction, resulting in a planar cell polarity and relocation of the MTOC by cytoskeletal motors (Tkachenko et al. 2013). The distance of the displaced nuclei by shear stress was 8 μm on average within 5 s; this rapid movement of the nuclei was unlikely to be caused by intracellular signaling events. These results suggest that EC nuclei may serve as mechanosensory organelles and that the shear stress-induced displacement of the nuclei contributes to the triggering of intracellular signaling events and eventually the relocation of the MTOC (Tzima et al. 2003; McCue et al. 2006).
Recent studies indicated that several nuclear envelope proteins were involved in the mechanosensing pathway , including nuclear lamins, emerin, and the LINC complexes (Fedorchak et al. 2014). The A-type lamins are the major nuclear mechanosensors of the mechanosensing pathway from the ECM into the nucleus (outside-in) and are key molecules of the nucleocytoskeletal coupling machinery. Poh et al. demonstrated local dynamic forces based on the matrix elasticity of transmitted forces from the ECM into the nuclear body; these forces were transmitted to the nuclear envelope via the F-actin/LINC/nuclear lamina structural pathways. Moreover, the forces triggered the dissociation of the SMN protein and coilin from the nuclear body. Lamin A knockout abolished the force-induced dissociation of SMN and coilin from the nuclear body, indicating an essential role for lamin A in the transmission of force to the nuclear body (Poh et al. 2012). The cells not only adjusted to the increase of mechanical forces by remodeling the structure of the nucleus and lamin A but also increased the level of lamin A in response to mechanical forces (Swift et al. 2013). Matrix elasticity directs the lineage specification of human bone marrow-derived mesenchymal stem cells (MSCs) in culture towards bone, fat, or other tissue types. A soft matrix promotes increased adipogenesis and decreased lamin A expression. Overexpression of lamin A in MSCs on the soft matrix led to an increase in osteogenesis, suggesting that lamin A enhanced matrix elasticity-directed differentiation. These studies showed that lamin A served as a nuclear mechanosensor for both the outside-in signal and the inside-out signal in the mechanosensing pathway. Transmission of mechanical forces into the nucleus requires the mechanical connection of the nucleus to the cytoskeleton; these connections are mediated by the LINC complexes. The LINC complexes consist of SUM domain proteins and KASH domain proteins, and intact LINC complexes are required for nuclear position, cell polarization, and force propagation (Jaalouk and Lammerding 2009; Lombardi and Lammerding 2011). There are five SUM domain proteins and six KASH domain proteins (nesprins 1–4, LRMP, and KASH5) in mammals, although only SUN1 and SUM2 have been demonstrated to associate with the nuclear lamina and nesprin 1–3 by directly binding to the cytoskeleton (Fig. 2.2) (Jaalouk and Lammerding 2009; Wang et al. 2012; Sosa et al. 2012). Disruption of these LINC complexes results in impaired cell motility, shear stress-induced cell polarization, defects in nuclear positioning and centrosome orientation, and disrupted perinuclear organization of actin and vimentin. The LINC complexes have been suggested to play prominent roles in force transmission between the nucleus and the cytoskeleton (Chancellor et al. 2010; Chambliss et al. 2013; Morgan et al. 2011; Lombardi et al. 2011). Interestingly, depletion of nesprin-1 caused an increase in the nuclear height, focal adhesion assembly, and traction in ECs. Actomyosin tension has been suggested to be balanced by the nucleus due to mechanical links mediated by nesprin-1. In the absence of these connections, actomyosin forces are assumed to be balanced by an additional number of focal adhesions, resulting in a decrease in cell motility. This evidence implies that nesprin-1 functions as a nuclear mechanosensor by linking mechanical force transmission between the nucleus and the cytoskeleton.
The mechanosensing system in the endothelial nucleus. LINC complexes include the SUN (Sad1p, UNC-84) domain proteins that span the inner membrane and the KASH (Klarsicht/ANC-1/Syne Homology, nesprins 1–4, LRMP, and KASH5) domain proteins on the outer membrane. Nesprin-1 and nesprin-2 connect F-actin, whereas Nesprin-3 connects the intermediate filament to the outer nuclear membrane
In addition to the nuclear envelope proteins, transmembrane actin-associated nuclear (TAN) lines , which are assembled from nesprin-2G and SUN-2, act as linkers between the actin bundles on the top of the nuclear surface and the nucleus. TAN lines are anchored by lamin A and allow the forces generated by the actin cytoskeleton to be transmitted to the nuclear envelope, consequently resulting in the movement of the nucleus. Super-resolution microscopy revealed that the structure of the TAN lines across the nuclear membrane was similar to the focal adhesions that crossed the cellular membrane. It is likely that the TAN lines are composed of additional cytoplasmic and nucleoplasmic proteins (Luxton et al. 2011). The perinuclear actin cap (actin cap) is composed of thick, parallel, and highly contractile actomyosin filament bundles that are anchored to the apical surface of the nucleus by LINC complexes and terminate at actin cap-associated focal adhesions (ACAFAs) at the basal surface of the adherent cell (Kim et al. 2013). Chambliss et al. showed that the conventional basal stress fiber reformed and organized in response to fluid shear stress at 0.5 dyne/cm2. The actin cap formed at shear stress 50 times lower (as low as 0.01 dyne/cm2 within 5 min) than the formation of the conventional basal stress fiber. This evidence suggests that the actin cap plays a key role in the fast and efficient transmission of mechanical forces from the ECM to the nucleus (Chambliss et al. 2013).
6 Summary and Conclusion
Most endothelial mechanosensors are located at the plasma membrane and serve as primary sensors in response to shear stress (Fig. 2.3). The cytoskeleton and the nucleus act as secondary sensors to accomplish the mechanotransduction. These mechanosensors activate upstream signaling molecules and mediate intracellular signaling through phosphorylation cascades via their associated adaptor proteins or elicited signaling proteins, eventually leading to the maintenance of endothelial homeostasis (Table 2.1). In this manuscript, we listed the endothelial mechanosensors and mechanosensing systems involved in the EC response to shear stress. Although these mechanosensors were identified one decade ago, the detailed mechanisms of the effects of ion channels in response to shear stress remain unclear. Ion channels are fast mechanoresponsive molecules and may play a role in the regulation of atherosclerosis. Due to the limitations of technical approaches and in vivo studies, little is known about which ion channels have the most important functional impacts on vascular physiology/pathophysiology in health and disease. Recent studies indicated novel mechanosensing in the nucleus. Several nuclear proteins, such as lamin A and the LINC complexes, have been identified as molecules that serve as nuclear mechanosensors and regulate signaling pathways in the nucleus. The mechanisms by which these nuclear mechanosensors regulate gene expression by transmitting signals into the nucleus and inducing a nuclear conformational remain unclear. These issues deserve further investigation.
The endothelial primary mechanosensors in response to shear stress . In ECs, shear stress is sensed by the primary mechanosensor on the membrane, including the ion channels, GPCRs , glycocalyx, caveolae, primary cilia, RTKs, BMPRs, mechanosensory complexes of cell-to-cell junctions (PECAM-1 , VE-cadherin, and VEGFR-2 ), and integrin. The shear stress-induced activity of ion channels results in a Ca2+ influx and leads to NO production. G proteins are rapidly activated within 1 s by shear stress, leading to Ras activation and NO production. Activation of the glycocalyx and primary cilia results in NO production. Shear stress induces the phosphorylation and activation of caveolae and triggers NO production and ERK activation. The oscillatory flow induces the activation of BMPR-specific Smad1/5. The activation of RTKs and mechanosensory complexes of the cell-to-cell junction by shear stress induces PI3K /Akt activation, leading to NO production. PECAM-1 is phosphorylated by shear stress, resulting in SHP-2 recruitment and ERK activation. Activated integrins associate with the adaptor protein Shc and FAK and mediate the integrin-dependent activation of downstream MAPKs
There is evidence indicating that different mechanosensors can interact with one another to transmit mechanical stimuli into the cell interior. Integrins and the ECM are highly interactive, and this interaction causes integrin activation. Computational models predict that the glycocalyx largely mediates the interaction of integrins and the ECM , suggesting that the glycocalyx may be a key regulator of integrin functions (Paszek et al. 2009). Cilia sense mechanical forces through polycystins and trigger intracellular calcium signaling and nitric oxide synthesis. Recent studies showed that the ECM and integrins surrounded the primary cilia, implying that integrins might be involved in shear stress-induced cilia activation (McGlashan et al. 2006; Drummond 1812). The oscillatory flow induces the association of BMPR1B-integrin αvβ3 with the intracytoplasmic kinase domain of BMPRII, resulting in the activation of BMPRII and phosphorylation of Smad1/5. PECAM-1 can interact with VE-cadherin and VEGFR-2 to form a mechanosensory complex that transduces shear stress-dependent signals into cells. The GPCRs , G-protein subunits Gαq/11, and PECAM-1 form a mechanosensory complex in response to shear stress. The shear stress-induced activation of integrins is required for the involvement of VEGFR-2 and Cav-1 to transduce signaling pathways. These results indicate that integrins are able to associate with other mechanosensors to transduce mechanical stimuli into the cell interior. Nuclear envelope proteins, such as nuclear lamins, emerin, and LINC complexes, and cytoskeleton molecules, such as TAN lines and the actin cap, are involved in the nuclear mechanosensing systems. These findings indicate that multiple endothelial mechanosensors work together to accomplish mechanosensing and mechanotransduction rather than a single mechanosensor. Indeed, a single endothelial mechanosensor is unlikely to exist.
In vivo studies revealed that these mechanosensors are involved in atherosclerotic formation. PECAM-1 and cilia were suggested to contribute to atherosclerotic progression. PECAM-1 was required for the activation of NF-kB and the downstream inflammatory responses induced by shear stress. PECAM-1 knockdown reduced lesion formation. Single-nucleotide polymorphisms in the human PECAM-1 gene revealed links to early atherosclerosis and cardiovascular diseases because these polymorphisms influenced the tyrosine phosphorylation of PECAM-1 and leukocyte transmigration (Elrayess et al. 2003, 2004). Primary cilia were located in increased numbers in the atheroprone region and were disrupted by high shear stress. Moreover, primary cilia are unlikely to play a role in the atheroprotective region, which is characterized by high shear stress and a uniform flow. These results strongly imply the correlation of atherosclerotic formation with PECAM-1 and cilia. Whether other mechanosensors in addition to PECAM-1 and cilia, such as ion channels and nuclear mechanosensing systems, are involved in atherosclerosis deserves further investigation.
References
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104:860–869
Alam S, Lovett DB, Dickinson RB, Roux KJ, Lele TP (2014) Nuclear forces and cell mechanosensing. Prog Mol Biol Transl Sci 126:205–215
Ankeny RF, Thourani VH, Weiss D, Vega JD, Taylor WR, Nerem RM, Jo H (2011) Preferential activation of smad1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low bmp antagonists and smad6. PLoS One 6, e20969
Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B (1994) Spatial and temporal relationships between cadherins and pecam-1 in cell-cell junctions of human endothelial cells. J Cell Biol 126:247–258
Baeyens N, Mulligan-Kehoe MJ, Corti F, Simon DD, Ross TD, Rhodes JM, Wang TZ, Mejean CO, Simons M, Humphrey J, Schwartz MA (2014) Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc Natl Acad Sci U S A 111:17308–17313
Barakat AI, Leaver EV, Pappone PA, Davies PF (1999) A flow-activated chloride-selective membrane current in vascular endothelial cells. Circ Res 85:820–828
Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M (1999) Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729–777
Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, Chien S (1998) Fluid shear stress activation of ikappaB kinase is integrin-dependent. J Biol Chem 273:30544–30549
Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M (1996) On the molecular basis and regulation of cellular capacitative calcium entry: roles for trp proteins. Proc Natl Acad Sci U S A 93:15195–15202
Brakemeier S, Kersten A, Eichler I, Grgic I, Zakrzewicz A, Hopp H, Kohler R, Hoyer J (2003) Shear stress-induced up-regulation of the intermediate-conductance ca(2+)-activated k(+) channel in human endothelium. Cardiovasc Res 60:488–496
Butcher DT, Alliston T, Weaver VM (2009) A tense situation: forcing tumour progression. Nat Rev Cancer 9:108–122
Bystrevskaya VB, Lichkun VV, Krushinsky AV, Smirnov VN (1992) Centriole modification in human aortic endothelial cells. J Struct Biol 109:1–12
Campbell ID, Humphries MJ (2011) Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 3, pii: a004994
Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367:463–467
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157
Chachisvilis M, Zhang YL, Frangos JA (2006) G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A 103:15463–15468
Chambliss AB, Khatau SB, Erdenberger N, Robinson DK, Hodzic D, Longmore GD, Wirtz D (2013) The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci Rep 3:1087
Chancellor TJ, Lee J, Thodeti CK, Lele T (2010) Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys J 99:115–123
Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB (2003) Shear stress increases expression of a katp channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285:C959–C967
Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY (1999) Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and shc. J Biol Chem 274:18393–18400
Chien S (2007) Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292:H1209–H1224
Chiu YJ, McBeath E, Fujiwara K (2008) Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited pecam-1 phosphorylation. J Cell Biol 182:753–763
Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D (2006) Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 172:41–53
Dahl KN, Engler AJ, Pajerowski JD, Discher DE (2005) Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 89:2855–2864
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6:16–26
Deguchi S, Maeda K, Ohashi T, Sato M (2005) Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. J Biomech 38:1751–1759
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Dejana E, Corada M, Lampugnani MG (1995) Endothelial cell-to-cell junctions. FASEB J 9:910–918
dela Paz NG, Melchior B, Shayo FY, Frangos JA (2014) Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (pecam-1) and the galphaq/11 subunits of heterotrimeric g proteins. J Biol Chem 289:7413–7424
Doyle DA (2004) Molecular insights into ion channel function (review). Mol Membr Biol 21:221–225
Drummond IA (1812) Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta 2011:1322–1326
Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X (2014) Trpv4, trpc1, and trpp2 assemble to form a flow-sensitive heteromeric channel. FASEB J 28:4677–4685
Dyer LA, Pi X, Patterson C (2014) The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol Metab 25:472–480
Ebong EE, Macaluso FP, Spray DC, Tarbell JM (2011) Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol 31:1908–1915
Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP (2011) Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108:1093–1101
Egorova AD, van der Heiden K, Poelmann RE, Hierck BP (2012) Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 83:S56–S61
Elrayess MA, Webb KE, Flavell DM, Syvanne M, Taskinen MR, Frick MH, Nieminen MS, Kesaniemi YA, Pasternack A, Jukema JW, Kastelein JJ, Zwinderman AH, Humphries SE (2003) A novel functional polymorphism in the pecam-1 gene (53g > a) is associated with progression of atherosclerosis in the LOCAT and REGRESS studies. Atherosclerosis 168:131–138
Elrayess MA, Webb KE, Bellingan GJ, Whittall RA, Kabir J, Hawe E, Syvanne M, Taskinen MR, Frick MH, Nieminen MS, Kesaniemi YA, Pasternack A, Miller GJ, Humphries SE (2004) R643g polymorphism in pecam-1 influences transendothelial migration of monocytes and is associated with progression of CHD and CHD events. Atherosclerosis 177:127–135
Falcone JC, Kuo L, Meininger GA (1993) Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol 264:H653–H659
Fedorchak GR, Kaminski A, Lammerding J (2014) Cellular mechanosensing: getting to the nucleus of it all. Prog Biophys Mol Biol 115:76–92
Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93:e136–e142
Forsyth SE, Hoger A, Hoger JH (1997) Molecular cloning and expression of a bovine endothelial inward rectifier potassium channel. FEBS Lett 409:277–282
Fu BM, Tarbell JM (2013) Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med 5:381–390
Fujioka K, Azuma N, Kito H, Gahtan V, Esato K, Sumpio BE (2000) Role of caveolin in hemodynamic force-mediated endothelial changes. J Surg Res 92:7–10
Gautam M, Gojova A, Barakat AI (2006a) Flow-activated ion channels in vascular endothelium. Cell Biochem Biophys 46:277–284
Gautam M, Shen Y, Thirkill TL, Douglas GC, Barakat AI (2006b) Flow-activated chloride channels in vascular endothelium. Shear stress sensitivity, desensitization dynamics, and physiological implications. J Biol Chem 281:36492–36500
Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10:21–33
Glass R, Burnstock G (2001) Immunohistochemical identification of cells expressing atp-gated cation channels (p2x receptors) in the adult rat thyroid. J Anat 198:569–579
Goehring NW, Grill SW (2013) Cell polarity: mechanochemical patterning. Trends Cell Biol 23:72–80
Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK (2008) Site-specific effects of pecam-1 on atherosclerosis in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol 28:1996–2002
Goetz JG, Steed E, Ferreira RR, Roth S, Ramspacher C, Boselli F, Charvin G, Liebling M, Wyart C, Schwab Y, Vermot J (2014) Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep 6:799–808
Gudi SR, Clark CB, Frangos JA (1996) Fluid flow rapidly activates g proteins in human endothelial cells. Involvement of g proteins in mechanochemical signal transduction. Circ Res 79:834–839
Gudi S, Nolan JP, Frangos JA (1998) Modulation of gtpase activity of g proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A 95:2515–2519
Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA (2003) Rapid activation of ras by fluid flow is mediated by galpha(q) and gbetagamma subunits of heterotrimeric g proteins in human endothelial cells. Arterioscler Thromb Vasc Biol 23:994–1000
Hahn C, Schwartz MA (2009) Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10:53–62
Hansen CG, Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20:177–186
Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R (2007) Arterial response to shear stress critically depends on endothelial trpv4 expression. PLoS One 2, e827
Helmke BP, Davies PF (2002) The cytoskeleton under external fluid mechanical forces: Hemodynamic forces acting on the endothelium. Ann Biomed Eng 30:284–296
Helmke BP, Goldman RD, Davies PF (2000) Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res 86:745–752
Hoger JH, Ilyin VI, Forsyth S, Hoger A (2002) Shear stress regulates the endothelial kir2.1 ion channel. Proc Natl Acad Sci U S A 99:7780–7785
Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J (2012) Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 196:641–652
Hynes RO (1999) Cell adhesion: old and new questions. Trends Cell Biol 9:M33–M37
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687
Ingber DE (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59:575–599
Ingber DE (2003a) Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116:1157–1173
Ingber DE (2003b) Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116:1397–1408
Iomini C, Tejada K, Mo W, Vaananen H, Piperno G (2004) Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 164:811–817
Jaalouk DE, Lammerding J (2009) Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10:63–73
Jackson DE, Ward CM, Wang R, Newman PJ (1997) The protein-tyrosine phosphatase shp-2 binds platelet/endothelial cell adhesion molecule-1 (pecam-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between pecam-1- and integrin-mediated cellular signaling. J Biol Chem 272:6986–6993
Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC (2003) Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res 93:354–363
Jow F, Numann R (1999) Fluid flow modulates calcium entry and activates membrane currents in cultured human aortic endothelial cells. J Membr Biol 171:127–139
Kim DH, Chambliss AB, Wirtz D (2013) The multi-faceted role of the actin cap in cellular mechanosensation and mechanotransduction. Soft Matter 9:5516–5523
Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J (2006) Evidence for a functional role of endothelial transient receptor potential v4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26:1495–1502
Koo A, Dewey CF Jr, Garcia-Cardena G (2013) Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol 304:C137–C146
Kuchan MJ, Frangos JA (1994) Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol 266:C628–C636
Kuchan MJ, Jo H, Frangos JA (1994) Role of g proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol 267:C753–C758
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–217
Li S, Kim M, Hu YL, Jalali S, Schlaepfer DD, Hunter T, Chien S, Shyy JY (1997) Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem 272:30455–30462
Li YS, Haga JH, Chien S (2005) Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38:1949–1971
Lieu DK, Pappone PA, Barakat AI (2004) Differential membrane potential and ion current responses to different types of shear stress in vascular endothelial cells. Am J Physiol Cell Physiol 286:C1367–C1375
Liu CL, Huang Y, Ngai CY, Leung YK, Yao XQ (2006) Trpc3 is involved in flow- and bradykinin-induced vasodilation in rat small mesenteric arteries. Acta Pharmacol Sin 27:981–990
Lombardi ML, Lammerding J (2011) Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem Soc Trans 39:1729–1734
Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J (2011) The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 286:26743–26753
Luxton GW, Gomes ER, Folker ES, Worman HJ, Gundersen GG (2011) Tan lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus 2:173–181
Maniotis AJ, Chen CS, Ingber DE (1997) Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 94:849–854
Masuda M, Fujiwara K (1993) The biased lamellipodium development and microtubule organizing center position in vascular endothelial cells migrating under the influence of fluid flow. Biol Cell 77:237–245
McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL (2006) Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res 98:939–946
McGlashan SR, Jensen CG, Poole CA (2006) Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem 54:1005–1014
Miyazono K, Maeda S, Imamura T (2005) Bmp receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 16:251–263
Moccia F, Villa A, Tanzi F (2000) Flow-activated Na(+)and K(+)current in cardiac microvascular endothelial cells. J Mol Cell Cardiol 32:1589–1593
Montell C (2001) Physiology, phylogeny, and functions of the trp superfamily of cation channels. Sci STKE 2001:re1
Moon JJ, Matsumoto M, Patel S, Lee L, Guan JL, Li S (2005) Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol 203:166–176
Morgan JT, Pfeiffer ER, Thirkill TL, Kumar P, Peng G, Fridolfsson HN, Douglas GC, Starr DA, Barakat AI (2011) Nesprin-3 regulates endothelial cell morphology, perinuclear cytoskeletal architecture, and flow-induced polarization. Mol Biol Cell 22:4324–4334
Mulivor AW, Lipowsky HH (2009) Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation 16:657–666
Nakao M, Ono K, Fujisawa S, Iijima T (1999) Mechanical stress-induced Ca2+ entry and Cl- current in cultured human aortic endothelial cells. Am J Physiol 276:C238–C249
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117:1161–1171
Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296:1636–1639
Newman PJ, Berndt MC, Gorski J, White GC 2nd, Lyman S, Paddock C, Muller WA (1990) Pecam-1 (cd31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247:1219–1222
Nilius B, Droogmans G (2001) Ion channels and their functional role in vascular endothelium. Physiol Rev 81:1415–1459
Nilius B, Droogmans G, Wondergem R (2003) Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium 10:5–15
Noria S, Cowan DB, Gotlieb AI, Langille BL (1999) Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res 85:504–514
North RA (2002) Molecular physiology of p2x receptors. Physiol Rev 82:1013–1067
Numata T, Shimizu T, Okada Y (2007) Direct mechano-stress sensitivity of trpm7 channel. Cell Physiol Biochem 19:1–8
Oancea E, Wolfe JT, Clapham DE (2006) Functional trpm7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98:245–253
Ohno M, Gibbons GH, Dzau VJ, Cooke JP (1993) Shear stress elevates endothelial cGMP. Role of a potassium channel and g protein coupling. Circulation 88:193–197
Okamoto T, Schlegel A, Scherer PE, Lisanti MP (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422
Okuda M, Takahashi M, Suero J, Murry CE, Traub O, Kawakatsu H, Berk BC (1999) Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-src activation. J Biol Chem 274:26803–26809
Olesen SP, Clapham DE, Davies PF (1988) Haemodynamic shear stress activates a k+ current in vascular endothelial cells. Nature 331:168–170
Osawa M, Masuda M, Kusano K, Fujiwara K (2002) Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 158:773–785
Osmanagic-Myers S, Dechat T, Foisner R (2015) Lamins at the crossroads of mechanosignaling. Genes Dev 29:225–237
Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA (2009) Rapid changes in shear stress induce dissociation of a g alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587:2365–2373
Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H (2000) Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol 278:H1285–H1293
Parton RG (1996) Caveolae and caveolins. Curr Opin Cell Biol 8:542–548
Paszek MJ, Boettiger D, Weaver VM, Hammer DA (2009) Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput Biol 5, e1000604
Pazour GJ, Witman GB (2003) The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15:105–110
Pedersen SF, Owsianik G, Nilius B (2005) Trp channels: an overview. Cell Calcium 38:233–252
Poh YC, Shevtsov SP, Chowdhury F, Wu DC, Na S, Dundr M, Wang N (2012) Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun 3:866
Radel C, Rizzo V (2005) Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol 288:H936–H945
Ray FR, Huang W, Slater M, Barden JA (2002) Purinergic receptor distribution in endothelial cells in blood vessels: a basis for selection of coronary artery grafts. Atherosclerosis 162:55–61
Romanenko VG, Davies PF, Levitan I (2002) Dual effect of fluid shear stress on volume-regulated anion current in bovine aortic endothelial cells. Am J Physiol Cell Physiol 282:C708–C718
Rubanyi GM, Romero JC, Vanhoutte PM (1986) Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 250:H1145–H1149
Runnels LW, Yue L, Clapham DE (2001) Trp-plik, a bifunctional protein with kinase and ion channel activities. Science 291:1043–1047
Schwartz MA, Ginsberg MH (2002) Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4:E65–E68
Schwarz G, Droogmans G, Nilius B (1992) Shear stress induced membrane currents and calcium transients in human vascular endothelial cells. Pflugers Arch 421:394–396
Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N (2002) Vegf receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A 99:9462–9467
Shyy JY, Chien S (1997) Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol 9:707–713
Shyy JY, Chien S (2002) Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91:769–775
Sosa BA, Rothballer A, Kutay U, Schwartz TU (2012) LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric sun proteins. Cell 149:1035–1047
Sowa G (2012) Caveolae, caveolins, cavins, and endothelial cell function: new insights. Front Physiol 2:120
Stevens HY, Melchior B, Bell KS, Yun S, Yeh JC, Frangos JA (2008) Pecam-1 is a critical mediator of atherosclerosis. Dis Model Mech 1:175–181, discussion 179
Sun D, Huang A, Koller A, Kaley G (2001) Endothelial k(ca) channels mediate flow-dependent dilation of arterioles of skeletal muscle and mesentery. Microvasc Res 61:179–186
Sun RJ, Muller S, Stoltz JF, Wang X (2002) Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur Biophys J 30:605–611
Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE (2013) Nuclear lamin-a scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104
Tai LK, Okuda M, Abe J, Yan C, Berk BC (2002) Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol 22:1790–1796
Takagi J, Petre BM, Walz T, Springer TA (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110:599–611
Tarbell JM, Pahakis MY (2006) Mechanotransduction and the glycocalyx. J Intern Med 259:339–350
Thi MM, Tarbell JM, Weinbaum S, Spray DC (2004) The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci U S A 101:16483–16488
Thomas CH, Collier JH, Sfeir CS, Healy KE (2002) Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc Natl Acad Sci U S A 99:1972–1977
Tkachenko E, Gutierrez E, Saikin SK, Fogelstrand P, Kim C, Groisman A, Ginsberg MH (2013) The nucleus of endothelial cell as a sensor of blood flow direction. Biol Open 2:1007–1012
Tolbert CE, Thompson PM, Superfine R, Burridge K, Campbell SL (2014) Phosphorylation at y1065 in vinculin mediates actin bundling, cell spreading, and mechanical responses to force. Biochemistry 53:5526–5536
Trebak M, Vazquez G, Bird GS, Putney JW Jr (2003) The trpc3/6/7 subfamily of cation channels. Cell Calcium 33:451–461
Tsuruta D, Jones JC (2003) The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci 116:4977–4984
Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA (2001) Activation of integrins in endothelial cells by fluid shear stress mediates rho-dependent cytoskeletal alignment. EMBO J 20:4639–4647
Tzima E, Kiosses WB, del Pozo MA, Schwartz MA (2003) Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem 278:31020–31023
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437:426–431
Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (2008) Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 196:542–550
Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB (2014) Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem 289:9547–9559
Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S (2002) Interplay between integrins and flk-1 in shear stress-induced signaling. Am J Physiol Cell Physiol 283:C1540–C1547
Wang Y, Chang J, Li YC, Li YS, Shyy JY, Chien S (2004) Shear stress and vegf activate ikk via the flk-1/cbl/akt signaling pathway. Am J Physiol Heart Circ Physiol 286:H685–H692
Wang S, Meng F, Mohan S, Champaneri B, Gu Y (2009) Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation 16:276–287
Wang W, Shi Z, Jiao S, Chen C, Wang H, Liu G, Wang Q, Zhao Y, Greene MI, Zhou Z (2012) Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res 22:1440–1452
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B (2002) Activation of trpv4 channels (hvrl-2/mtrp12) by phorbol derivatives. J Biol Chem 277:13569–13577
Weinbaum S, Tarbell JM, Damiano ER (2007) The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9:121–167
Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J (2000a) P2x(4) receptors mediate atp-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol 279:H285–H292
Yamamoto K, Korenaga R, Kamiya A, Ando J (2000b) Fluid shear stress activates ca(2+) influx into human endothelial cells via p2x4 purinoceptors. Circ Res 87:385–391
Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J (2006) Impaired flow-dependent control of vascular tone and remodeling in p2x4-deficient mice. Nat Med 12:133–137
Yao Y, Rabodzey A, Dewey CF Jr (2007) Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293:H1023–H1030
Yeh JC, Otte LA, Frangos JA (2008) Regulation of g protein-coupled receptor activities by the platelet-endothelial cell adhesion molecule, pecam-1. Biochemistry 47:9029–9039
Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116:1284–1291
Zhou J, Lee PL, Tsai CS, Lee CI, Yang TL, Chuang HS, Lin WW, Lin TE, Lim SH, Wei SY, Chen YL, Chien S, Chiu JJ (2012) Force-specific activation of smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc Natl Acad Sci U S A 109:7770–7775
Zhou J, Lee PL, Lee CI, Wei SY, Lim SH, Lin TE, Chien S, Chiu JJ (2013) Bmp receptor-integrin interaction mediates responses of vascular endothelial smad1/5 and proliferation to disturbed flow. J Thromb Haemost 11:741–755
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 The American Physiological Society
About this chapter
Cite this chapter
Chen, LJ., Wang, WL., Chiu, JJ. (2016). Vascular Endothelial Mechanosensors in Response to Fluid Shear Stress. In: Chien, S., Engler, A., Wang, P. (eds) Molecular and Cellular Mechanobiology. Physiology in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-5617-3_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-5617-3_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-5615-9
Online ISBN: 978-1-4939-5617-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)