Abstract
For many tissues, cyclic mechanical stimulation is considered necessary to maintain the normal morphology in vitro. The aim of this study was to design and evaluate a simple bioreactor system capable of medium-term (more than 2 weeks) culture of native and engineered aortic valves. The system consists of three pistons in separate cylindrical chambers that are simultaneously driven through the culture medium by a crank and cam assembly. The faces of these pistons have unidirectional valves mounted in opposing orientations that permit flow from one side of the face to the other. A custom designed stent was employed to secure either native or engineered trileaflet valves to the pistons. Computational fluid dynamics and finite element modeling was used to assist selection of materials and components in the system. Finally, sterility testing using base culture medium was performed to verify the ability of the system to retain sterile conditions. The current design permits the cyclic opening and closing of three aortic valves, however this device can be modified to accommodate up to 12 valves simultaneously. This new bioreactor system has applications not only for development of tissue-engineered valves, but for also studying disease models in the aortic valve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aortic valve consists of three leaflets continuous with three aortic sinus walls and is located between the left ventricle and the aorta. It functions as a one-way valve, allowing oxygenated blood to flow from the left ventricle to the aorta. The normal in vivo mechanical loading of aortic valves is responsible for varied biosynthetic responses of valvular cells and heterogeneous distribution of extracellular matrix throughout the leaflets.12 For example, protein and glycosaminoglycan synthesis is greater at locations in the leaflet demonstrating the greatest functional stresses.9 Furthermore, mechanical stimulation has been shown to be necessary for the maintenance of tissue phenotype as well as regulating matrix synthesis for a variety of tissues when cultured in vitro.6,10,13 Cultured valve leaflets respond to hydrostatic pressure in a magnitude dependent manner by altering their sulfated glycosaminoglycan (sGAG), collagen, and DNA contents.14 Similarly, engineered tissue surrogates containing valvular interstitial cells (VICs) react to cyclic stretch in a reversible manner by altering their GAG and proteoglycan production.3 The need to improve our understanding of the true role that mechanical stimuli play on the extracellular matrix of the aortic valve has motivated the design and creation of the in vitro mechanical environment. The effects of varied mechanical stimuli on valves have only been studied in the past few years, and further investigations in this area will have far ranging impacts from advancing knowledge of basic valve biology to understanding valve disease progression. Mechanical stimulation culture systems have wide utility not only as systems to study models of valve disease and remodeling, but also for use in the development of tissue engineered heart valves.
A number of bioreactor systems have been designed to provide physical stimuli to engineered aortic valve tissues. These systems vary greatly in complexity, from very simple models that provide an isolated mechanical stimulus2,3 to very complex flow loops with automated resistance and feedback controls.1,4,8,11 For example, Engelmayr et al. have detailed a system that allows the application of flexure, thereby elucidating the effect of this particular type of mechanical stimulus.2 In contrast, the flow loop described by Hildebrand et al. allows robust control over the physiologic environment, with control of both pressure and flow rates, which are achieved with computer controlled pumps and flow resistors.4 Previously developed bioreactors and flow loops each serve a specific purpose and are well-characterized in the literature1–5,8,11; however, these systems generally fail to address the need for a simple, scalable design. The newly developed bioreactor described here lacks the robust level of control achieved in the most complicated flow loops, but can be easily scaled to allow a large number of valves to be cultured in pseudo-physiologic conditions. The design objectives of this aortic valve culture system were simple operation, high efficiency multivalve culture, low-cost, and a sterile culture environment.
Physical Design of Bioreactor System
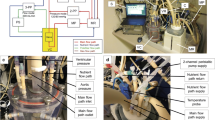
The custom designed bioreactor system provides mechanical stimulation to aortic valves through a cyclic opening and closing action (Fig. 1). This action, caused by fluid flow, is accomplished by raising and lowering a piston containing a securely mounted aortic root through culture medium. Motion is achieved using a Parker BE233FJ servo motor (Parker Compumotor, Rohnert Park, CA) coupled to a 25:1 planetary gearbox (Parker Compumotor, Rohnert Park, CA), which in turn drives a cam-crank (cam diameter 9.0 cm) assembly. The servo motor was chosen for its capacity for high torque (4.48 Nm) in a relatively small frame (5.7 × 5.7 × 14 cm, 1.9 kg). This servo provides sufficient torque to operate the system across a range of 0–110 equivalent BPM. The crank is attached to a cross-member (40 × 2.54 × 6.35 cm), which slides in one dimension along two hardened steel alignment shafts. The drive components (cam, crank, cross-member) were made of 316L steel for high corrosion resistance as well as durability and low deformation under continuous load. The structural components of the bioreactor are made from anodized 6061 aluminum, chosen for its combination of low weight, strength and corrosion resistance. By varying the crank length and shaft speed of the motor, stroke volume (651 mL) and frequency (0–110 BPM) can be controlled. For example, with the current cam and crank, a flow rate of 5 L/min can be achieved with a cam speed of 7.7 rpm. Currently the system is being run at 5 rpm resulting in 3.3 L/min of flow through the valves. These flow rates are comparable to the current state of the art flow loop described by Hildebrand et al.4
Motion of the three valve-containing pistons ensures that the valves open and close during each cycle (Fig. 1). The piston makes a tight seal with the wall of the culture chamber to ensure all flow is through the valve. The piston faces are attached to the driven cross-member with steel shafts and a double seal is used at the air/chamber interface. The piston faces were machined from polycarbonate and can be autoclaved with the connecting shafts partially assembled. Each piston has one tissue valve mounted for flow in the positive z-direction, and one mechanical valve (Carbomedics, Austin, TX) mounted opposite to the tissue valve to allow flow in the negative z-direction.
A method of securing the aortic valve to the piston was established by adapting a concept put forth by Hildebrand et al.4 An aortic root (prepared as described in the following paragraph) is sutured to a custom designed stent-like device (Fig. 2), which consists of three arms, each containing holes that allow a swaged suture needle to pass through. These holes were curved to match the radius of curvature of a 2-0 suture needle. Due to the complex geometry of the arms, this stent was fabricated using stereolithography (Laser Reproductions, Columbus, OH) from a high temperature nanoparticle filled polyurethane resin (Nanoform 15120, DSM Somos, Elgin, IL) to ensure both sufficient mechanical and thermal stability at high temperatures (autoclave 121 °C). The base of the stent contains a recess for a silicone rubber gasket (thickness 0.125′′) that ensures a tight seal between the piston and aortic root.
The dissection and preparation of the aortic root allows the tissue to be efficiently loaded into the organ culture system with minimal contamination for cyclic culture. First, the root is grossly dissected from porcine hearts obtained from a local abattoir. The root complete with valve is rinsed in PBS containing 2% antibiotic/antimycotic for 15 min. Next, the coronary arteries are ligated, and the root is then sutured to the stent using 2-0 Prolene sutures (Ethicon, Cincinnati, OH). After the root has been secured to the stent, the ventricular annulus is sutured to the silicone gasket. The aortic valve/stent assembly is then locked into the piston face with spring clips (Fig. 3). This valve is mounted to allow flow in an opposite direction to a permanently installed bileaflet mechanical valve (Carbomedics 19 mm aortic valve, Sulzer Carbomedics, Austin, TX). The oppositely oriented valves both function in one direction only, thereby ensuring that flow proceeds entirely through one valve per half revolution of the cam. During the first half of the cam revolution (the “downstroke”), culture medium will be present on both sides of the piston surface and will flow from the bottom portion of the culture vessel to the top. Once the second half of the revolution begins (the “upstroke”), the mechanical valve will open, the tissue valve will close, and fluid will flow from the top portion of the culture vessel to the bottom.
The pistons are located inside the cylindrical culture chambers, each of which is filled with 1.3 L of culture medium (Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 2% antibiotic/antimycotic solution and 10% bovine growth serum (BGS); Mediatech, Manassas, VA and Thermo Scientific, Waltham, MA respectively).7 The culture chambers are sealed by a rubber plug with an in-line 0.2 μm syringe filter in line to allow adequate gas exchange. Silicone o-rings are used to create a seal between sterile, fluid-filled areas and non-sterile areas of the bioreactor.
Computational Analyses
Computational Fluid Dynamics
Computational models were used to investigate the forces due to fluids contained within the cylindrical culture chambers, which ultimately guided the selection of materials and the servo motor. Basic computational fluid dynamic (CFD) models of each direction of flow were performed using a simplified culture chamber in Floworks (Solidworks Corporation, Concord, MA), which will approximate solutions to Navier–Stokes equations under specific boundary conditions and initial values. Models representing several states of the system were developed. In state 1, an open 19 mm Carbomedics aortic valve was modeled, and the porcine valve was assumed to be completely closed, with no leakage between chambers. The flow was modeled as an internal flow (Q = 0.193 L/s), with static pressure (761 mmHg) and flow rate input as boundary conditions. This flow rate was chosen to be more than two times higher than physiologic or roughly 2.5 times what would be used experimentally6 in order to generate a “safety factor” for load presented to the motor and to prevent material failure. Additionally, this state was modeled as an adiabatic system, and water was used as the test medium. Average transvalvular pressure was measured from areas on either side of the valve where laminar flow was observed (Fig. 4a). In state 1 this value was approximately 61.658 mmHg. The highest normal force due to fluid resistance in state 1 was 168.087 N. This system was recalculated in state 2 of the model—in which the porcine valve was open, and the mechanical valve was closed (Fig. 4b) but conditions were otherwise identical to state 1. It was assumed that the orifice area of the porcine valve was identical to the aortic cross-sectional area, and the mechanical valve was completely closed with no leakage. Both average transvalvular pressure and normal force on the piston were lower in state 2—18.394 mmHg and 28.484 N respectively. Based on the calculated normal force exerted on the piston face, materials were selected to ensure consistent mechanical performance under load. An estimate of the maximum torque required to complete a stroke was calculated from the state 1 data, and was subsequently used in selection of the servo motor and gearbox.
Finite Element Analysis
Results obtained from CFD studies were entered into a finite element analysis (FEA) of the behavior of the system under loaded conditions. The maximum calculated normal force on the disc was applied to a simplified internal model of the piston assembly. Material parameters for polycarbonate and steel were obtained from ASTM specifications provided by the supplier (McMaster-Carr, Atlanta, GA) and input into the model of the piston face and the connecting threaded shaft, respectively. The steel shaft was assumed to be fixed, which was used as a boundary condition. Von Mises stress and material deformations for these components were then calculated. Upon analysis of the von Mises stress resulting from a normal force on the piston face of 168.087 N, it was determined that the interface of the steel and polycarbonate threads resulted in an unacceptably high value, and would likely crack during loading. A design revision was made using a steel washer to distribute the load, and then fix the axial location of the disc with a steel nut. This revision was modeled and the system was recalculated. This revision minimized the von Mises stress to more than an order of magnitude below the yield strength of polycarbonate (71–120 MPa, Fig. 5). Deformation of the disk was also investigated since the alignment of the system is crucial to ensure no stiction between the circumferential surface of the piston and the inner wall of the culture chamber. There was no detectable bending deformation at modeled loads of the disc therefore the design was deemed acceptable.
Bearing lock-up was observed frequently when evaluating the prototype design (Fig. 1a). In response to these problems, CosmosWorks (Dassault Systèmes, Concord, MA) was used to analyze displacement and deformation of drive components using a finite element model of the system throughout a complete revolution of the cam. First, the peak torque required as a function of the stroke was calculated using a motion study with a 1 RPS shaft speed. The system was then virtually “assembled” in this configuration and deformation and displacement studies were performed. It was observed that purely due to the size of the link, placement of the cam, and location where these parts were pinned, there was a significant bending moment placed upon the sliding plate. The system was redesigned to increase the length:diameter ratio spanned by the bearings on the alignment shaft. Additionally, the link was thickened from 0.25 to 0.5 in. and the location for pinning to the sliding plate was changed to be located in the center, rather than on the face of the part. Lastly, the cam’s diameter was reduced to decrease the force exerted on the drive assembly. This new design (Fig. 1b) was then subjected to the same analysis in CosmosWorks, where the maximum resultant displacement was observed to be reduced from 0.075 to ~0.035 mm (Fig. 6).
Sterility Challenge
The final design criteria was that the system provide a sterile culture environment for valves throughout the duration of stimulation. Thus, all developed components of the bioreactor are able to be steam sterilized. After all components of the bioreactor are removed from the autoclave, the system is assembled in a laminar flow hood using autoclaved tools and sterile gloves. The ability of the bioreactor system to provide a sterile culture environment was tested by filling the chambers with culture medium, placing the system in a humidified incubator (37 °C with 5% CO2), and enabling the drive. No valves were mounted in the system for the purposes of sterility evaluation. Medium samples were taken at one, two and four days and were plated on agar gels. Gels were inspected visually for colony formation at 7 days post inoculation. No colony formation was observed indicating that no contamination of the culture environment occurred. Subsequently, the system has been run for up to 3 weeks (10 bpm, 4.2 L/min) with porcine valves loaded, and no visible signs of contamination have been observed.
Future Studies
Scalability was a major motivating factor in the design of this system, therefore this system can easily accommodate six tissue valves. In order to accommodate large sample sizes, the mechanical valve can be replaced with another tissue valve, wherein that each culture chamber would have two biologic valves mounted in opposite directions, thereby permitting cyclic stimulation of six valves per bioreactor (Fig. 1c). Furthermore, with the addition of a twin-output gearbox and an additional three culture chambers, this system will permit the culture of up to 12 tissue valves per run. This configuration has a small footprint (40 × 40 cm) for the number of samples, and even with a doubled number of culture chambers will still fit on a standard incubator shelf (Fig. 1d). In the future, this system will be evaluated for its ability to maintain the normal state of valves ex vivo and also used as a system to study TEHV scaffolds. Additionally, this system could be used to investigate the effects of biochemical agents on valve biology and tissue remodeling. This wide range of bioreactor applications has implications for both the study of valve pathologies as well as tissue engineering of heart valves.
Conclusions
The design of the system evolved as prototypes were fabricated and empirical results on system performance were obtained. The initial design of the system allowed the bearings to freeze, which ultimately resulted in sheared or bent parts. This failure was due to two design flaws. First, variability between the porcine valves meant that slightly different forces were exerted on the sliding plate as a result of resistance in each culture chamber, resulting in an unbalanced load. Second, the prototype of the system did not have an optimal design to prevent bearing lockup in sliding and the pinning geometry contributed to an unbalanced bending moment. This failure was examined by studying the system using the CosmosWorks software package, thus leading to the redesign of the pinning geometry and location of alignment shaft bearings. After the new design was tested in the software package, the system was fabricated and run for 3 weeks, with no lockup problems observed.
The design of a simple bioreactor system that can perform both organ culture and tissue engineering duties has yet to be realized. Many designs exist, but are inadequate for the design objectives listed here due to complicated mechanical designs, high implementation costs, lengthy setup times, low sample numbers, and complicated loading procedures. Flow loops offer the most precise control over culture conditions due to their use of pulsatile pumps and closed-loop feedback systems (both pressure and flow rate), however these systems require custom software to be created. These sorts of feedback systems require nontrivial software to both acquire and process signals before this information can be integrated into meaningful changes in the pump’s output. Furthermore, pulsatile pumps are more complicated than rotating shafts to design and maintain. In contrast, the newly described system is the first to allow rapid loading of aortic valve roots due to the use of the spring-locked stent. This system can be pre-assembled and left under UV light in a BSC before dissection of the tissue. Since the system does not need to be completely disassembled to load the samples, it can be loaded and operational in minutes after tissue dissection.
The bioreactor presented here is simple to replicate, has the ability to scale to accommodate varying sample sizes, and uses very simple drive and control mechanisms. Limitations of this system include the lack of robust flow control and feedback systems, the requirement that all valves are subjected to the same stroke volume and flow rate, and imprecise waveform control. Furthermore, this system requires large volumes of media and an entire half-height incubator to run. Nonetheless, potential uses for a simple, scalable aortic valve organ culture system are wide ranging and the presented system will find applications for study of valve biology and tissue engineering.
References
Dumont, K., J. Yperman, E. Verbeken, P. Segers, B. Meuris, S. Vandenberghe, W. Flameng, and P. R. Verdonck. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif. Org. 26:710–714, 2002.
Engelmayr, Jr., G. C., D. K. Hildebrand, F. W. Sutherland, J. E. Mayer, Jr., and M. S. Sacks. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials 24:2523–2532, 2003.
Gupta, V., J. A. Werdenberg, B. D. Lawrence, J. S. Mendez, E. H. Stephens, and K. J. Grande-Allen. Reversible secretion of glycosaminoglycans and proteoglycans by cyclically stretched valvular cells in 3D culture. Ann. Biomed. Eng. 36:1092–1103, 2008.
Hildebrand, D. K., Z. J. Wu, J. E. Mayer, Jr., and M. S. Sacks. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann. Biomed. Eng. 32:1039–1049, 2004.
Hoerstrup, S. P., R. Sodian, J. S. Sperling, J. P. Vacanti, and J. E. Mayer, Jr. New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng. 6:75–79, 2000.
Konduri, S., Y. Xing, J. N. Warnock, Z. He, and A. P. Yoganathan. Normal physiological conditions maintain the biological characteristics of porcine aortic heart valves: an ex vivo organ culture study. Ann. Biomed. Eng. 33:1158–1166, 2005.
Merryman, W. D., I. Youn, H. D. Lukoff, P. M. Krueger, F. Guilak, R. A. Hopkins, and M. S. Sacks. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am. J. Physiol. 290:H224–H231, 2006.
Mol, A., N. J. Driessen, M. C. Rutten, S. P. Hoerstrup, C. V. Bouten, and F. P. Baaijens. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann. Biomed. Eng. 33:1778–1788, 2005.
Schneider, P. J., and J. D. Deck. Tissue and cell renewal in the natural aortic valve of rats: an autoradiographic study. Cardiovasc. Res. 15:181–189, 1981.
Stegemann, J. P., and R. M. Nerem. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 31:391–402, 2003.
Warnock, J. N., S. Konduri, Z. He, and A. P. Yoganathan. Design of a sterile organ culture system for the ex vivo study of aortic heart valves. J. Biomech. Eng. 127:857–861, 2005.
Weber, K. T., Y. Sun, L. C. Katwa, J. P. Cleutjens, and G. Zhou. Connective tissue and repair in the heart. Potential regulatory mechanisms. Ann. NY Acad. Sci. 752:286–299, 1995.
Weston, M. W., and A. P. Yoganathan. Biosynthetic activity in heart valve leaflets in response to in vitro flow environments. Ann. Biomed. Eng. 29:752–763, 2001.
Xing, Y., J. N. Warnock, Z. He, S. L. Hilbert, and A. P. Yoganathan. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann. Biomed. Eng. 32:1461–1470, 2004.
Acknowledgments
This work was funded by NIH HL080080 and the Rice University NIH Biotechnology Graduate Training Program (5T32 GM008362). Thanks to Dr. R. E. Phillips at Carbomedics, Inc. for generously providing the mechanical heart valves. Thanks to Dwight Dear for aiding in fabrication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durst, C.A., Jane Grande-Allen, K. Design and Physical Characterization of a Synchronous Multivalve Aortic Valve Culture System. Ann Biomed Eng 38, 319–325 (2010). https://doi.org/10.1007/s10439-009-9846-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9846-3