Abstract
Muscle artifacts are typically associated with sleep arousals and awakenings in normal and pathological sleep, contaminating EEG recordings and distorting quantitative EEG results. Most EEG correction techniques focus on ocular artifacts but little research has been done on removing muscle activity from sleep EEG recordings. The present study was aimed at assessing the performance of four independent component analysis (ICA) algorithms (AMUSE, SOBI, Infomax, and JADE) to separate myogenic activity from EEG during sleep, in order to determine the optimal method. AMUSE, Infomax, and SOBI performed significantly better than JADE at eliminating muscle artifacts over temporal regions, but AMUSE was independent of the signal-to-noise ratio over non-temporal regions and markedly faster than the remaining algorithms. AMUSE was further successful at separating muscle artifacts from spontaneous EEG arousals when applied on a real case during different sleep stages. The low computational cost of AMUSE, and its excellent performance with EEG arousals from different sleep stages supports this ICA algorithm as a valid choice to minimize the influence of muscle artifacts on human sleep EEG recordings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of arousals in sleep is a matter of interest among basic researchers and clinicians not only for its implications on the normal sleep physiology,7,8,21,39 but also for its central role in the diagnosis and follow-up of some prevalent sleep disorders.36,41,43,51 For instance, arousals are often associated with sleep disruptions which frequently intrude as muscle artifacts into the ongoing electroencephalographic (EEG) activity of many neurological and sleep disorders.14,19,27,29,36,41,51 Even though the enhancement of muscle tone is not always a prerequisite for EEG arousal identification (e.g., in non-REM sleep),1 the inclusion of this criterion has been shown to shorten the scoring time without altering the inter-scorer agreement and reliability results.42 Consequently, muscle artifacts will distort oscillatory EEG activity during the arousal period.
Previous results highlight the relevance of studying spatiotemporal changes of EEG activity during the arousal episodes. Evidence suggests that spectral variations associated with arousals provide an effective and reliable method of identifying sleep-disordered breathing events.47,57 The influence of arousals on EEG spectra has also demonstrated to be of clinical value in the diagnosis of the restless legs syndrome.28 Indeed, periodic leg movements exert a differential impact on sleep continuity and daytime functioning when they are accompanied by arousals.46 Additional examples emphasizing the importance of studying EEG activity during arousals come from epilepsy research. Terzaghi and colleagues50 have recently found that highly stereotyped minor motor events and seizure discharges frequently occur in association with arousal fluctuations in nocturnal frontal lobe epilepsy patients. In this particular case, minimization of muscle artifacts has been shown to improve the source localization of the pre-ictal and ictal seizure onset which is critical for subsequent surgical resection of epileptogenic tissue.26
Sleep EEG segments contaminated with muscle artifacts are typically ruled out, by hand or with automatic procedures, when they are larger than an arbitrarily preset threshold, generating a substantial amount of data loss.20 Although different technical approaches have been employed for correcting muscle artifacts, none of them has yielded satisfactory results in sleep research. Digital filters have been typically applied on EEG signals to eliminate high-frequency bands (above 15 Hz) where muscle artifacts are supposed to contribute.25,58 It is well known, however, that EMG activity not only obscures fast EEG oscillations, typical in the sleep-to-wake transition,44,45 but also modifies slower EEG frequency bands.24,59 Regression in either the time or frequency domain has been extensively used to eliminate ocular artifacts from human EEG recordings,16 but these methods are not applicable to muscle activity since no regressing channels exist for these scalp-recorded artifact sources.35 Alternatively, there is a regression method which reduces the influence of muscle artifacts on fast EEG frequencies (51–69 Hz) but it does not apply to the time domain.23 Principal component analysis (PCA) methods perform better than regression at correcting ocular artifacts from EEG,6 but the assumption that EEG sources are linearly decorrelated and spatially orthogonal to one another makes PCA techniques inappropriate for our purpose.34
An alternative approach for artifact reduction is based on blind source separation techniques (BSS). Independent Component Analysis (ICA)15 has been commonly applied to both EEG source isolation3 and artifact correction.30,32,35,55 ICA-based analysis methods are able to separate out EEG signals from an estimate of the overlapping projections of the artifact in all scalp EEG electrodes, assuming that sources are statistically independent.12 ICA preserves more brain activity than other correction techniques (i.e., PCA), and is able to separate a wide variety of EEG artifacts simultaneously, without needing of reference channels. Though distinct ICA methods pursue identical goals, they model different sources, which may critically affect the effective elimination of extracerebral artifacts from EEG recordings.
A number of previous studies have employed different ICA algorithms to reduce muscle activity and other artifacts from EEG recordings in clinical conditions.30,56 ICA reliability has also been reported for artifact correction in sensory evoked and event-related potential studies,33,35,52 but the performance of different ICA algorithms on removing muscle artifacts from sleep EEG recordings remains largely unexplored.20
In the present study, we evaluated the performance of four ICA algorithms to minimize artifacts from temporalis muscles appearing in human sleep EEG during spontaneous arousals. We focused on temporalis because together with frontalis muscles represent the most common sources of muscle artifacts during resting EEG conditions.2,37 The four selected ICA algorithms have been previously proposed as valid methods for EEG artifact correction,30,32,33,35,48,49,52,56 but they differ in both their theoretical assumptions and the underlying statistic fundamentals. Two of them (AMUSE and SOBI) are based on second-order statistics (SOS) and can separate independent sources by minimizing correlations between signals. The other two (Infomax and JADE) employ higher-order statistics (HOS) and the probability density functions of the sources, assuming their non-gaussianity distribution. As HOS-based techniques need a higher number of samples than SOS algorithms to reach an accurate signal separation, we would expect that the former showed a lower performance when applied to the short-duration EEG segments of sleep arousals.
To aim this goal, a semi-simulation study was designed by combining sleep EEG recordings with different levels of muscle contamination extracted from real EMG signals. Then, the most efficient ICA algorithm was applied to typical spontaneous arousals identified in different sleep stages of a healthy elderly subject. To test the performance of the selected ICA algorithm on real sleep data, power spectra of temporal electrodes were compared before and after minimizing muscle activity from scalp EEG recordings.
Methods
Subjects
Four healthy volunteers (two men; mean age = 24,75 ± 1,5 year) and one additional healthy elderly woman (64 year) participated in this study. Subjects were evaluated by a structured interview and questionnaires, disregarding those with any medical and/or psychological disorders. Medication or drugs consumption was also an exclusion criteria. Subjects were asked to refrain from naps, drugs, alcohol, or drinking caffeinated beverages during 48 h prior to the day of recording. To control the absence of sleep disorders, subjects completed daily records in the week prior to sleeping in the laboratory. All participants gave informed consent after a full explanation of the experimental protocol.
Recording Protocol
Polysomnographic recordings in the four young participants included 24 EEG derivations referenced to linked mastoids, horizontal electrooculogram (EOG), and submental electromyography (EMG). 10 EEG electrodes were placed longitudinally and regularly spaced over scalp in left hemisphere (Fp1-O1) and 10 over right hemisphere (Fp2-O2) following identical electrode separation as in the contralateral hemisphere. 4 additional electrodes were placed over temporal regions (T3, T4, T5, and T6) following the International 10–20 system.31 Electrode impedances were kept below 5 KΩ. Filters were set between 0.5 and 100 Hz for EEG, 5–100 Hz for EMG and 0.3–30 Hz for EOG. Signals were amplified and digitized using a MEDICID® 4 system (Neuronic®, S.A.) at a sampling rate of 256 Hz.
For the elderly subject, sleep was recorded from 59 scalp EEG derivations referenced to linked mastoids. The recording protocol also included horizontal and vertical EOG channels, and a bipolar montage for submental electromyography (EMG). Electrode impedances were kept below 5 KΩ, and filter settings were identical to the young participants. Signals were acquired with BrainAmp MR amplifiers (Brain Vision®) at a sampling rate of 250 Hz. Two different sleep technologists performed independent visual sleep scoring according to standard criteria40 in all recordings.
Simulation Study
To compare the performance of different ICA algorithms on removing muscle artifacts from sleep EEG recordings, a semi-simulation study was carried out by combining real sleep EEG recordings with different levels of EMG contamination. Sixteen artifact-free EEG segments (4 20-s segments per subject) were extracted from tonic REM sleep periods characterized by the absence of rapid eye movements and muscular atonia. Each EEG segment was selected by careful visual inspection considering the total lack of artifacts.
Artifacts from temporalis muscles were modeled using submental EMG activity recorded during a spontaneous awakening (20-s epoch) and multiplied by a typical muscle scalp map for both right and left temporal muscle components. The scalp map was isolated as follows. First, ICA (Infomax algorithm) was applied to a 20-s segment of sleep EEG (20 s) highly contaminated by muscle activity in temporal channels, obtaining the separation matrix and the different ICA components. Two ICA components were located over temporal regions (right and left) based on their scalp topography and power spectral distribution. After identification of temporal muscle components, they were replaced by previously selected EMG signals. Values of remaining ICA components were set to zero. The new set of components was multiplied by the mixing matrix to obtain the scalp distribution of muscle artifacts. Amplitude of the muscle contamination was maximum over temporal electrodes (T3 and T4), and near zero in the remaining ones.
Since performance of different ICA algorithms may be affected by the strength of muscle contribution, this variable was manipulated with four power ratio conditions (100, 10, 1, and 0.1). Power ratios were computed dividing the power of the muscle artifact signal by the power of the EEG signal recorded in T4. Signal-to-noise ratios (SNR) corresponding to the above power ratios result in 20, 10, 0, and −10 dB, respectively. Altogether, 64 20-s datasets (16 EEG segments × 4 SNR conditions) were obtained before ICA application.
ICA Algorithms
Each ICA algorithm employs a different approach to estimating independence18 which may affect the sensibility of each method on separating muscle artifacts from sleep EEG activity. Four ICA algorithms were tested in the present study considering its statistical properties and frequency of use in EEG studies for artifact correction: AMUSE (Algorithm for Multiple Unknown Source Extraction) is a second-order statistic (SOS) method, useful to separate temporally uncorrelated sources.53,54 It only employs one time delay to compute correlation between signals, in addition to the instantaneous correlation. SOBI (Second Order Blind Identification) is another SOS algorithm that exploits the time coherence of the signal sources to decompose the mixture of sources. Because such cross-correlations are sensitive to the temporal EEG features, detailed characteristics of the ongoing activity provide useful information for source separation.4 SOBI performance has demonstrated to improve EEG source separation when both long time intervals (300 ms) and large number of time delays were considered.48,49 Since no studies have determined the optimal time delays to separate muscle activity from EEG signals, the maximum amount of consecutive time delays (77) was applied every 300 ms considering our sampling rate. Infomax algorithm is based on high-order statistics (HOS) to estimate the probability distributions of the independent component. It assumes a priori the probability density functions of the sources (supergaussian and subgaussian for the extended version).38 Contrary to SOS algorithms, temporal information from signals is not considered. Estimation of independence is based on minimization of mutual information between sources. JADE (Joint Approximate Diagonalization of Eigen-matrices) is another HOS algorithm that uses fourth-order statistics and minimizes cross-cumulants to achieve independence among estimated components. It assumes that sources of interest are non-gaussian whereas noise is a gaussian independent source. Although no parameter-tuning is required, a considerable data length seems necessary for reliability of source separation.11
All ICA algorithms run in MATLAB® v. 7.4 (The MathWorks, Inc.) and are freely downloaded from the Internet. Infomax was implemented in the runica function of the EEGLAB toolbox v. 5.3 (http://www.sccn.ucsd.edu/eeglab/).17 The remaining ICA algorithms are available in the ICALAB toolbox for Signal Processing v.3 (http://www.bsp.brain.riken.jp/ICALAB/).13 All the analyses were performed on a Dell™ workstation with 4 Intel Xeon™ Dual Core processors, 2.66 GHz each, and 16 GB of RAM, under Microsoft Windows XP 64 bits.
Muscle Component Selection
Criteria for identifying muscle components in practice were based on time-domain features, scalp topography, and power spectrum.33–35 Muscle components were characterized by the presence of fast EEG activities together with an abrupt increase in amplitude simultaneously to the arousal episode. Furthermore, topographic distribution of fast EEG activity associated with muscle contamination showed their maximal values over fronto-temporal areas, and near zero in the remaining cortical regions. EEG segments contaminated with muscle activity suffered a drastic enhancement of spectral power above 50 Hz. Overall, the entire process (ICA application on EEG signals, muscle component identification, and subsequent artifact removal) takes about 15 min when performed by an experienced person. In our simulation experiment, the muscle component selection was faster since the number of muscle components (two) and the time-domain representation of the original muscle signals were known a priori by the experimenters.
Efficacy Indicators
The efficacy of each ICA algorithm was determined considering three different indexes: (i) computation time; (ii) Pearson’s correlation coefficient (R 2) and mean square error (MSE) between scalp EEG signals recorded from temporal regions; and (iii) Pearson’s correlation coefficient and MSE between the remaining electrodes (EEG signals recorded from non-temporal areas). Correlation and MSE were computed between artifact-free EEG segments extracted from tonic REM sleep before and after applying each ICA algorithm for the four SNR conditions. Both correlation and MSE are two different statistical approaches to evaluate similarity between two signals. Algorithm efficacy is near optimal when the values of correlation coefficients are close to 1 and MSE approximates to zero.
Statistical Analysis
Correlations and MSE data were statistically tested by using a two-way ANOVA with repeated measurements, including the algorithm (AMUSE, SOBI, Infomax, JADE) and the SNR (20, 10, 0, −10 dB) as within-subject factors. Multiple comparisons were corrected with the Greenhouse-Geisser estimator. Differences between conditions were analyzed by using Newman-Keuls post hoc tests.
Data Visualization
To verify the performance of the most efficient ICA algorithm in realistic conditions, 20-s EEG epochs corresponding to arousals from stage 2, slow wave sleep (SWS) and REM sleep were selected from the sleep recording of a healthy 64-year woman. In all cases, the EEG arousal was accompanied by an increment of the EMG activity in association with scalp-recorded muscle contamination. To provide information about gross variations of spectral power caused by state-dependent EEG arousals, Fourier spectra were computed on the 7-s period preceding and following the arousal onset for each sleep stage before and after applying the ICA algorithm to the signal recorded at electrode T8 (the most contaminated EEG location in these data sets). To further determine the time–frequency changes of state-dependent EEG arousals, wavelet power analysis was applied to 14-s EEG segments (7 s preceding the arousal and 7 s from the arousal initiation). For this analysis, a continuous complex Morlet was used with 0.5 Hz of spectral resolution. The wavelet difference was also computed to evaluate the time–frequency evolution of spectral power caused by the muscle contamination before and after ICA application for each state-dependent EEG arousal.
Results
Computation Time
AMUSE demonstrated to be considerably faster, whereas Infomax took significantly longer than the remaining ICA algorithms used in the present study. In particular, AMUSE took 0.03 s at separating muscle artifacts from EEG activity in a 20-s EEG segment with 24 electrodes. However, Infomax lasted longer than 1 min for the same data set, whereas SOBI was almost fourfold faster than JADE (1.3 vs. 4.7 s, respectively). Differences in computation time between ICA algorithms are unlikely due to implementations in different toolboxes, since similar results were observed when the four ICA algorithms were run in EEGLAB.
Muscle Artifact Removal from Temporal Regions
To determine the efficacy of different ICA algorithms on removing muscle contamination from temporal regions during awakenings, correlations between artifact-free sleep EEG and ICA-processed sleep EEG were computed. Electrodes T3 and T4 were chosen for the analysis because they presented the highest strength of muscle contamination in our simulation experiment. Since results were quite similar in both temporal locations, T4 data was just reported for simplicity matter. Figure 1 depicts mean correlation values across ICA algorithms and contamination levels for T4. A principal effect of the factor ‘ICA algorithm’ was obtained [F(3,45) = 73.09, p < 10−7, ε = 0.41], mainly due to the lower performance of JADE as compared to the remaining algorithms [F(3,252) > 6, p < 10−3]. This result was confirmed for all contamination levels (p < 0.04), except when the spectral power of the muscle signal was 10 times below the real EEG amplitude. Statistical results provided by MSE data were highly similar to those obtained with correlations (Table 1), pointing out the equivalence between both statistical indexes to evaluate efficacy between different ICA algorithms. JADE showed the maximal MSE values in T4 signals [F(3,45) = 45.7, p < 10−5, ε = 0.34], specifically for SNR ≥ 0 (p ≤ 0.02).
Mean correlation values (R 2) between artifact-free T4 signals and same EEG signals after being processed with AMUSE, SOBI, Infomax, and JADE across different levels of muscle contamination. Mean correlation values between artifact-free T4 signals and contaminated T4 signals are also displayed (no ICA, gray square). Vertical bars indicate mean errors
Muscle Artifact Removal from Non-temporal Regions
Muscle activity also spreads to extratemporal electrodes but in a lesser degree. To comply with this premise, non-temporal EEG regions were slightly contaminated by muscle activity in the simulation study. Despite similarities (all correlations were higher than 0.95 due to the low artifact contribution in extra-temporal regions), differences between algorithms were statistically significant [F(3,45) = 21.3, p < 10−5, ε = 0.69]. AMUSE and SOBI showed the highest performance for non-temporal EEG locations, followed by Infomax [F(3,252) = 15.7, p < 10−8]. Again, JADE was the least efficient ICA algorithm at separating muscle signals from sleep EEG recordings. As expected, performance varied as a function of contamination strength [F(3,252) = 9.3, p < 10−5]. Interaction between ‘ICA algorithm’ and ‘contamination level’ was also significant [F(9,135) = 4.3, p < 10−2, ε = 0.36] mainly due to the lower correlation values obtained with JADE for the three higher contamination levels (p < 10−3). For the −10 dB SNR (muscle contamination 10 times below EEG amplitude), AMUSE performed better than Infomax and JADE (p < 10−2). Figure 2 depicts mean correlation values across ICA algorithms and contamination levels for extratemporal electrodes.
Mean correlation values (R 2) between artifact-free extratemporal EEG signals and same EEG signals after being processed with AMUSE, SOBI, Infomax, and JADE across different levels of muscle contamination. Mean correlation values between artifact-free extratemporal EEG signals and the same EEG signals but contaminated are also displayed (no ICA, gray square). Vertical bars indicate mean errors
Similar results were obtained for the MSE index. An interaction effect between ‘ICA algorithm’ and ‘contamination level’ [F(9,135) = 4.3, p < 10−2, ε = 0.36] allowed to determine that MSE results shown by JADE were significantly worse than those obtained with the remaining algorithms for SNR ≥ 0 (p < 10−3). In contrast, AMUSE performed significantly better than both JADE and Infomax in the least contaminated conditions. With the exception of AMUSE, the rest of ICA algorithms showed an impaired efficacy in the lowest contamination level (SNR −10 dB) over non-temporal EEG locations. As shown by the two statistical indexes, AMUSE performance was independent of the strength of contamination for non-temporal EEG derivations.
Application of AMUSE to Real Data
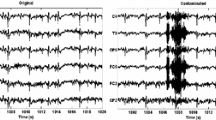
To qualitatively validate the abovementioned results, the AMUSE algorithm was applied to human sleep EEG recordings during spontaneous arousals from stage 2, SWS, and REM sleep (Fig. 3). Figure 3a shows examples of EEG recordings from a 64-year healthy woman containing arousals from different sleep stages before (top panel) and after reduction of muscle artifacts with AMUSE (bottom panel). Note that most of the high frequency muscle contamination was eliminated in all sleep stages after AMUSE application, as displayed in the raw EEG data and wavelet power spectra plotted in Fig. 3a (bottom panel).
Muscle artifact removal by AMUSE applied to human sleep EEG recordings during spontaneous arousals recollected from different sleep stages in a healthy 64-year woman. (a) EEG segments (14 s) during spontaneous arousals from stage 2, SWS and REM sleep before (top panel) and after removal of muscle artifacts with AMUSE (bottom panel). Raw EEG data for each sleep stage represents a subset of 4 EEG electrodes highly contaminated with muscle activity from a set of 59 scalp EEG montage. Wavelet power spectra of T8 signals for each EEG segment are additionally shown before (top panel) and after AMUSE application (bottom panel). (b) Fourier power spectra of T8 signals computed before and after the arousal onset (identical time window of 7 s) for the raw EEG data (solid line) and for the same signals after muscle artifact removal with AMUSE (dashed line). Independent component scalp maps of muscle contribution removed from arousals in each sleep stage are also displayed. (c) Difference wavelet spectral power in T8 signals before and after AMUSE application for each variant of EEG arousal
During the stage 2 of sleep, muscle contamination was also present several seconds preceding the EEG arousal, particularly on temporal locations. This is in agreement with previous indications that not only phasic EMG events but also tonic aspects of myogenic activity are likely to affect cortical EEG activity during non-REM sleep.10 In this case, AMUSE not only attenuated the high frequency events intermingled with the cortical EEG activity but also those slower EEG components, including theta and alpha oscillations, which have also been demonstrated to be affected by muscle activity.59
Interestingly, the EEG arousal during non-REM sleep was characterized by an increase in delta power, mostly evident in stage 2 (Fig. 3b, left panel). These slower frequency components were also removed by AMUSE, but only during the arousal from SWS. Although K-complexes can also be present in arousals from stage 2, the enhanced delta power observed in our study likely came from different extracerebral sources such as breathing, which also contribute to activity within the delta band. Breathing can produce two kinds of EEG artifacts. One of the them in the form of slow and rhythmic activity, synchronous with the body movements of respiration and mechanically affecting the impedance of usually one electrode. The other type can be slow or sharp waves that occur synchronously with inhalation or exhalation and involve those electrodes on which the patient is lying. As shown in the ICA maps (Fig. 3b), the sources of these artifacts, usually asymmetric, were not included among the ICA components that were removed from the EEG, which might explain the enhanced delta power.
The arousal from REM sleep included an increase in EMG amplitude together with an enhanced power of fast EEG frequencies. However, as indicated by the small effect of AMUSE, the contribution of the EMG activity to cortical EEG activity was negligible in this sleep stage as compared with stage 2 and SWS. The amount of muscle activity removed from the electrode T8 during non-REM and REM arousals is shown in the difference wavelet power spectra plotted in Fig. 3c.
Discussion
This study evaluated, for first time, the efficacy of different ICA algorithms at minimizing muscle activity from human sleep EEG recordings by using simulated and experimental data. We found that AMUSE, Infomax, and SOBI performed significantly better than JADE when the strength of the muscle contamination was above the EEG amplitude over temporal regions. Additionally, AMUSE demonstrated to be fairly independent of the contamination level as indicated by the high performance at removing muscle artifacts from non-temporal EEG electrodes even when the amplitude of muscle contamination was 10 times lower than EEG activity. The low computational cost of AMUSE (44–2000 times faster than the remaining ICA algorithms tested in this study), and its excellent performance supports this ICA algorithm as the best choice to remove muscle artifacts from sleep EEG recordings.
We tested ICA algorithms based on both second-order measures (such as the autocorrelation function and power spectrum) and higher-order statistics (such as the nth order cumulants and the nth order spectrum). Since EEG and muscular sources are assumed to be temporally uncorrelated, both SOS- and HOS-based methods should provide adequate BSS estimations to face up this problem. Results showed that AMUSE, SOBI (both SOS algorithms), and Infomax (HOS algorithm) performed better than JADE (HOS algorithm) at separating muscle artifacts from sleep EEG recordings. Pilot studies revealed that FastICA (another HOS algorithm broadly used to remove EEG artifacts)18,22 yielded similar results to those obtained with JADE. Ting and colleagues52 compared AMUSE (SOS algorithm) and FastICA (HOS algorithm) performance at separating genuine EEG activity from typical artifact sources (EOG and EMG) over single-trial event-related potential epochs with a small number of samples. Signal sources obtained with FastICA were not as accurately estimated as compared with AMUSE, probably due to the fact that HOS-based ICA algorithms need larger data sets to provide robust source separation between EEG activity and artifacts. Considering that muscle artifacts in sleep EEG recordings can appear during short sleep-to-wake transitions (arousals) and longer awakenings, the use of SOS-based ICA algorithms, like AMUSE, should work efficiently in both scenarios.
Infomax, a HOS-based ICA algorithm, yielded similar results to those provided by the two SOS algorithms used in the current study (SOBI and AMUSE). It is worth noting that Infomax uses higher-order moments to estimate the probability density functions of the obtained components, assuming that sources have a supergaussian distribution.3 Our muscle signals were markedly supergaussian (positive kurtosis of 11.84) when compared with the almost gaussian distributions observed in the rest of EEG signals (averaged kurtosis of 0.99), allowing Infomax to show a similar performance as SOS-based ICA algorithms.
Multiple time delays make SOBI a more robust choice than AMUSE (just one time delay) in the presence of temporally uncorrelated additive white (random) noise.5 However, multiple time delays employed by SOBI did not make the difference relative to AMUSE. Likely, EEG signals considered as noise in our study are composed by different temporally correlated sources, and due to their oscillating nature are far from being random signals. Finally, AMUSE shows a number of attractive advantages over other ICA algorithms: (i) it relies only on second-order statistics of the input signals, which are expected to provide more reliable estimations in adverse signal-to-noise ratios; (ii) it allows—in contrast to HOS-based ICA algorithms—the separation of gaussian sources; (iii) it requires relative small datasets to identify artifacts; (iv) it is much faster than the vast majority of BSS algorithms, and (v) it is straightforward to apply because no parameter-tuning is needed.
Extracerebral artifacts affecting sleep EEG recordings can be originated from a variety of sources including motion of different body parts, sweat, 50–60 Hz noise from electrical line, slow and rapid ocular movements, cardiac pulse, as well as glossokinetic and respiratory events. ICA-based methods are able to remove artifacts from different sources simultaneously,9 which would not only improve sleep classification but also accuracy in the diagnosis of both neurological and sleep disorders.
In summary, our results revealed that ICA algorithms provide an efficient tool for identification and minimization of muscle artifacts from sleep EEG recordings. Artifact identification has relevance for automatic sleep scoring systems, whereas reduction of myogenic activity is crucial at determining EEG mechanisms associated with normal and pathological sleep fragmentations, which might be of helpful to get further insights about the pathophysiology of prevalent sleep disorders. Furthermore, efficient methods to reduce muscle contamination from sleep EEG recordings would enhance the reliability of quantitative EEG results on cortical dynamics underlying arousals and awakenings from sleep.
References
American Sleep Disorders Association (ASDA). EEG arousals: scoring rules and examples. Sleep 15:173–184, 1992
Barlow, J. S. Artifact processing (rejection and minimization) in EEG data processing, In: Handbook of Electroencephalography and Clinical Neurophysiology. Revised series, vol. 2, edited by F. H. Lopes da Silva, W. Storm van Leeuwen, A. Remond. Amsterdam: Elsevier, 1986, pp. 15–62
Bell A. J., T. J. Sejnowski. An information-maximization approach to blind separation and blind deconvolution. Neural. Comput. 7:1129–1159, 1995
Belouchrani A., K. Abed-Meraim, J. F. Cardoso, E. Moulines. A blind source separation technique using second order statistics. IEEE Trans. Signal Process. 45:434–444, 1997
Belouchrani A., A. Cichocki. Robust whitening procedure in blind source separation context. Electron. Lett. 36:2050–2051, 2000
Berg P., M. Scherg. Dipole models of eye activity and its application to the removal of eye artifacts from the EEG ad MEG. Clin. Physiol. Meas. 12:49–54, 2000
Bonnet M. H., D.L. Arand. EEG arousal norms by age. J. Clin. Sleep Med. 3:271–274, 2007
Boselli M., L. Parrino, A. Smerieri, M. G. Terzano. Effect of age on EEG arousals in normal sleep. Sleep 21:351–357, 1998
Boudet S., L. Peyrodie, P. Gallois, C. Vasseur. A global approach for automatic artifact removal for standard EEG record. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1:5719–5722, 2006
Brunner D. P., R. C. Vasko, C. S. Detka, J. P. Monahan, C. F. Reynolds, D. J. Kupfer. Muscle artifacts in the sleep EEG: automated detection and effect on all-night EEG power spectra. J. Sleep Res. 5:155–164, 1996
Cardoso J. F., A. Souloumiac. Blind beam-forming for non gaussian signals. IEEE Proc. Radar Signal Process. 140:362–370, 1993
Cichocki C., S. Amari. Adaptive Blind Signal and Image Processing: Learning Algorithms and Applications. Chichester: John Wiley and Sons, Ltd., 2002
Cichocki, C., S. Amari, K. Siwek, and T. Tanaka. ICALAB Toolboxes for Signal and Image Processing. http://www.bsp.brain.riken.go.jp, 2002
Cohrs S., T. Rasch, S. Altmeyer, J. Kinkelbur, T. Kostanecka, A. Rothenberger, E. Rüther, G. Hajak. Decreased sleep quality and increased sleep related movements in patients with Tourette’s syndrome. J. Neurol. Neurosurg. Psychiatry 70:192–197, 2001
Comon P. Independent component analysis, a new concept? Signal Process. 36:287–314, 1994
Croft R. J., J. S. Chandler, R. J. Barry, N. R. Cooper, A. R. Clarke. EOG correction: a comparison of four methods, Psychophysiology 42:16–24, 2005
Delorme A., S. Makeig. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134:9–21, 2004
Delorme A., T. Sejnowski, S. Makeig, Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage 34:1443–1449, 2007
Fantini M. L., L. Ferini-Strambi. Idiopathic rapid eye movement sleep behaviour disorder. Neurol. Sci. 1:15–20, 2007
Fatourechi M., A. Bashashati, R. K. Ward, G. E. Birch. EMG and EOG artifacts in brain computer interface systems: a survey. Clin. Neurophysiol. 118:480–494, 2007
Ferri R., O. Bruni, S. Miano, M. G. Terzano. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP). Sleep Med. 6:29–36, 2005
Frank R. M., G. A. Frishkoff. Automated protocol for evaluation of electromagnetic component separation (APECS): application of a framework for evaluating statistical methods of blink extraction from multichannel EEG. Clin. Neurophysiol. 118(1):80–97, 2007
Gasser T., J. C. Schuller, U. S. Gasser. Correction of muscle artifacts in the EEG power spectrum, Clin. Neurophysiol. 116:2044–2050, 2005
Goncharova I. I., D. J. McFarland, T. M. Vaughan, J. R. Wolpaw. EMG contamination of EEG: spectral and topographical characteristics. Clin. Neurophysiol. 114:1580–1593, 2003
Gotman J., D. R. Skuce, C. J. Thompson, P. Gloor, J. R. Ives, W. F. Ray. Clinical applications of spectral analysis and extraction of features from electroencephalograms with slow waves in adult patients. Electroencephalogr. Clin. Neurophysiol. 35:225–235, 1973
Hallez H., A. Vergult, R. Phlypo, P. Van Hese, W. De Clercq, Y. D’Asseler, R. Van de Walle, B. Vanrumste, W. Van Paesschen, S. Van Huffel, I. Lemahieu. Muscle and eye movement artifact removal prior to EEG source localization. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1:1002–1005, 2006
Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clin. Neurophysiol. 115:1965–1974, 2004
Hornyak M., B. Feige, U. Voderholzer, D. Riemann. Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin. Neurophysiol. 116:1265–1272, 2005
Ikeda T., K. Nishigawa, K. Kondo, H. Takeuchi, G.T. Clark. Criteria for the detection of sleep-associated bruxism in humans. J. Orofac. Pain 10:270–282, 1996
Iriarte J., E. Urrestarazu, M. Valencia, M. Alegre, A. Malanda, C. Viteri, J. Artieda. Independent component analysis as a tool to eliminate artefacts in EEG: a quantitative study. J. Clin. Neurophysiol. 20:249–257, 2003
Jasper H. H. The 10–20 electrode system of the international federation. EEG Clin. Neurophysiol. 10: 371–375, 1958
Joyce C. A., I. F. Gorodnitsky, M. Kutas. Automatic removal of eye movement and blink artifacts from EEG data using blind component separation. Psychophysiology 41:313–325, 2004
Jung T. P., C. Humphries, T. W. Lee, S. Makeig, M. J. McKeown, V. Iragui, T. J. Sejnowski. Extended ICA removes artifacts from electroencephalographic recordings. Adv. Neural Inf. Process. Syst. 10:894–900, 1998
Jung T. P., C. Humphries, T.-W. Lee, M. McKeown, V. Iragui, S. Makeig, T. J. Sejnowski. Removing electroencephalographic artifacts: comparison between ICA and PCA, IEEE Int. Workshop Neural Netw. Signal Process. 8:63–72, 1998
Jung T. P., S. Makeig, C. Humphries, T. W. Lee, M. J. Mckeown, V. Iragui, T. J. Sejnowski. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37:163–178, 2000
Karadeniz D., B. Ondze, A. Besset, M. Billiard. EEG arousals and awakenings in relation with periodic leg movements during sleep. J. Sleep Res. 9:273–277, 2000
Klass D. W. The continuing challenge of artifacts in the EEG. Am. J. EEG Technol. 35:239–269, 1995
Lee T. W., M. Girolami, T. J. Sejnowski. Independent component analysis using an extended Infomax algorithm for mixed subgaussian and supergaussian sources. Neural. Comput. 11:417–441, 1999
Mathur R., N. J. Douglas. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep 18:330–333, 1995
Rechtschaffen A, A. Kales. A Manual for Standardized Terminology, Technique and Scoring for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/Brain Research Institute, 1968
Schwartz D. J., P. Moxley. On the potential clinical relevance of the length of arousals from sleep in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2:175–180, 2006
Smurra M. V., M. Dury, G. Aubert, D. O. Rodenstein, G. Liistro. Sleep fragmentation: comparison of two definitions of short arousals during sleep in OSAS patients. Eur. Respir. J. 17:723–727, 2001
Stepanski E., J. Lamphere, P. Badia, F. Zorick, T. Roth. Sleep fragmentation and daytime sleepiness. Sleep 7:18–26, 1984
Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb. Cortex 7:583–604, 1997
Steriade M., D. A. McCormick, T. J. Sejnowski. Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685, 1993
Stiasny K., W. H. Oertel, C. Trenkwalder. Clinical symptomatology and treatment of restless legs syndrome and periodic limb movement disorder. Sleep Med. Rev. 6:253–265, 2002
Swarnkar V., U. R. Abeyratne, C. Hukins. Inter-hemispheric asynchrony of the brain during events of apnoea and EEG arousals. Physiol. Meas. 28(8):869–880, 2007
Tang A. C., J.-Y. Liu, M. T. Sutherland. Recovery of correlated neuronal sources from EEG: the good and bad ways of using SOBI. NeuroImage 28:507–519, 2005
Tang A.C., M. T. Sutherland, C. J. McKinney. Validation of SOBI components from high density EEG. NeuroImage 25:539–553, 2005
Terzaghi, M., I. Sartori, R. Mai, L. Tassi, S. Francione, F. Cardinale, L. Castana, M. Cossu, G. Lorusso, R. Manni, and L. Nobili. Coupling of minor motor events and epileptiform discharges with arousal fluctuations in NFLE. Epilepsia 2007 (in press).
Terzano M. G., L. Parrino, M. C. Spaggiari, V. Palomba, M. Rossi, A. Smerieri. CAP variables and arousals as sleep electroencephalogram markers for primary insomnia. Clin. Neurophysiol. 114:1715–1723, 2003
Ting K. H., P. C. W. Fung, C. Q. Chang, F. H. Y. Chan. Automatic correction of artifact from single-trial event-related potentials by blind source separation using second order statistics only. Med. Eng. Phys. 28:780–794, 2006
Tong L., Y. Inouye, R. Liu. Waveform-preserving blind estimation of multiple independent sources. IEEE Trans. Signal Process. 41:2461–2470, 1993
Tong L., V. Soon, Y. F. Huang, R. Liu. Indeterminacy and identifiability of blind identification. IEEE Trans. Circuits Syst. 38:499–509, 1991
Tran Y., A. Craig, P. Boord, D. Craig. Using independent component analysis to remove artifact from electroencephalographic measured during stuttered speech. Med. Biol. Eng. Comput. 42:627–633, 2004
Urrestarazu E., J. Iriarte, M. Alegre, M. Valencia, C. Viteri, J. Artieda. Independent component analisis removing artifacts in ictal recordings. Epilepsia 45:1071–1078, 2004
Xavier P., K. Behbehani, D. Watenpaugh, J. R. Burk. Detecting electroencephalography variations due to sleep disordered breathing events. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1:6097–6100, 2007
Zhou W., J. Gotman. Removing eye-movements artifacts from the EEG during the intracarotid amobarbital procedure. Epilepsia 46:409–414, 2005
Zimmermann R., E. Scharein. MEG and EEG show different sensitivity to myogenic artifacts. Neurol. Clin. Neurophysiol. 2004:78, 2004
Acknowledgments
This research was supported by grants from European Union (FP6-2005-NEST-Path 043309), Spanish Ministry of Education and Science (SAF2005-00398), and Regional Ministry of Innovation, Science and Enterprise, Junta de Andalucia (CTS-229).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crespo-Garcia, M., Atienza, M. & Cantero, J.L. Muscle Artifact Removal from Human Sleep EEG by Using Independent Component Analysis. Ann Biomed Eng 36, 467–475 (2008). https://doi.org/10.1007/s10439-008-9442-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9442-y