Abstract
Endothelial cells (ECs) line the innermost of the blood vessel wall and are constantly subjected to shear stress imposed by blood flow. ECs were also influenced by the neighboring vascular smooth muscle cells (VSMCs). The bidirectional communication between ECs and VSMCs modulates vascular homeostasis. In this study, the involvement of histone deacetylase 6 (HDAC6) in modulating migration of ECs co-cultured with VSMCs by the normal level of laminar shear stress (NSS) was investigated. ECs was either cultured alone or co-cultured with VSMCs under static conditions or subjected to NSS of 15 dyne/cm2 by using a parallel-plate co-culture flow chamber system. It was demonstrated that both NSS and VSMCs could increase EC migration. The migration level of ECs co-cultured with VSMCs under NSS was not higher than that under the static condition. The process of EC migration regulated by VSMCs and NSS was associated with the increased expression of HDAC6 and low level of acetylated tubulin. The increase in HDAC6 expression was accompanied by a time-dependent decrease in the acetylation of tubulin in ECs co-cultured with VSMCs. Inhibition of the HDAC6 by siRNA or tributyrin, an inhibitor of HDACs, induced a parallel alteration in the migration and the acetylated tubulin of ECs co-cultured with VSMCs. It was observed by immunofluorescence staining that the acetylated tubulin was distributed mostly around the cell nucleus in ECs co-cultured with VSMCs. The results suggest that the NSS may display a protective function on the vascular homeostasis by modulating EC migration to a normal level in a VSMC-dependent manner. This modulation process involves the down-regulation of acetylated tubulin which results from increased HDAC6 activity in ECs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial dysfunction, including the elevation of permeability and migration, plays a crucial role in all stages of atherosclerosis.6,20 Pathological studies have shown that atherosclerotic lesions occur preferentially at vessel branch points, bifurcations and regions of high curvature, suggesting that low and disturbed shear stress is an inducer in atherogenesis, whereas the normal level of laminar shear stress (NSS) is atheroprotective.3,9
Many studies have shown that flow shear stress causes an increase in endothelial cell (EC) migration.13,28,32 However, all these studies used the monolayer of ECs cultured alone as an experimental model, which may not accurately reflect the environment of ECs in vivo. ECs and vascular smooth muscle cells (VSMCs) are neighbors to each other in the vessel wall. The interaction between ECs and VSMCs plays an important role in maintaining the normal vascular structure and function.15,23,34,35 It had been demonstrated that ECs modulate VSMC migration in a co-culture system of ECs and VSMCs.34 However, we have a particular interest in the migration behavior of ECs under NSS and its mechanisms in such co-culture system.
Cell migration is a multistep process which includes the protrusion of the leading edge, the formation of new adhesions at the front, the contraction of the cell, and the release of adhesions at the rear. Each step has a close relation with three types of cytoskeleton.24 When ECs were treated with taxol to change the stability of microtubule, the protrusion of lamellipodia and the migration speed of ECs are all reduced. Furthermore, microtubule depolymerization leads to the rapid formation of stress fibers and adhesion plaques in phorbol ester-treated fibroblasts.10 These results showed that a suitable level of microtubule polymerization may be necessary for the migration of ECs. Acetylated tubulin is one of the characteristics of stabilized microtubules. Reversible acetylation on the ε-amino group of α-tubulin Lys40 marks stabilized microtubule structures and may contribute to regulating microtubule dynamics.25 It has been demonstrated histone deacetylase 6 (HDAC6), one member of the class II HDACs, is mainly present in the cytoplasm and participates in the control of acetylation of cytoplasmic proteins.16 The discovery of α-tubulin as a HDAC6 substrate was an important step forward to understand the mechanisms controlling tubulin acetylation.14 As acetylated tubulin has been shown to regulate cell motility in some cases,21,26 it is reasonable to hypothesize that HDAC6 and actylated tubulin may be involved in the migration of ECs modulated by NSS and VSMCs.
In the present study, the effects of NSS and co-cultured VSMCs on EC migration were investigated. Subsequently, it was demonstrated whether HDAC6, a unique enzyme, was involved in the process of EC migration through regulation of the acetylation level of tubulin. These studies may help to explain the atheroprotective effect of NSS on the migration of ECs and its underlying mechanism.
Materials and Methods
Cell Culture
ECs and VSMCs were isolated from fresh human umbilical veins as described previously.34 ECs and VSMCs between passages 2–7 were used in all experiments. Cell populations with more than 95% purity were used in all experiments. The investigation complies with the principles outlined in the Declaration of Helsinki for use of human tissue.
EC-VSMC Co-culture Model and NSS Loading
A co-culture model was established using a cell culture insert which has a 10 μm thick porous polyethylene terephthalate (PET) membrane (BectonDickinson Labware, NJ) as described previously.34 Briefly, each PET membrane contains 1.6 million pores/cm2, and each pore is 0.4-μm in diameter. ECs were first seeded onto the outer side of the PET membrane at a density of 3 × 105 cells/cm2. After allowing 4–5 h for attachment, the membrane was placed with the EC side down into a 6-well plate containing the completed culture medium, and then VSMCs were seeded on the inside of the membrane at a density of 1 × 105 cells/cm2. ECs and VSMCs were maintained in their respective medium and grown to confluence.
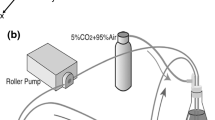
To introduce NSS to the ECs side of the co-culture model, the co-culture insert and parallel-plate flow chamber were assembled into a parallel-plate co-culture flow chamber system as described previously.34 The design and construction of the chamber are illustrated in Fig. 1. In the test section of the chamber system, the flow path is 28 mm in width (w) and 0.2 mm in height (h). Shear stress (τ) intensity (dyne/cm2) was calculated by using the formula τ = 6μQ/wh 2, where μ is the viscosity of the medium and Q is the flow rate (milliliters per second). NSS produced by the chamber in the present study was estimated to be 15 dyne/cm2 and applied to the ECs side. Flow-loading experiments were performed at 37 °C and 5% CO2 in a humidified incubator, and maintained at pH 7.4.
Schematic diagram of the test section of the parallel-plate flow chamber system for the co-culture of ECs and VSMCs (adapted from Wang et al.34)
Four experimental groups were divided: (1) a control group, Ø/EC, ECs cultured alone under the static condition; (2) VSMC/EC group, ECs co-cultured with VSMCs under the static condition; (3) Ø/EC + NSS group, NSS was applied to the EC side of Ø/EC; (4) VSMC/EC + NSS group, NSS was applied to the EC side of VSMC/EC.
Transwell Migration Assay
EC migration was performed with Transwell system (Costar Inc), which allows cells to migrate through the microporous membrane with a pore size of 8 μm, which constitutes the bottom of Transwell insert in 6-well plates as described previously.22 Briefly, ECs were trypsinized from the co-culture model, washed, and resuspended in serum-free M199 after being cultured alone or with VSMCs under the static or NSS conditions for 12 h. Then, the EC suspension was added to the upper compartment of the Transwell insert at a concentration of 2 × 105 cells/well, and the lower compartment was filled with 600 μL M199 containing 50% heat-inactivated FBS to serve as the chemoattractant.17,22 After 6 h of incubation, the cells which remained in the upper side of the microporous membrane were removed with a cotton swab, and cells that had migrated to the lower side of the microporous membrane was stained with Mayer’s Hematoxylin, and the number of migrated cells was counted under a microscope (magnification, 200×, Olympus IX71, Japan). For each group, 12 randomly chosen fields from duplicate wells were photographed. At least three independent experiments were performed for each group.
Inhibitor Studies
For inhibitor studies, tributyrin, a pharmacological specific inhibitor of HDAC was prepared. DMSO, a solvent for tributyrin, was present at equal concentrations in all groups, including the control. ECs were treated with tributyrin (8 μmol/L, Sigma) or vehicle (1% FBS/medium + DMSO) for 1 h before co-culturing with VSMCs. The tributyrin or DMSO was kept in the medium throughout the experiment.19
HDAC6 Knockdown by siRNA
ECs were transfected with 100 nmol/L of HDAC6 siRNA (GenePharma) or scrambled siRNA (GenePharma) for 4 h using lipofectamine 2000 (Invitrogen) in Opti-MEM media (Gibco) according to the manufacturer’s instruction. The sequence of siRNA oligo is as follows: HDAC6 siRNA: sense 5′-CUU CGA AGC GAA AUA UUA ATT-3′ and anti-sense 5′-UUA AUA UUU CGC UUC GAA GTG-3′; scramble siRNA: sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and anti-sense 5′-ACG UGA CAC GUU CGG AGA ATT-3′.
Fluorescence Microscopy
After ECs were cultured with or without VSMCs on the outer side of the co-culture insert for 12 h, the VSMCs located at the inner side of the insert were removed with a cotton swab, and the ECs were fixed for 30 min in 2% formaldehyde in PBS, permeabilized in 0.2% Triton X-100/PBS and blocked with 10% goat serum. The cells were stained with an antibody against the acetylated tubulin (1:500, Sigma) followed by FITC-conjugated goat anti-mouse IgG (Jackson Immunoresearch). Images were acquired using a confocal microscope (LSM 510 METALaser Scanning Microscope, Zeiss, Germany).
SDS-PAGE and Western Blot Analysis
EC lysates were prepared and analyzed by Western blot as previously described.34 Proteins (30 μg/lane) were fractionated by SDS-PAGE on a 10% acrylamide gel under reducing conditions and blotted onto nitrocellulose membrane (Amersham). And the membrane was incubated with polyclonal antibodies against HDAC6 (1:1000, Cell Signaling Technology), goat polyclonal against GAPDH (1:500, Santa Cruz Biotechnology) and monoclonal antibody against acetylated tubulin (1:1000, Sigma).
Statistical Analysis
Each experiment was performed at least in triplicates, and all values were expressed as mean ± SD. The student’s t-test was used to compare two groups and ANOVA was used for comparisons between multiple groups. Values of p < 0.05 were accepted as statistically significant.
Results
The Effects of NSS and VSMCs on EC Migration
In present study, ECs cultured either in the presence (VSMC/EC) or absence (Ø/EC) of VSMCs were subjected to NSS of 15 dyne/cm2 or kept in the static for 12 h respectively and then EC migration was evaluated using the Transwell assay. As shown in Fig. 2A, ECs co-cultured with VSMCs in the static showed an increase in migration as compared to ECs cultured alone (p < 0.05, VSMC/EC vs. the control Ø/EC, Fig. 2B). The application of NSS to ECs cultured alone also promoted EC migration (p < 0.05, Ø/EC + NSS vs. Ø/EC, Fig. 2B). However, the migration of ECs co-cultured with VSMCs and subjected to NSS not only was not increased than that of ECs cultured alone with application of NSS (p > 0.05, VSMC/EC + NSS vs. Ø/EC + NSS, Fig. 2B), but it also was significantly diminished in comparison with ECs co-cultured with VSMCs in the static (p < 0.05, VSMC/EC + NSS vs. VSMC/EC, Fig. 2B). The results clearly demonstrate that NSS and VSMCs could promote migration of ECs separately, but the presence of NSS inactivates the effect of VSMCs on EC migration.
The effects of NSS and VSMCs on migration of ECs. (A) EC migration was detected by Transwell assay. Representative microscopic images of ECs had migrated to the bottom of the Transwell insert illustrate how NSS and VSMCs affected EC migration; 200× magnification. (a) ECs cultured alone in the static (the control, Ø/EC); (b) ECs cultured alone with NSS (Ø/EC + NSS); (c) ECs co-cultured with VSMCs were maintained in the static (VSMC/EC); (d) ECs co-cultured with VSMCs were exposed to NSS for 12 h (VSMC/EC + NSS). (B) Histogram shows fold change in EC migration relative to the control. Values shown are the mean ± SD for each group from at least three independent experiments. * p < 0.05 vs. the control Ø/EC, and # p < 0.05 vs. VSMC/EC
The Effects of NSS and VSMCs on Expression of HDAC and Acetylated Tubulin in ECs
We hypothesize that the modulation of EC migration by NSS and VSMCs is through activation of HDAC6 expression in ECs, and then down-regulation acetylated tubulin, which leads to a less stable microtubule network and an accelerated treadmiling process. The expression of HDAC6 and acetylated tubulin in ECs was examined in order to confirm this hypothesis. As shown in Fig. 3A, there was a significant increase of the HDAC6 expression in ECs co-cultured with VSMCs for 12 h compared with the ECs cultured alone (p < 0.05, VSMC/EC vs. the control Ø/EC, Fig. 3A). When NSS was applied to ECs which were cultured alone, the increased expression of HDAC6 in ECs was also detected (p < 0.05, Ø/EC + NSS vs. the control Ø/EC, Fig. 3A). However, the HDAC6 level of ECs co-cultured with VSMCs under NSS was no more than that of EC cultured alone under NSS (p > 0.05, VSMC/EC + NSS vs. Ø/EC + NSS). Contrast to HDAC6, the co-cultured ECs showed nearly 45% lower of acetylated tubulin protein expression compared with the control Ø/EC (p < 0.001, Fig. 3A). While the NSS was applied to the ECs co-cultured with VSMCs, the tubulin acetylation in ECs was close to the level of ECs cultured alone under NSS (p > 0.05, VSMC/EC + NSS vs. Ø/EC + NSS, Fig. 3A), which had been reduced by about 20% of tubulin acetylation compared with ECs cultured alone (p < 0.05, Ø/EC + NSS vs. Ø/EC, Fig. 3A). The results show that HDAC6 activation and associated down-regulation of microtubule acetylation in ECs are induced by VSMCs and NSS respectively. However, the presence of NSS could protect against VSMCs regulation of HDAC6 and acetylated tubulin expression in ECs.
The effects of NSS and VSMCs on expression of HDAC6 and acetylated tubulin in ECs. ECs cultured either in the presence (VSMC/EC) or absence (Ø/EC) of VSMCs were subjected to a shear stress of 15 dyne/cm2 or kept in the static for 12 h. The expression of HDAC6 and acetylated tubulin was determined by Western blot (A). The expression of HDAC6 and acetylated tubulin proteins in ECs was presented as band densities (normalized to the GAPGH protein levels). The results shown are mean ± SD from three independent experiments. * p < 0.05 vs. the control Ø/EC, # p < 0.01 vs. VSMC/EC. (B) The distribution of acetylated tubulin in ECs was showed by immunofluorescence. (a) ECs cultured alone in the static; (b) ECs co-cultured with VSMCs were maintained in the static. Bar = 10 μm
The distribution of acetylated tubulin in the ECs between each group was also examined. ECs were fixed and the acetylated tubulin distribution was observed through immunostaining and examined subsequently by confocal microscopy. The acetylated tubulin in ECs cultured alone in the static, as seen in Fig. 3Ba, was universal in the cytoplast of the cells. However, the acetylated tubulin in ECs co-cultured with VSMCs was distributed mainly around the nucleus of the cell (Fig. 3Bb).
These results demonstrate that HDAC6 up-regulation is associated with down-regulation of the acetylated tubulin in ECs co-cultured with VSMCs.
VSMCs Modulate the Expression of HDAC6 Associated with Down-regulation of Acetylated Tubulin in ECs is Time-dependent
The previous results demonstrated that HDAC6 up-regulation accompanied the down-regulation of acetylated tubulin in ECs co-cultured with VSMCs at 12 h. Subsequently, the relationship between the expression of HDAC6 and acetylased tubulin was determined at other time points. Figure 4a shows that the expression of the HDAC6 was increased slightly in the ECs co-cultured with VSMCs at 5 min, and the level of HDAC6 expression increased steadily up to 6 h (Fig. 4b, p < 0.001). The increase in HDAC6 expression (maximal induction of nearly 3-fold to the control in Fig. 4b) was associated with a gradual decrease in the acetylation of tubulin, with a minimal expression (nearly 50% down-regulation, p < 0.001 vs. the control Ø/EC, Fig. 4b) at 6 h in the ECs co-cultured with VSMCs. These results show that VSMCs increase the expression of HDAC6 associated with down-regulation of acetylated tubulin in ECs, which is in a time-dependent manner.
Time course of HDAC6 and acetylated tubulin expression in ECs activated by the co-cultured VSMCs in the static. ECs were kept as the control (Ø/EC) or co-cultured with VSMCs (VSMC/EC) for the times indicated in the static. Expression of HDAC6 and acetylated tubulin were determined by Western blot analysis. The amount of HDAC6 and acetylated tubulin proteins in VSMC/EC was presented as band densities (normalized to the GAPDH protein level). The results shown are mean ± SD from three independent experiments. * p < 0.05, # p < 0.01 vs. the control Ø/EC
Silencing HDAC6 Modulating VSMC-induced Migration and Microtubule Acetylation in ECs
Whether or not HDAC6 was responsible for VSMCs mediated EC migration was examined by using an HDAC pharmacological inhibitor, tributyrin, and an HDAC6 specific siRNA transfection respectively. ECs were incubated with tributyrin for 1 h at dose of 8 μmol/L prior to co-culturing with VSMCs. As shown in Fig. 5a, this treatment resulted in significant inhibition of HDAC6 protein level in the ECs (Fig. 5a, p < 0.05) as well as EC migration (Figs. 6a, 6c, and 6e, p < 0.001). Similar results were obtained when specific siRNA for HDAC6 was used. HDAC6 protein level was significantly reduced in the ECs co-cultured with VSMCs in the static by siRNA of HDAC6, but not by scramble siRNA as determined by Western blot (Fig. 5b, p < 0.05), which showed its specificity for HDAC6. Silencing HDAC6 significantly inhibited the VSMC enhanced-EC migration (Figs. 6b, 6d, and 6f, p < 0.05) and with a concomitant elevation of acetylation level of tubulin in the ECs (Fig. 5b, p < 0.05). These results strongly suggest that HDAC6 play an important role on the migration of ECs modulated by VSMCs through regulation of the acetylated level of tubulin.
The up-regulation of acetylated tubulin in ECs co-cultured with VSMCs by inhibition of the activity of HDAC6 using tributyrin (a) or specific siRNA (b). The cell lysates were immunoblotted with anti-HDAC6, anti-acetylated tubulin, anti-GAPDH antibodies. ECs co-cultured with VSMCs maintained in the static for 12 h before assay. Quiescent ECs were pretreated with or without tributyrin (8 μmol/L) for 1 h (the DMSO or tributyrin was maintained during experiment time and the transwell assay), or transfected with control siRNA or a specific siRNA of HDAC6 (100 nmol/mL) for 24 h. Values shown are the mean ± SD for each group from at least three independent experiments. * p < 0.05 vs. the control VSMC/EC + DMSO in (a); * p < 0.05 vs. the control VSMC/EC + negative siRNA in (b)
Inhibition of HDAC6 activity by tributyrin or specific siRNA for HDAC6 decreased the migration of ECs. Micrographs of migrating ECs treated with DMSO (a), niRNA (b), tributyrin (c) and specific siRNA (d), respectively, 200× magnification. Histograms shown are fold change in EC migration, and the treatments are as indicated in (e) and (f). Values shown are the mean ± SD for each group from at least three independent experiments. * p < 0.001 vs. the control VSMC/EC + DMSO, # p < 0.01 vs. the control VSMC/EC + negative siRNA
Discussion
Our present study provide a systematic analysis of the relationship between the expression of HDAC6, the acetylation level of tubulin, and the migration of ECs, which were co-cultured with and without VSMCs under both static conditions or in response to NSS. In this study, the results clearly demonstrate that NSS and VSMCs could induce an increase of ECs migration separately, but NSS could inactivate the effect of VSMCs on EC migration. In other words, NSS protects against the migration of ECs co-cultured with VSMCs to maintain the endothelial function at a normal level. HDAC6 plays an important role in this process through down-regulation of the acetylation level of tubulin.
Flow shear stress is sensed by the endothelium and plays an important role in normal physiological responses as well as disease pathologies. Many studies have shown that flow shear stress causes an increase in the migration of ECs.13,28,32 This is consistent with our findings that NSS and VSMCs could promote EC migration separately. But EC migration is not increased accumulatively when NSS and VSMCs act together on ECs. In our study, the effect of shear stress on EC migration appears to be conflictive with others at first glance. Virtually, all other studies of increase in EC migration used the monolayer of ECs cultured alone, which may not simulate the environment of ECs in vivo. A physical contact, via myoendothelial bridges, between ECs and VSMCs has been demonstrated in vivo.29 Increasing studies in vitro have confirmed that bidirectional cross-talk and paracrine effect between ECs and VSMCs may influence ECs response to hemodynamic forces and affect VSMCs function.8,34 Therefore, it is necessary to investigate the role of flow shear stress in modulating EC function, including migration, in the presence of VSMCs. In the current study, a parallel-plate co-culture flow chamber system was used, which provides a very close interface between ECs and VSMCs and mimics the environment of ECs in vivo. Generally, the phenotype of VSMCs used in experiments in vitro is synthetic.8,31 After changing from contractile phenotype to the synthetic, VSMCs would release some growth factors to interact with ECs lining the vessel wall and could affect EC growth, migration, and survival.1 However, Tsai et al. 31 have shown that application of shear stress to ECs could induce synthetic-to-contractile phenotypic modulation in VSMCs. The contractile phenotypic VSMCs secrete less growth factors than that of synthetic ones,2 which results in the inactivated migration of co-cultured ECs under NSS. Moreover, Otero et al.20 and Tressel et al.30 reported that the vascular permeability and EC migration were heightened during EC dysfunction process when the local hemodynamic force sensed by ECs was low and disturbed shear stress abnormally. The present study showed the presence of NSS inactivates the effect of VSMCs on EC migration. The result suggests that NSS is beneficial to maintaining the endothelial homeostasis.
Cell migration is a multistep process which includes the protrusion of the leading edge, the formation of new adhesions at the front, the contraction of the cell, and the release of adhesions at the rear. Each step has a close relation with three types of the cytoskeleton, microtubules, actin microfilaments and intermediate filaments.4,12,33 Dynamic microtubules are crucial for modulating cell motility,5 and the adaptation of cell to vary of mechanical level.27 Microtubule destabilization significantly enhanced aspects of their motility.5 Acetylation plays a role in the maintenance of stable populations of microtubules.7 Therefore, we hypothesized that the changed acetylation level of tubulin may participate in the NSS and VSMCs regulated migration of ECs. In our study, it was demonstrated that NSS and VSMCs could inhibit acetylation level of tubulin in ECs, and the migration of ECs was decreased when tubulin was hyperacetylated which was induced by either genetic or pharmacological inhibition of HDAC6. Treatment with tributyrin, one of HDAC inhibitors which yield hyperacetylated tubulin, was found to equivalently downregulate EC migration which had been promoted by VSMCs. From these data, the hyperacetylation of tubulin appears to be both necessary and sufficient to decrease cell motility.
HDACs, which were discovered as chromatin-modifying enzymes, are a huge family including three subtypes and over ten members. Among them, HDAC6 is a unique enzyme that is essentially cytoplasmic and participates in the control of the acetylation of cytoplasmic proteins,16 including tubulin.14 It could be possible that HDAC6 itself alters microtubule stability and then affect the EC motility. HDAC6 was also found to highly expressed in the testis, and contributes to the dynamic changes in microtubule configurations of the cells. In addition to microtubule acetylation changes, other proteins such as Hsp 90 and cortactin have been reported to have increased acetylation or altered activity when HDAC6 is inhibited or deleted from cells which also mediates cell adhesion and motility.18,36 HDAC6-dependent control of cell migration could involve the actin cytoskeleton by altering the acetylation level of cortactin,36 or link actin filaments and microtubule dynamics through its interaction with form in homology proteins, mDia1 and mDia2, which control actin polymerization.11 In the present study, we found that ECs co-cultured with VSMCs or subjected to NSS experience enhanced HDAC6 expression accompanied by changes of EC migration and expression of acetylated tubulin. Furthermore, pharmacological or genetical inhibition of HDAC6 activity also decreased the EC migration. Thus, our results are consistent with the role of HDAC6 protein in determining the dynamic characters of microtubule and the motile properties of ECs.
In summary, our findings indicate that the application of NSS or co-culturing with VSMCs independently cause the stimulation of EC migration. However, instead of producing a cumulative effect, ECs migration is significantly diminished when both NSS and co-culturing are applied simultaneously. Our results highlight the relevance of tubulin acetylation in mediating cell motility through a HDAC6-dependent manner. VSMC/EC interaction is required for the activation of HDAC6, which plays a key role in EC migration. It is shown that NSS could modulate migration of ECs co-cultured with VSMCs to a normal level, which exerts a protective role in maintaining the vascular endothelial function at a homeostaic state.
References
Babic, A. M., C. C. Chen, and L. F. Lau. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alpha(v)beta(3), promotes endothelial cell survival, and induces angiogenesis in vivo. Mol. Cell. Biol. 19:2958–2966, 1999.
Badier-Commander, C., A. Couvelard, D. Henin, T. Verbeuren, J. B. Michel, and M. P. Jacob. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J. Pathol. 193:398–407, 2001.
Barakat, A., and D. Lieu. Differential responsiveness of vascular endothelial cells to different types of fluid mechanical shear stress. Cell Biochem. Biophys. 38:323–343, 2003.
Barberis, L., C. Pasquali, D. Bertschy-Meier, A. Cuccurullo, C. Costa, C. Ambrogio, F. Vilbois, R. Chiarle, M. Wymann, F. Altruda, C. Rommel, and E. Hirsch. Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur. J. Immunol. 39:1136–1146, 2009.
Bazzoni, G., P. Tonetti, L. Manzi, M. R. Cera, G. Balconi, and E. Dejana. Expression of junctional adhesion molecule—a prevents spontaneous and random motility. J. Cell Sci. 118:623–632, 2005.
Brevetti, G., V. Schiano, and M. Chiariello. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis 197:1–11, 2008.
Cambray-Deakin, M. A., and R. D. Burgoyne. Posttranslational modifications of alpha-tubulin: acetylated and detyrosinated forms in axons of rat cerebellum. J. Cell Biol. 104:1569–1574, 1987.
Chiu, J. J., L. J. Chen, S. F. Chang, P. L. Lee, C. I. Lee, M. C. Tsai, D. Y. Lee, H. P. Hsieh, S. Usami, and S. Chien. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-kappaB. Arterioscler. Thromb. Vasc. Biol. 25:963–969, 2005.
Cunningham, K. S., and A. I. Gotlieb. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85:9–23, 2005.
Danowski, B. A. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 93(Pt 2):255–266, 1989.
Destaing, O., F. Saltel, B. Gilquin, A. Chabadel, S. Khochbin, S. Ory, and P. Jurdic. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 118:2901–2911, 2005.
Eiseler, T., H. Doppler, I. K. Yan, K. Kitatani, K. Mizuno, and P. Storz. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat. Cell Biol. 11:545–556, 2009.
Hu, Y. L., S. Li, H. Miao, T. C. Tsou, M. A. del Pozo, and S. Chien. Roles of microtubule dynamics and small GTPase Rac in endothelial cell migration and lamellipodium formation under flow. J. Vasc. Res. 39:465–476, 2002.
Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X. F. Wang, and T. P. Yao. HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458, 2002.
Johnson, T. L., and R. M. Nerem. Endothelial connexin 37, connexin 40, and connexin 43 respond uniquely to substrate and shear stress. Endothelium 14:215–226, 2007.
Khochbin, S., A. Verdel, C. Lemercier, and D. Seigneurin-Berny. Functional significance of histone deacetylase diversity. Curr. Opin. Genet. Dev. 11:162–166, 2001.
Kouchi, H., K. Nakamura, K. Fushimi, M. Sakaguchi, M. Miyazaki, T. Ohe, and M. Namba. Manumycin A, inhibitor of ras farnesyltransferase, inhibits proliferation and migration of rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 264:915–920, 1999.
Kovacs, J. J., P. J. Murphy, S. Gaillard, X. Zhao, J. T. Wu, C. V. Nicchitta, M. Yoshida, D. O. Toft, W. B. Pratt, and T. P. Yao. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell 18:601–607, 2005.
Li, C., Y. Hu, G. Sturm, G. Wick, and Q. Xu. Ras/Rac-dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arterioscler. Thromb. Vasc. Biol. 20:E1–E9, 2000.
Otero, K., F. Martinez, A. Beltran, D. Gonzalez, B. Herrera, G. Quintero, R. Delgado, and A. Rojas. Albumin-derived advanced glycation end-products trigger the disruption of the vascular endothelial cadherin complex in cultured human and murine endothelial cells. Biochem. J. 359:567–574, 2001.
Palazzo, A., B. Ackerman, and G. G. Gundersen. Cell biology: tubulin acetylation and cell motility. Nature 421:230, 2003.
Qi, Y. X., M. J. Qu, D. K. Long, B. Liu, Q. P. Yao, S. Chien, and Z. L. Jiang. Rho-GDP dissociation inhibitor alpha downregulated by low shear stress promotes vascular smooth muscle cell migration and apoptosis: a proteomic analysis. Cardiovasc. Res. 80:114–122, 2008.
Redmond, E. M., J. P. Cullen, P. A. Cahill, J. V. Sitzmann, S. Stefansson, D. A. Lawrence, and S. S. Okada. Endothelial cells inhibit flow-induced smooth muscle cell migration: role of plasminogen activator inhibitor-1. Circulation 103:597–603, 2001.
Ridley, A. J. Rho GTPases and cell migration. J. Cell Sci. 114:2713–2722, 2001.
Rosenbaum, J. Cytoskeleton: functions for tubulin modifications at last. Curr. Biol. 10:R801–R803, 2000.
Saji, S., M. Kawakami, S. Hayashi, N. Yoshida, M. Hirose, S. Horiguchi, A. Itoh, N. Funata, S. L. Schreiber, M. Yoshida, and M. Toi. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene 24:4531–4539, 2005.
Schroder, E. A., K. Tobita, J. P. Tinney, J. K. Foldes, and B. B. Keller. Microtubule involvement in the adaptation to altered mechanical load in developing chick myocardium. Circ. Res. 91:353–359, 2002.
Simmers, M. B., A. W. Pryor, and B. R. Blackman. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. Am. J. Physiol. Heart Circ. Physiol. 293:H1937–H1946, 2007.
Spagnoli, L. G., S. Villaschi, L. Neri, and G. Palmieri. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia 38:124–125, 1982.
Tressel, S. L., R. P. Huang, N. Tomsen, and H. Jo. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 27:2150–2156, 2007.
Tsai, M. C., L. Chen, J. Zhou, Z. Tang, T. F. Hsu, Y. Wang, Y. T. Shih, H. H. Peng, N. Wang, Y. Guan, S. Chien, and J. J. Chiu. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ. Res. 105:471–480, 2009.
Urbich, C., E. Dernbach, A. Reissner, M. Vasa, A. M. Zeiher, and S. Dimmeler. Shear stress-induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits alpha(5) and beta(1). Arterioscler. Thromb. Vasc. Biol. 22:69–75, 2002.
Vinogradova, T., P. M. Miller, and I. Kaverina. Microtubule network asymmetry in motile cells: role of Golgi-derived array. Cell Cycle 8:2168–2174, 2009.
Wang, H. Q., L. X. Huang, M. J. Qu, Z. Q. Yan, B. Liu, B. R. Shen, and Z. L. Jiang. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium 13:171–180, 2006.
Williams, C., and T. M. Wick. Endothelial cell-smooth muscle cell co-culture in a perfusion bioreactor system. Ann. Biomed. Eng. 33:920–928, 2005.
Zhang, X., Z. Yuan, Y. Zhang, S. Yong, A. Salas-Burgos, J. Koomen, N. Olashaw, J. T. Parsons, X. J. Yang, S. R. Dent, T. P. Yao, W. S. Lane, and E. Seto. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell 27:197–213, 2007.
Acknowledgment
This research was supported by grants from the National Natural Science Foundation of China, Nos. 10732070, 10772120 and 10572096.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Larry V. McIntire oversaw the review of this article.
Yan-Hua Wang and Zhi-Qiang Yan contributed equally to this article.
Rights and permissions
About this article
Cite this article
Wang, YH., Yan, ZQ., Qi, YX. et al. Normal Shear Stress and Vascular Smooth Muscle Cells Modulate Migration of Endothelial Cells Through Histone Deacetylase 6 Activation and Tubulin Acetylation. Ann Biomed Eng 38, 729–737 (2010). https://doi.org/10.1007/s10439-009-9896-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9896-6