Abstract
The objective of this study was to investigate the hypothesis that the application of dynamic compression following transforming growth factor-β3 (TGF-β3) induced differentiation will further enhance chondrogenesis of mesenchymal stem cells (MSCs). Porcine MSCs were encapsulated in agarose hydrogels and cultured in a chemically defined medium with TGF-β3 (10 ng/mL). Dynamic compression (1 Hz, 10% strain, 1 h/day) was initiated at either day 0 or day 21 and continued until day 42 of culture; with TGF-β3 withdrawn from some groups at day 21. Biochemical and mechanical properties of the MSC-seeded constructs were evaluated up to day 42. The application of dynamic compression from day 0 inhibited chondrogenesis of MSCs. This inhibition of chondrogenesis in response to dynamic compression was not observed if MSC-seeded constructs first underwent 21 days of chondrogenic differentiation in the presence of TGF-β3. Spatial differences in sGAG accumulation in response to both TGF-β3 stimulation and dynamic compression were observed within the constructs. sGAG release from the engineered construct into the surrounding culture media was also dependent on TGF-β3 stimulation, but was not effected by dynamic compression. Continued supplementation with TGF-β3 appeared to be a more potent chondrogenic stimulus than the application of 1 h of daily dynamic compression following cytokine initiated differentiation. In the context of cartilage tissue engineering, the results of this study suggest that MSC seeded constructs should be first allowed to undergo chondrogenesis in vitro prior to implantation in a load bearing environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Once damaged articular cartilage has a limited reparative capacity and thus lesions often progress to arthritis.8 This has motivated the development of cell-based therapies for the repair of cartilage defects such as autologous chondrocyte implantation.5,57 A major limiting factor in extending the use of such therapies is obtaining sufficient numbers of viable chondrocytes, particularly in elderly and more osteoarthritic patients. An age-related loss in chondrocyte yield, proliferation and functional capacity has been observed in culture-expanded chondrocytes,4 while chondrocytes isolated from osteoarthritic patients exhibit reduced collagen synthesis.60 Mesenchymal stem cells (MSCs) are a promising alternative cell source for cartilage repair due to both their ease of isolation and expansion, and their chondrogenic differentiation capacity.13,58,63 Chondrogenic differentiation of MSCs from different tissue sources has been shown in the presence of members of the transforming growth factor-β (TGF-β) superfamily.25,27,28,42,51,54,68,69
The same functional demands which put cartilage at risk of injurious damage and frequent degenerative changes must be considered in the design and appraisal of any therapeutic intervention. In the context of cell-based tissue engineering therapies, mechanical factors are of particular importance. While chondrogenesis of MSCs has been demonstrated in different three-dimensional scaffolds and hydrogels,15,18,35,39,46,67 it has been shown that matrix accumulation and the subsequent mechanical properties of MSC laden constructs are lower than those of chondrocyte seeded controls.18,46 This suggests that further optimization of the cellular environment is required if MSCs are to be used to engineer cartilaginous tissues with functional properties similar to those obtainable with chondrocytes. Furthermore, if MSCs are to be implanted into the joint as part of a clinical repair strategy, the biomechanical and biochemical influences of the joint environment on MSC differentiation and extracellular matrix (ECM) synthesis must be determined.
Chondrocytes generally respond to physiological levels of dynamic compressive loading through enhanced cartilage-specific macromolecule biosynthesis.10,16,20,33,45,55,59 The differentiation pathway and biosynthetic activity of MSCs is also at least partially regulated by the biophysical environment.30,37 For example, the mechanical properties of repair tissue generated by MSCs transplanted into full-thickness cartilage defects have been shown to depend on the location of the injury,65 suggesting that the local mechanical environment is regulating cellular activity. MSCs have also been cultured in vitro in bioreactors designed to mimic certain aspects of in vivo joint loading, most commonly dynamic compression or hydrostatic pressure.2,23,43 It has been demonstrated that dynamic compressive loading in the absence of TGF-β family members can increase chondrogenic gene expression12,23,24,43,56,61 and stimulate the accretion of cartilage-like extra-cellular matrix (ECM) components34,43,56 relative to unloaded controls. However, the combined effects of dynamic compression and chondrogenic growth factor supplementation on chondrogenesis of MSCs are generally more complex.12 Dynamic compression in the presence of TGF-β has resulted in increases in both chondrogenic gene expression1,23,61 and ECM secretion.1,61 Other studies, however, indicate the contrary, with the combination of dynamic compression and TGF-β resulting in a down-regulation of chondrogenic gene-expression12 and inferior ECM accumulation34,62 when compared to unloaded controls. Previous work in our laboratory investigating the effects of long-term application of dynamic compression on MSC differentiation in the presence of TGF-β3 showed that chondrogenesis was significantly inhibited as evidenced by lower ECM deposition and resulting mechanical properties when compared to unloaded controls.62
It has been shown that the application of dynamic compression to MSC seeded constructs in the presence of TGF-β1 applied at early time-points (day 8) results in decreased aggrecan gene expression; while loading at later time-points (day 16) leads to increases in chondrogenic gene expression.49 This suggests that the mechano-sensitivity of MSCs changes depending on the stage of chondrogenesis. In similar bioreactor studies, it has been shown that delaying the application of dynamic compression to chondrocyte seeded constructs allowing ECM accumulation can enhance subsequent sulfated glycosaminoglycan (sGAG) synthesis17 or the mechanical properties of the engineered tissue.41 In this study, MSCs were allowed to undergo differentiation in the presence of TGF-β3 for 21 days prior to initiation of delayed dynamic compression. The objective of this study was to test the hypothesis that the application of dynamic compression following TGF-β3 induced differentiation would further enhance chondrogenesis of MSCs in agarose hydrogel culture. To comprehensively address this hypothesis, the study (i) investigated the spatial accumulation of ECM within agarose hydrogels; (ii) compared continued TGF-β3 supplementation and dynamic compression as potential regulators of post-cytokine initiated MSC chondrogenic differentiation; and (iii) assessed the effect of compression and TGF-β3 supplementation on sGAG retention within, and release from, MSC seeded agarose hydrogels.
Materials and Methods
Experimental Design
This study comprised of two parts. One, termed “transient TGF-β3” received TGF-β3 supplementation for the first 21 days of culture, after which it was removed. The second termed “continuous TGF-β3” received continuous TGF-β3 supplementation for the 42 day culture period. Both parts involved dynamic compression applied after a 21 day period of unloaded free-swelling (FS) culture in the presence of TGF-β3. Within each part, half the samples taken through 21 days of FS culture were subjected to dynamic compressive culture for a further 21 days; termed delayed dynamic compression (DDC). The remainder were kept in FS conditions as a control. An additional group also received dynamic compression culture from day 0 to day 42, termed continuous dynamic compression (CDC). (Details of loading magnitude and duration are provided below. The term ‘continuous’ refers to samples loaded from day 0.) The timeline and conditions are illustrated in Fig. 1. Samples were assessed at day 0, day 21, and day 42.
Schematic of experimental design. −TGF-β3: without TGF-β3 supplementation. +TGF-β3: with TGF-β3 supplementation. FS: free-swelling unloaded samples. DDC: delayed dynamic compression (maintained in free-swelling conditions for 21 days prior to dynamic compressive loading). CDC: continuous dynamic compression. −: transient TGF-β3 supplementation. +: continuous TGF-β3 supplementation. n = 5 or 6 constructs per group per time point
Cell Isolation and Expansion
MSCs were isolated from the femora of three porcine donors (4-month old; ~50 kg) within 3 h of sacrifice. Porcine MSCs were isolated and expanded according to a modified method developed for human MSCs.38 MSCs were plated at a seeding density of 5 × 103 cells/cm2 in high-glucose Dulbecco’s modified eagles medium (4.5 mg/mL d-Glucose, 200 mM l-Glutamine; hgDMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/mL)–streptomycin (100 μg/mL) (all GIBCO, Biosciences, Dublin, Ireland) and expanded to passage three in a humidified atmosphere at 37 °C and 5% CO2.
Agarose Hydrogel Encapsulation
MSCs from three donors were pooled, suspended in hgDMEM and mixed with 4% agarose (Type VII, Sigma-Aldrich, Arklow, Ireland) in phosphate buffered saline (PBS) at a ratio of 1:1 at ~40 °C, to yield a final gel concentration of 2% and a cell density of 15 × 106 cells/mL. The agarose-cell suspension was cast in a stainless steel mold to produce cylindrical constructs (∅ 5 mm × 3 mm thickness). Constructs were maintained separately in 12-well plates with 2.5 mL chondrogenic medium (CM) which consisted of hgDMEM supplemented with penicillin (100 U/mL)–streptomycin (100 μg/mL), 100 μg/mL sodium pyruvate, 40 μg/mL l-proline, 1.5 mg/mL bovine serum albumin, 4.7 μg/mL linoleic acid, 1× insulin–transferrin–selenium, 50 μg/mL l-ascorbic acid-2-phosphate (all Sigma-Aldrich, Arklow, Ireland). In addition, 100 nM dexamethasone (Sigma-Aldrich) and 10 ng/mL TGF-β3 (R&D Systems, Abingdon, UK) were added 72 h after construct fabrication; the day 0 time point. Medium was exchanged every 3 or 4 days with 500 μL samples taken from three wells for biochemical analysis.
Dynamic Compression Application
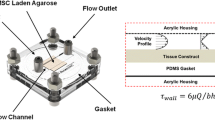
Dynamic compressive loading was applied to constructs as outlined in Fig. 1. Intermittent dynamic compression (DC) was carried out in an incubator-housed, custom-built compressive loading bioreactor, as shown in Fig. 2. Medium used for loading was the same as that in which the constructs were maintained (enabling media sampling for sGAG release as described below). Axial compression was applied via impermeable platens using an electric linear actuator with 0.05 μm resolution (Zaber Technologies Inc., Vancouver, Canada). A 1000 g load cell (RDP Electronics Ltd, Wolverhampton, UK) positioned beneath the constructs sensed the load applied. The system was controlled and data logged using LabVIEW 7 control and data acquisition software (National Instruments Corp., Newbury, UK). The dynamic compression protocol consisted of 10% strain amplitude superimposed on a 0.01 N/construct preload at a frequency of 1 Hz employed for a period of 1 h/day, 5 days/week.
Mechanical Testing and Analysis of Physical Parameters
Constructs were mechanically tested (n = 4 or 5) in unconfined compression between impermeable platens using a standard materials testing machine with a 5 N load cell (Zwick Roell Z005, Herefordshire, UK) as previously described.6 Briefly, constructs were kept hydrated through immersion in a PBS bath maintained at room temperature. A preload of 0.01 N was applied to ensure that the construct surface was in direct contact with the impermeable loading platens. Stress relaxation tests were performed consisting of a ramp displacement of 1 μm/s up to 10% strain, which was maintained until equilibrium was reached (~30 min). This was followed by a dynamic test where cyclic strain amplitude of 1% (10–11% total strain) was applied for 10 cycles at 1 Hz.
Biochemical Content
The biochemical content of constructs (n = 3 or 4) was assessed at each time point. Constructs were cored using a 3 mm biopsy punch; the wet mass of each component recorded and the construct frozen at −85 °C for later analyses. Samples were digested with papain (125 μg/mL) in 0.1 M sodium acetate, 5 mM l-cysteine HCl, 0.05 M EDTA, pH 6.0 (all Sigma-Aldrich) at 60 °C under constant rotation for 18 h. DNA content was quantified using the Hoechst Bisbenzimide 33258 dye assay as previously described.32 The proteoglycan content was estimated by quantifying the amount of sulfated glycosaminoglycan (sGAG) in constructs using the dimethylmethylene blue dye-binding assay (Blyscan, Biocolor Ltd., Carrickfergus, UK), with a shark chondroitin sulfate standard. Total collagen content was determined through measurement of the hydroxyproline content.29 A hydroxyproline-to-collagen ratio of 1:7.69 was used.26 Samples of cell culture medium taken for analysis at each media exchange (n = 3) were analyzed for sGAG secreted to the media. Total media volume was accounted for and the data is presented as the average sGAG released into the media per construct.
Histology and Immunohistochemistry
Constructs (n = 2) were fixed in 4% paraformaldehyde (Sigma-Aldrich), wax embedded and sectioned at 5 μm to produce a cross section perpendicular to the disk face. Sections were stained for sGAG with 1% alcian blue 8GX (Sigma-Aldrich) in 0.1 M HCl, and for collagen with picro-sirius red. The deposition of collagen type I and type II was identified through immunohistochemistry. Briefly, sections were quenched of peroxidase activity, rinsed with PBS before treatment with chondroitinase ABC (Sigma-Aldrich) in a humidified environment at 37 °C. Slides were rinsed with PBS and non-specific sites were blocked with goat serum (Sigma-Aldrich). Sections were then incubated overnight at 4 °C with the primary antibody; mouse monoclonal collagen type I antibody (1:400; 1.4 mg/mL; Abcam, Cambridge, UK) or mouse monoclonal anti-collagen type II (1:100; 1 mg/mL; Abcam). After washing in PBS, sections were incubated for 1 h in the secondary antibody; anti-mouse IgG biotin antibody produced in goat (1:400; 1 mg/mL; Sigma-Aldrich). Color was developed using the Vectastain ABC reagent (Vectastain ABC kit, Vector Laboratories, Peterborough, UK) followed by exposure to peroxidase DAB substrate kit (Vector Laboratories). Negative and positive controls of porcine ligament and cartilage were included for each batch.
Statistical Analysis
The study was repeated twice. Unless otherwise stated, results presented are from a single replicate which is representative of both in terms of the main study findings. Statistics were preformed using MINITAB 15.1 software package (Minitab Ltd., Coventry, UK). Groups were analyzed for significant differences using a general linear model for analysis of variance with factors of group, construct region, growth factor supplementation, dynamic compression condition and interactions between these factors examined. Tukey’s test for multiple comparisons was used to compare conditions. Significance was accepted at a level of p ≤ 0.05. Numerical and graphical results are presented as mean ± standard deviation.
Results
Differences in construct physical parameters were observed across groups (Table 1). Removal of growth factor at day 21 had no effect on construct mass, while loading did have a significant effect with FS and DDC in both studies having greater wet weights than constructs receiving dynamic compression from day 0 (CDC) at days 21 (p < 0.05) and 42 (p < 0.00005). Loading condition had an effect on thickness with continuous dynamic compression (CDC) resulting in inhibition of axial swelling; p < 0.00005.
Staining of construct sections for sGAG and collagen revealed a steady accretion of positive staining for all conditions at each time point beyond day 0. At day 21, stronger straining for proteoglycan, collagen, and specifically collagen type II was observed in the free swelling constructs (FS+) when compared to the continuous dynamic compression constructs (CDC+) (Fig. 3). This staining was especially prevalent in the pericellular region. At day 42, both FS constructs and constructs subjected to delayed dynamic compression (DDC) exhibited more intense staining for proteoglycan than continuously loaded (CDC) constructs (Fig. 4). Pericellular staining for collagen was evident in all regimes. A significant amount of extra-cellular matrix (ECM) staining was evident in FS and DDC constructs which was not present in the CDC constructs (Fig. 4).
Histological and immunohistochemical analysis for FS+ and CDC+ at day 21 with alcian blue staining for sulfated proteoglycan (top), picro-sirius red for collagen (second from top), collagen type I immunochemistry (third from top) and collagen type II immunohistochemistry (bottom). Representative images taken at the center of each construct section. Scale bar: 100 μm
Histological analysis of constructs at day 42 with alcian blue staining for sulfated proteoglycan (top) and picro-sirius red for collagen (bottom). FS: free-swelling. DDC: delayed dynamic compression, initiated on day 21. CDC: continuous dynamic compression, initiated on day 0. Representative images taken of approx. ¼ of each construct section. Scale bar: 500 μm
Immunohistochemistry indicated a strong presence of collagen type II in both the pericellular matrix (PCM) and the ECM of both FS and DDC groups for either TGF-β3 supplementation condition. Weaker collagen type II staining was evident in the CDC group with transient TGF-β3. For FS groups, the core region exhibited more intense staining than the annular region (Fig. 5). A more intense collagen type II staining was evident in constructs receiving continued TGF-β3 supplementation when compared to transient supplementation. These same constructs stained weakly for collagen type I, while FS and DDC constructs receiving transient TGF-β3 supplementation exhibited stronger staining for collagen type I, especially in the annular region (Fig. 5).
Immunohistochemical analysis of constructs at day 42 with collagen type I immunohistochemistry (top) and collagen type II immunohistochemistry (bottom). FS: free-swelling. DDC: delayed dynamic compression, initiated on day 21. CDC: continuous dynamic compression, initiated on day 0. Representative images taken of approx. ¼ of each construct section. Scale bar: 500 μm
There was a net increase in DNA content indicating cell proliferation at day 42 in groups receiving continued TGF-β3 supplementation (p = 0.0059); resulting in significantly higher DNA content than groups where TGF-β3 was removed (transient TGF-β3; p < 0.00005; Fig. 6a). sGAG content increased over day 0 values for all experimental conditions; p < 0.05 (Figs. 6b, 6c, and 8b). Removal of TGF-β3, loading condition, and construct region all had a significant effect on sGAG accumulation; p < 0.0005. Regardless of TGF-β3 supplementation condition, both FS and DDC constructs had significantly greater sGAG content than CDC constructs; p < 0.002 (Figs. 6b and 6c). At day 42, sGAG accumulation (% wet weight) was significantly greater in constructs continuously supplemented with TGF-β3 (Fig. 6b). Following the removal of TGF-β3 (transient TGF-β3), delayed dynamic compression (DDC−) resulted in a non-significant trend toward higher sGAG/DNA in comparison to free-swelling conditions (FS−); p = 0.067 (Fig. 6c). This difference was significant in the construct cores; p < 0.05 (Figs. 7b and 7c). There was no difference between FS+ and DDC+ groups at day 42.
Collagen content increased from day 0 values for all constructs; p < 0.0001 (Figs. 6d and 6e). As with sGAG, loading condition and construct region were seen to have a significant effect on collagen content; however, TGF-β3 supplementation had no effect on collagen accumulation (% wet weight). Collagen accumulation was no different in FS or DDC constructs; however, both FS− and DDC− constructs contained higher collagen/DNA than the corresponding CDC− constructs; p < 0.05 (Fig. 6e, transient TGF-β3). Increases in collagen accumulation were greatest in the construct core; p < 0.0002 (Figs. 7d and 7e).
sGAG secreted to the media increased with time in culture with significantly more sGAG secreted for all experimental conditions from day 21 to day 42 than day 0 to day 21; p < 0.0005 (Fig. 8a). Significantly more sGAG was secreted to the media from samples where TGF-β3 supplementation ceased at day 21 (transient TGF-β3), than receiving continuous supplementation; p < 0.00005 (Fig. 8a). Both FS and DDC constructs under both TGF-β3 conditions secreted significantly more sGAG to the medium than the corresponding constructs compressed from day 0 (CDC); p < 0.05 (Fig. 8).
(a) sGAG secreted per construct to medium over the experimental time period (μg). a p < 0.05 vs. Day 0–Day 21; b p < 0.05 vs. continuous TGF-β3; c p < 0.05 vs. CDC (same TGF-β3 condition); d p < 0.05 vs. DDC (same TGF-β3 condition). (b) sGAG retained per construct (μg). a p < 0.05 vs. day 0; b p < 0.05 vs. day 21; c p < 0.05 vs. CDC; d p < 0.05 vs. continuous TGF-β3. Medium data cannot be matched to individual construct data as media is pooled during bioreactor culture. Data presented in this figure are pooled from multiple experimental runs
The compressive equilibrium modulus increased with time for all conditions relative to day 0; p < 0.05 (Fig. 9a). On day 42, the compressive equilibrium modulus of FS− constructs reached a value of 16.17 ± 1.21 kPa; significantly greater than either continuous dynamic compression (CDC) group. There were no differences between FS and DDC constructs. Increases in the 1 Hz dynamic modulus with time were only seen at day 42 for FS and DDC constructs; p < 0.05 (Fig. 9b). The 1 Hz dynamic modulus was not different for both FS and DDC constructs, however, both were significantly greater than CDC constructs; p < 0.00005. The 1 Hz dynamic modulus was affected by TGF-β3 removal, with continued TGF-β3 supplementation resulting in greater dynamic moduli than transient TGF-β3 supplementation; p = 0.031.
Discussion
The chondrogenic potential of MSCs appears to be regulated by their mechanical environment, but this response depends on the stage of chondrogenesis. In the presence of TGF-β3, continuous dynamic compression was observed to inhibit chondrogenesis, corroborating our previous finding.62 By delaying the application of dynamic compression for 3 weeks until chondrogenic differentiation had been initiated (as evidenced by histological and immunohistochemical staining for proteoglycan and type II collagen), this inhibition of chondrogenesis in response to dynamic compression was not observed. There are a number of possible explanations for this temporal response to dynamic compression. The phenotype of the MSCs on initiation of dynamic compression at day 0 (CDC) and day 21 (DDC) may be one such explanation; as a greater level of chondrogenic differentiation may result in a more anabolic response to loading similar to that of chondrocytes.9,10,16,20,33,44,45,55,59 Mouw et al. demonstrated that dynamic compression applied at day 8 resulted in a decrease in aggrecan gene expression in MSCs; however, when loading was applied at day 16, an increase in chondrogenic gene expression was observed.49 Related to this, the different levels of matrix accumulation at day 0 and day 21 could also be responsible for the differential response to dynamic compression in CDC and DDC constructs. The level of PCM and ECM accumulation would affect both the local mechanical stimuli developed during loading,21 and biochemical signaling from the ECM. For example, in a study of dynamically compressed single chondrocytes and chondrons (chondrocytes with attached PCM), the presence of a PCM regulated the response to mechanical compression.66 It has also been demonstrated that the stiffness of isolated chondrons seeded into agarose is higher than that of the surrounding extracellular agarose environment, leading to stress shielding of the chondrocytes during loading.36
Akin to our previous studies, sGAG and collagen accumulation in MSC laden constructs was greatest in the construct core.7,62 This may be due to a lower oxygen tension in the construct core, as low oxygen tension has been shown to promote chondrogenesis of MSCs.14,19,52,53 Another explanation for this spatial difference in matrix deposition is that a large percentage of the sGAG produced in the annulus is simply secreted into the culture medium. Indeed, significant levels of sGAG were measured in the culture media. Similar levels of sGAG release to the media from MSC seeded constructs has been observed in other studies.3,40 Interestingly, while sGAG accumulation at day 42 was greater in constructs continuously maintained in TGF-β3; sGAG secreted to the culture medium was greatest in constructs transiently supplemented with TGF-β3. This suggests that continued supplementation of TGF-β3 encourages retention of sGAG within the construct. This may be due to an increase in type I collagen production following removal of TGF-β3, and possibly the formation of a collagen network less able to retain sGAG within the construct. Intense collagen type II staining was seen throughout the center of both FS+ and DDC+ constructs continuously supplemented with TGF-β3. However, when TGF-β3 supplementation ceased at day 21, a slight reduction in type II staining was evident by day 42; with significant type I staining observed, particularly in the annular region. Future studies will investigate whether this is indicative of a failure to achieve a stable chondrogenic phenotype following 21 days of culture in the presence of TGF-β3.
sGAG release into the media was lower in the continuous dynamic compression (CDC) groups compared to FS controls. This demonstrates that the inhibition of chondrogenesis observed due to CDC from day 0 is not simply due to greater sGAG release into the media due to loading. Similarly the application of delayed dynamic compression did not lead to increases in sGAG release to the media, indicating that mechanical loading does not negatively influence sGAG retention in these immature chondrogenically primed constructs.
The application of 1 h of daily dynamic compression to MSC-laden constructs following three weeks of TGF-β3 induced differentiation did not enhance overall levels of cartilage matrix accumulation within the constructs. While a trend toward higher levels of sGAG/DNA was observed in constructs subjected to delayed dynamic compression, these differences were not significant. We were therefore unable to corroborate the initial hypothesis of this study. A more complex picture emerges from the spatial analysis of sGAG accumulation within constructs subjected to delayed dynamic compression. In the construct core, dynamic compression was seen to have a small positive effect on sGAG accumulation following the withdrawal of TGF-β3. No statistical differences were observed between construct annuli. These differences may be due in part to spatial variations in the mechanical signals developed in cell seeded agarose hydrogels in response to dynamic unconfined compression.23,43 Even in a homogenous tissue or hydrogel, MSCs in the annulus of the construct will experience higher levels of fluid flow in response to dynamic unconfined compression.43 Furthermore, as greater tissue maturation is observed in the construct core compared to the annulus, this could potentially alter cell-level deformation and lead to increased fluid pressurization within the construct core due to dynamic compression. Such hydrostatic fluid pressure is known to promote chondrogenesis of MSCs.2,47,48,64 However, uncoupling the exact role of mechanical signals in regulating MSC chondrogenesis in this model system is further complicated by spatial gradients in oxygen, nutrients, and growth factors that will develop within the engineered construct.
When TGF-β3 was continuously maintained in the culture media, no significant differences in spatial sGAG accumulation were observed in DDC constructs compared to FS controls. The finding that MSC response to loading depends on TGF-β3 supplementation agrees with the observation of Li et al., who demonstrated greater differences between loaded and FS constructs at 0 or 1 ng/mL TGF-β1 concentrations.40 They suggest that surface motion superimposed on 10% dynamic compression promotes chondrogenesis of MSCs seeded onto fibrin-polyurethane scaffolds through the TGF-β pathway by up-regulating TGF-β gene expression and protein synthesis. This may explain why delayed dynamic compression with the continued supplementation of TGF-β3 did not enhance sGAG accumulation in construct cores in the present study; as there may have been an overabundance of the growth factor above what was required to stimulate maximal chondrogenesis, rendering any TGF-β production stimulated through dynamic compression ineffective.
Large increases in the equilibrium modulus of any of the experimental groups were not observed despite increases in total sGAG and collagen within the constructs. This may be due to the inhomogeneous accumulation of matrix; with the majority of sGAG and collagen accumulation in the construct core. Inhomogeneous tissue development has been shown to influence the apparent mechanical properties of engineered cartilaginous tissue.31 In addition, an important finding of this study is that the application of delayed dynamic compression did not lead to increases in the mechanical properties of the engineered tissue in either the continuous or transient TGF-β supplementation groups. This is in contrast to the results of studies subjecting chondrocytes seeded in agarose to delayed dynamic compression following pre-culture in a chemically defined medium supplemented with TGF-β.41 There are a number of possible explanations for this finding. If cell-mediated reorganization of the engineered tissue in response to dynamic compression is responsible for the superior mechanical properties in chondrocyte seeded hydrogels, it may be that MSCs are unable to actively remodel their ECM in a similar manner in response to only 1 h of daily dynamic compression. It may be that longer durations of loading are necessary for improvements in the mechanical properties of cartilaginous constructs engineered using MSCs. A recent study using bovine MSCs in a similar agarose model system demonstrated that 4 h of delayed dynamic compression is necessary for improvements in tissue mechanical properties to be observed.22 Another possible explanation is that other matrix molecules not investigated here such as cartilage oligomeric matrix protein (COMP), which may play a key role in determining the functional properties of engineered cartilage tissues,50 may be differentially expressed by MSCs and chondrocytes in response to loading. Alternatively, it may simply be that the beneficial effects of dynamic compression on the mechanical properties of tissue engineered cartilage will only become apparent once a certain minimum threshold of ECM is accumulated. Given that the total biochemical content of the cartilaginous tissues generated in this study are significantly lower than that observed with primary chondrocytes cultured under similar conditions,11,41 future studies will investigate if altered culture conditions and MSC sources will lead to greater ECM accumulation prior to the application of dynamic compression.
To conclude, in the presence of TGF-β3, the application of 10% dynamic compression for 1 h per day from day 0 to day 42 inhibited chondrogenesis. In contrast, the application of 1 h of daily dynamic compression from day 21 to day 42 following TGF-β3 induced differentiation did not inhibit chondrogenesis of MSCs; however, neither did it lead to any increases in the functional properties of the tissue. In the context of cartilage tissue engineering, this study also demonstrated superior chondrogenesis in MSC-laden constructs maintained in TGF-β3 for the duration of the 42-day culture period; suggesting that continued supplementation with TGF-β3 at supra-physiological levels is a more potent chondrogenic stimulus than the dynamic compression regime utilized in this study. It has yet to be established if this is true for other types of mechanical loading regimes. Finally, this study could also be viewed as an in vitro model of how MSC-laden constructs might respond to a load bearing environment. In this case, allowing for a period of TGF-β induced differentiation produced a tissue that responded more positively to a load bearing environment.
References
Angele, P., D. Schumann, M. Angele, B. Kinner, C. Englert, R. Hente, B. Fuchtmeier, M. Nerlich, C. Neumann, and R. Kujat. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology 41:335–346, 2004.
Angele, P., J. U. Yoo, C. Smith, J. Mansour, K. J. Jepsen, M. Nerlich, and B. Johnstone. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 21:451–457, 2003.
Babalola, O. M., and L. J. Bonassar. Effects of seeding density on proteoglycan assembly of passaged mesenchymal stem cells. Cell. Mol. Bioeng. Epub March 2, 2010. doi:10.1007/s12195-010-0107-1.
Barbero, A., S. Grogan, D. Schafer, M. Heberer, P. Mainil-Varlet, and I. Martin. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage 12:476–484, 2004.
Brittberg, M., A. Lindahl, A. Nilsson, C. Ohlsson, O. Isaksson, and L. Peterson. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331:889–895, 1994.
Buckley, C. T., S. D. Thorpe, O’ Brien F. J., A. J. Robinson, and D. J. Kelly. The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J. Mech. Behav. Biomed. Mater. 2:512–521, 2009.
Buckley, C. T., T. Vinardell, S. D. Thorpe, M. G. Haugh, E. Jones, D. McGonagle, and D. J. Kelly. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J. Biomech. 43:920–926, 2010.
Buckwalter, J. A., and H. J. Mankin. Articular cartilage: Part II. J. Bone Joint Surg. (American Volume) 79:612–632, 1997.
Buschmann, M. D., Y. A. Gluzband, A. J. Grodzinsky, and E. B. Hunziker. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 108(Pt 4):1497–1508, 1995.
Buschmann, M. D., Y. A. Gluzband, A. J. Grodzinsky, J. H. Kimura, and E. B. Hunziker. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J. Orthop. Res. 10:745–758, 1992.
Byers, B. A., R. L. Mauck, I. E. Chiang, and R. S. Tuan. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng. A 14:1821–1834, 2008.
Campbell, J. J., D. A. Lee, and D. L. Bader. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology 43:455–470, 2006.
Caplan, A. I. Mesenchymal stem cells. J. Orthop. Res. 9:641–650, 1991.
Clark, C. C., B. S. Tolin, and C. T. Brighton. The effect of oxygen tension on proteoglycan synthesis and aggregation in mammalian growth plate chondrocytes. J. Orthop. Res. 9:477–484, 1991.
Coleman, R. M., N. D. Case, and R. E. Guldberg. Hydrogel effects on bone marrow stromal cell response to chondrogenic growth factors. Biomaterials 28:2077–2086, 2007.
Davisson, T., S. Kunig, A. Chen, R. Sah, and A. Ratcliffe. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J. Orthop. Res. 20:842–848, 2002.
Demarteau, O., D. Wendt, A. Braccini, M. Jakob, D. Schafer, M. Heberer, and I. Martin. Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem. Biophys. Res. Commun. 310:580–588, 2003.
Erickson, I. E., A. H. Huang, C. Chung, R. T. Li, J. A. Burdick, and R. L. Mauck. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng. A 15:1041–1052, 2009.
Grimshaw, M. J., and R. M. Mason. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage 8:386–392, 2000.
Grodzinsky, A. J., M. E. Levenston, M. Jin, and E. H. Frank. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2:691–713, 2000.
Guilak, F., and V. C. Mow. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J. Biomech. 33:1663–1673, 2000.
Huang, A. H., M. J. Farrell, M. Kim, and R. L. Mauck. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur. Cell Mater. 19:72–85, 2010.
Huang, C. Y., K. L. Hagar, L. E. Frost, Y. Sun, and H. S. Cheung. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22:313–323, 2004.
Huang, C. Y., P. M. Reuben, and H. S. Cheung. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells 23:1113–1121, 2005.
Huang, C. Y. C., P. M. Reuben, G. D’Ppolito, P. C. Schiller, and H. S. Cheung. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat. Record A: Discov. Mol. Cell. Evol. Biol. 278:428–436, 2004.
Ignat’eva, N. Y., N. A. Danilov, S. V. Averkiev, M. V. Obrezkova, V. V. Lunin, and E. N. Sobol. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 62:51–57, 2007.
Iwasaki, M., K. Nakata, H. Nakahara, T. Nakase, T. Kimura, K. Kimata, A. I. Caplan, and K. Ono. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology 132:1603–1608, 1993.
Johnstone, B., T. M. Hering, A. I. Caplan, V. M. Goldberg, and J. U. Yoo. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 238:265–272, 1998.
Kafienah, W., and T. J. Sims. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol. Biol. 238:217–230, 2004.
Kelly, D. J., and C. R. Jacobs. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res. C: Embryo Today 90:75–85, 2010.
Kelly, D. J., and P. J. Prendergast. Effect of a degraded core on the mechanical behaviour of tissue-engineered cartilage constructs: a poro-elastic finite element analysis. Med. Biol. Eng. Comput. 42:9–13, 2004.
Kim, Y. J., R. L. Sah, J. Y. Doong, and A. J. Grodzinsky. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 174:168–176, 1988.
Kim, Y. J., R. L. Sah, A. J. Grodzinsky, A. H. Plaas, and J. D. Sandy. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch. Biochem. Biophys. 311:1–12, 1994.
Kisiday, J., D. D. Frisbie, W. McIlwraith, and A. Grodzinsky. Dynamic compression stimulates proteoglycan synthesis by mesenchymal stem cells in the absence of chondrogenic cytokines. Tissue Eng. A 15:2817–2824, 2009.
Kisiday, J. D., P. W. Kopesky, C. H. Evans, A. J. Grodzinsky, C. W. McIlwraith, and D. D. Frisbie. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J. Orthop. Res. 26:322–331, 2008.
Knight, M. M., D. A. Lee, and D. L. Bader. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim. Biophys. Acta Mol. Cell Res. 1405:67–77, 1998.
Knothe Tate, M. L., T. D. Falls, S. H. McBride, R. Atit, and U. R. Knothe. Mechanical modulation of osteochondroprogenitor cell fate. Int. J. Biochem. Cell Biol. 40:2720–2738, 2008.
Lennon, D. P., and A. I. Caplan. Isolation of human marrow-derived mesenchymal stem cells. Exp. Hematol. 34:1604–1605, 2006.
Li, Z., L. Kupcsik, S. J. Yao, M. Alini, and M. J. Stoddart. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng. A 15:1729–1737, 2009.
Li, Z., L. Kupcsik, S. J. Yao, M. Alini, and M. J. Stoddart. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J. Cell. Mol. Med. Epub May 13, 2009. doi:10.1111/j.1582-4934.2009.00780.x.
Lima, E. G., L. Bian, K. W. Ng, R. L. Mauck, B. A. Byers, R. S. Tuan, G. A. Ateshian, and C. T. Hung. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage 15:1025–1033, 2007.
Mackay, A. M., S. C. Beck, J. M. Murphy, F. P. Barry, C. O. Chichester, and M. F. Pittenger. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4:415–428, 1998.
Mauck, R. L., B. A. Byers, X. Yuan, and R. S. Tuan. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech. Model. Mechanobiol. 6:113–125, 2007.
Mauck, R. L., S. L. Seyhan, G. A. Ateshian, and C. T. Hung. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann. Biomed. Eng. 30:1046–1056, 2002.
Mauck, R. L., M. A. Soltz, C. C. Wang, D. D. Wong, P. H. Chao, W. B. Valhmu, C. T. Hung, and G. A. Ateshian. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122:252–260, 2000.
Mauck, R. L., X. Yuan, and R. S. Tuan. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14:179–189, 2006.
Miyanishi, K., M. C. Trindade, D. P. Lindsey, G. S. Beaupre, D. R. Carter, S. B. Goodman, D. J. Schurman, and R. L. Smith. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 12:2253–2262, 2006.
Miyanishi, K., M. C. Trindade, D. P. Lindsey, G. S. Beaupre, D. R. Carter, S. B. Goodman, D. J. Schurman, and R. L. Smith. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 12:1419–1428, 2006.
Mouw, J. K., J. T. Connelly, C. G. Wilson, K. E. Michael, and M. E. Levenston. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25:655–663, 2007.
Ng, K. W., R. L. Mauck, C. C. Wang, T. A. Kelly, M. M. Ho, F. Hui Chen, G. A. Ateshian, and C. T. Hung. Duty cycle of deformational loading influences the growth of engineered articular cartilage. Cell. Mol. Bioeng. 2:386–394, 2009.
Nishimura, K., L. A. Solchaga, A. I. Caplan, J. U. Yoo, V. M. Goldberg, and B. Johnstone. Chondroprogenitor cells of synovial tissue. Arthritis Rheum. 42:2631–2637, 1999.
Obradovic, B., R. L. Carrier, G. Vunjak-Novakovic, and L. E. Freed. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnol. Bioeng. 63:197–205, 1999.
Obradovic, B., J. H. Meldon, L. E. Freed, and G. Vunjak-Novakovic. Glycosaminoglycan deposition in engineered cartilage: experiments and mathematical model. Aiche J. 46:1860–1871, 2000.
Palmer, G. D., A. Steinert, A. Pascher, E. Gouze, J. N. Gouze, O. Betz, B. Johnstone, C. H. Evans, and S. C. Ghivizzani. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol. Ther. 12:219–228, 2005.
Palmoski, M. J., and K. D. Brandt. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis Rheum. 27:675–681, 1984.
Park, S. H., W. Y. Sim, S. W. Park, S. S. Yang, B. H. Choi, S. R. Park, K. Park, and B. H. Min. An electromagnetic compressive force by cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng. 12:3107–3117, 2006.
Peterson, L., T. Minas, M. Brittberg, A. Nilsson, E. Sjogren-Jansson, and A. Lindahl. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 374:212–234, 2000.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147, 1999.
Sah, R. L., Y. J. Kim, J. Y. Doong, A. J. Grodzinsky, A. H. Plaas, and J. D. Sandy. Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 7:619–636, 1989.
Tallheden, T., C. Bengtsson, C. Brantsing, E. Sjogren-Jansson, L. Carlsson, L. Peterson, M. Brittberg, and A. Lindahl. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res. Ther. 7:R560–R568, 2005.
Terraciano, V., N. Hwang, L. Moroni, H. B. Park, Z. Zhang, J. Mizrahi, D. Seliktar, and J. Elisseeff. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25:2730–2738, 2007.
Thorpe, S. D., C. T. Buckley, T. Vinardell, F. J. O’Brien, V. A. Campbell, and D. J. Kelly. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 377:458–462, 2008.
Tuan, R. S., G. Boland, and R. Tuli. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 5:32–45, 2003.
Wagner, D. R., D. P. Lindsey, K. W. Li, P. Tummala, S. E. Chandran, R. L. Smith, M. T. Longaker, D. R. Carter, and G. S. Beaupre. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann. Biomed. Eng. 36:813–820, 2008.
Wakitani, S., T. Goto, S. J. Pineda, R. G. Young, J. M. Mansour, A. I. Caplan, and V. M. Goldberg. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 76:579–592, 1994.
Wang, Q. G., J. L. Magnay, B. Nguyen, C. R. Thomas, Z. Zhang, A. J. El Haj, and N. J. Kuiper. Gene expression profiles of dynamically compressed single chondrocytes and chondrons. Biochem. Biophys. Res. Commun. 379:738–742, 2009.
Williams, C. G., T. K. Kim, A. Taboas, A. Malik, P. Manson, and J. Elisseeff. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 9:679–688, 2003.
Yoo, J. U., T. S. Barthel, K. Nishimura, L. Solchaga, A. I. Caplan, V. M. Goldberg, and B. Johnstone. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Joint Surg. Am. 80:1745–1757, 1998.
Zuk, P. A., M. Zhu, H. Mizuno, J. Huang, J. W. Futrell, A. J. Katz, P. Benhaim, H. P. Lorenz, and M. H. Hedrick. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7:211–228, 2001.
Acknowledgments
Funding was provided by Science Foundation Ireland (07-RFP-ENMF142 and the President of Ireland Young Researcher Award: 08/YI5/B1336).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Thorpe, S.D., Buckley, C.T., Vinardell, T. et al. The Response of Bone Marrow-Derived Mesenchymal Stem Cells to Dynamic Compression Following TGF-β3 Induced Chondrogenic Differentiation. Ann Biomed Eng 38, 2896–2909 (2010). https://doi.org/10.1007/s10439-010-0059-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-0059-6