Abstract
The pulmonary epithelium is exposed to mechanical strains during normal breathing or mechanical ventilation. While important for the regulation of cellular processes, excessive strains damage epithelial cells. To investigate the effects of strain on the epithelium, we developed a stretching device to apply equi-biaxial strains to cells cultured on elastic membranes. Following device validation, we exposed a murine epithelial cell line (MLE-12) to 30 min of cyclic stretch with 0, 25, 50, 75 and 100% change in surface area on pronectin or type I collagen coated membranes. Following stretch, we assessed cell viability using fluorescent immunocytochemisty and surfactant secretion using [3H] labeled phosphatidylcholine (PC). Cell injury increased with increasing strain with cells on pronectin showing more injury than on type I collagen. Stretching had no effect on surfactant secretion on either substratum suggesting MLE-12 cells are a poor model for stretch-induced surfactant secretion. The cells grown on pronectin, however, demonstrated a 3-fold increase in surfactant secretion compared to those grown on type I collagen at all strains. This suggests that, while this cell line does not demonstrate stretch-induced surfactant secretion, the underlying extracellular matrix plays a crucial factor in both cell death and signal transduction in response to strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alveolar epithelium is comprised of two distinct cell types. Alveolar type I (AEI) cells are large, thin cells that line more that 90% of the alveolar surface area and serve primarily as a barrier between the blood–gas interface.3 Alveolar type II (AEII) cells are smaller, cuboidal cells responsible for the production and secretion of pulmonary surfactant, while serving as progenitors for the AEI cell type.3,8,23 Recently, there has been increasing interest in the effects of mechanical strain on these cells, largely driven by the hypothesis that mechanical ventilation results in injurious forces being imparted on the pulmonary epithelium.
There is considerable evidence that mechanical ventilation in the setting of acute lung injury (ALI) can exacerbate pre-existing lung injury in the form of ventilator induced lung injury (VILI).4,5,10,24 In the case of severe atelectasis without reopening, or during the use of excessive tidal volumes, localized lung regions may receive non-physiologic volumes that may result in overdistention of the alveolar wall, well beyond that seen under healthy conditions. It is thought that a maneuver to total lung capacity results in an approximate 40% increase in surface area in the lung.19 Therefore it could be speculated that in the presence of significant atelectasis, mechanical ventilation could result in expansion of the non-collapsed regions of lung beyond the physiologic total lung capacity resulting in basement membrane surface area changes far greater than 40%. Any overdistention is directly transmitted to the pulmonary epithelium resulting in compromise of the epithelial barrier and/or direct injury to the epithelium. Indeed, Tschumperlin and Margulies19 demonstrated that this phenomenon may play a role by demonstrating in primary cell culture that rat AEII cells exposed to mechanical strain exhibited increasing cell injury with both increasing strain amplitude and duration of stretch.
While excessive stretch may result in injury to the epithelium, it has also been shown that mechanotransduction of strain plays a critical role in cell and lung function. For example, it has been shown that stretch can enhance surfactant secretion,6,9,23 reducing interfacial surface tension within the lung or upregulate Na+–K+-ATPase activity,7,21 aiding in the clearance of edema from the alveolar space.
To further investigate the effects of strain on the pulmonary epithelium, we have developed a device capable of applying equi-biaxial strains of up to 100% change in surface area (ΔSA) to cells grown on elastic, protein-coated membranes. To test the effects of larger than normal strains, we used this device to characterize the effects of cyclic strains on cell viability and surfactant secretion in the mouse lung epithelial-12 (MLE-12) cell line cultured on type I collagen and pronectin coated elastic membranes. This transformed, tumor-derived cell line has been used as a model of AEII cells and has been shown to secrete surfactant phospholipids as well as surfactant proteins in response to known secretagogues and hence is thought to be useful in the study of surfactant production and regulation.22
Methods
Cell Stretching Device
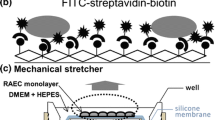
A mechanical stretching device was developed for cell stretching experiments inside the controlled environment of a cell incubator. This device is based, in principle, on similar devices described elsewhere.19 Cells are cultured on Bioflex culture plates (Flexcell Int, Hillsboro, NC) with each well of these six well plates consisting of a 0.5 mm thick clear elastic membrane with approximately 9.8 cm2 growing area. Two Bioflex culture plates can be secured on a sliding platform whose movement is determined by a computer-controlled linear actuator (Smartmotor, Ultra-Motion, Mattituck, NY) as shown in Fig. 1a. Situated directly below each well is an indentor post made of hard plastic. The top of each post is beveled in the center to minimize contact area between the indentor post and the elastic membrane with the contact point around each post rounded and lubricated (Bracote 804, Castrol, Commerce, CA) to minimize friction (Fig. 1b). Each indentor post can be removed to allow for unstretched cells within the same culture plate to be done in parallel with stretched cells, ensuring each sample is treated in identical manner. The vertical displacement of the moveable platform and culture plate results in sliding of the membrane over the indentor post and the expansion of the elastic membrane in an equibiaxial manner depicted in Fig. 1b. Having determined the relationship between the indentor depth and change in surface area of the elastic membrane (see below) the amplitude and frequency of stretch can be directly controlled via programming the displacement of the linear actuator.
Schematic side views (a) of the mechanical stretching device. The device consists of a sliding platform containing two 6-well bioflex plates supported by two vertical posts whose vertical displacement is controlled by a computer controlled linear actuator mounted at the bottom of the device. Directly below each well is a lubricated indentor post mounted on a stationary platform. Expansion of the flexible membrane (b) is achieved by vertical displacement of the moveable platform resulting in each membrane being stretched in a biaxial manner related solely to indentor depth. The membrane only comes in contact around the periphery of each indentor post.
Deformation Field
Six wells were examined within the stretching device to determine the relationship between indentor depth and radial strain as well as the change in membrane surface area. This procedure is similar to that described by Tschumperlin and Margulies.19 Briefly, each well was marked with a center point and two sets of four equidistant marks at 90° intervals with inner and outer radii of 0.46 cm and 0.91 cm, respectively from the center mark. The wells were imaged (Coolpix 5400, Nikon) in the unstretched state (Fig. 2, left) and at indentor depth intervals of 0.64 cm up to a maximum of 2.54 cm (Fig. 2, right). Using software developed in our laboratory, the distance between each of the eight peripheral marks and the center mark was calculated at each indentor depth and the radial strain (ε) was defined as the fractional length change between the peripheral and center marks,
where r 0 is the initial radius and Δr is the change in radius.
At each indentor depth the radial strain for each marker was compared to verify the uniformity of the strain field. After determining that the radial strain was uniform for all orientations, the ΔSA for the inner and outer radii were calculated and a 3rd order polynomial was fit to the average ΔSA as a function of indentor depth. Thus, we were able to convert a desired ΔSA waveform to indentor depth and deliver the adjusted displacement to the linear actuator in order to achieve the corresponding ΔSA.
To verify that this relationship can be applied to cells being stretched in a dynamic situation and that the relationship will not be affected by prolonged strains, two wells were marked as described before and stretched using a displacement waveform calculated to deliver a sinusoidal change in surface area with an amplitude of 50% and 100% ΔSA at 12 cycles/min for 1 h. The first and final cycles were dynamically imaged and the ΔSA versus time was calculated at intervals of 0.2 s as described previously.
Cell Culture
Mouse lung epithelial cells (MLE-12, American Type Culture Collection, Manassas VA) were seeded on either type I collagen or pronectin (presents 13 copies of a short peptide sequence from the cell-binding domain of human fibronectin (VTGRGDSPAS) separated by structural peptide sequences) coated bioflex plates. Ham’s F-12 50:50 mixed media supplemented with penicillin–streptomycin (100 U, 100 μg/ml), Insulin–Transferrin–Selenium (0.005 mg/ml, 0.01 mg/ml, 30 nM), HEPES (10 nM), l-glutamine (8 mmol), β-estradiol (10 nM), Hydrocortisone (10 nM) and 2% fetal bovine serum served as the growth medium as recommended by American Type Culture Collection and all reagents were acquired from Sigma unless noted otherwise. All cells were used within passage 4–8. The cells were seeded at a density of approximately 2 × 104 cells/cm2 and were restricted to grow within the center of the well using plastic inserts. When the cells reached approximately 90% confluence (approximately 3 days on pronectin and 4–5 days on type I collagen), the cells were used in experiments.
Determination of Cellular Injury
Prior to stretching, the cells were washed three times and the media was replaced with serum-free media containing 0.23 μM ethidium homodimer-1 and 0.12 μM calcein AM (Live/Dead, molecular probes, Eugene, Oregon). Ethidium homodimer−1 (528/617 nm) is non-membrane permeable and enters cells through a compromised plasma membrane and fluoresces bright red upon binding to nucleic acids, but is excluded from cells with an intact plasma membrane. Calcein AM (494/517 nm) is membrane permeable and well retained within live cells resulting in a bright green fluorescence. Following washing, cells were stretched for 30 min at 0% (n = 12 wells), 25% (n = 3 wells), 50% (n = 3 wells), 75% (n = 3 wells) or 100% (n = 3 wells) ΔSA at 12 cycles/min on both collagen or pronectin coated membranes. After stretching, the fluorescent dyes were added and the cells were incubated for 30 min at 37°C. The media was then removed and the cells were imaged using a Zeiss Axiovert 100TV with a 10× objective. Green and red images were taken from seven random locations within each well. Due to the large number of living cells per field of view only the red (injured) cells were counted for each field of view during stretching experiments. In order to quantify the total number of living cells per field of view three separate wells consisting of an unstretched control well, stretched to 50% strain and 100% strain were imaged at 20× magnification and the total number of living (green) cells were counted and the results were scaled to estimate the average number of living cells in the 10× images. Results are presented as percentage injured cells per field of view.
Phospholipid Secretion
The amount of [3H] phoshpatidylcholine (PC) secreted by cells was determined as previously described elsewhere.22 Briefly, 0.5 μCi/ml [3H] choline choloride (GE Healthcare, Piscataway NJ) were added to the media 20 h prior to the start of the experiment. Immediately prior to the experiment, the cells were washed three times and the media was replaced with serum-free media. Cells that underwent stretch were then stretched for 30 min at 0% (n = 12 wells), 25% (n = 6 wells), 50% (n = 6 wells), 75% (n = 6 wells) or 100% (n = 6 wells) change in surface area on both type I collagen and pronectin followed by a 1 h incubation period. Two additional groups of cells (n = 5 wells) were treated in the same manner described above, however grown on tissue culture plastic. One group was incubated in serum-free media, while the other was incubated in serum-free media containing 10 μM ATP, a known surfactant secretagogue, for 3 h. Following the incubation period, the media was removed and the cells were lifted and the lipids from both the media and the cells were isolated according to the Folch partition.11 Activity from the isolations was assessed with a scintillation counter (Beckman LS3801, Ramsey, Minnesota) and the percentage [3H] PC secreted was defined as the detections per minute of the media normalized by the total detections per minute of the cells and the media.
Statistical Analysis
All data are presented as mean ± standard deviation for each treatment group, unless otherwise specified. Each image was treated as a separate sample and data were analyzed by two-way anova (SigmaStat, San Rafael CA). Differences between groups were considered statistically significant for p < 0.05.
Results
Device Characterization
Figure 3a shows the average radial strain for both radii in each of the four orientations averaged over six wells when the indentor depth was increased from the unstretched condition to 2.56 cm. The dashed line indicates the average linear strain in each of the four orientations (41.3%). In all four cases the mean strain compared well to the global average with no significant difference between groups (p > 0.3). Thus, this device delivers a uniform strain field across the region imaged during this excursion. Figure 3b demonstrates the relationship between ΔSA for the regions covered by both the inner and outer sets of markers across the ranges of depths tested in the static testing. There was virtually no difference between the ΔSA for either set of markers hence the two groups were averaged together and the global average was fit to a 3rd order polynomial (ΔSA = 1.46x 3 + 9.08x 2 + 10.42x) shown as the solid line in Fig. 3b. The results from the two sets of dynamic stretching are shown in Fig. 3c. For both 50% and 100% ΔSA, the desired waveform is represented as a solid line with the initial stretch measurements represented with a dashed line and the final measurement with the dotted line. In the case of 50% ΔSA, the actual ΔSA fits well to the desired waveform with the maximum amplitude reaching 49.6% ΔSA before an hour of stretch and 49.1% ΔSA after. Both amplitudes are well within the error of measurement. In the case of the desired 100% ΔSA, there is little difference in measurement before and after stretch, however in each case, the actual ΔSA appears to overshoot the desired maximum amplitude by approximately 5% with a slight phase shift, likely due to viscoelastic properties of the membrane and/or friction between the membrane and indentor post, as the device approaches the maximum displacement.
Population averages (diamonds) and global average of all orientations (dashed line) of radial strain (a) as a function of orientation for six wells at 1.0 cm indentor depth and average change in surface area (b) for the inner (open circles) marks, outer marks (open squares) average (open diamonds) of all marks and 3rd order polynomial fit (solid line). Also, change in membrane surface area for two desired sinusoidal stretch waveforms (c) at 100% and 50% change in surface area before (dashed line) and after (dotted line) 1 h of cyclic stretching.
Characterization of Cellular Injury
Figure 4 shows representative 20× images of MLE-12 cells unstretched (left), stretched at 50% (middle) and 100% ΔSA (right) for 1 h cultured on pronectin-coated membranes. The top panel shows the injured cells stained red and the middle panel shows the living cells stained green with the bottom panel being a combination of the two. In the unstretched case, the cells nearly form a monolayer with few injured cells. The number of injured cells increases with increasing strain and demonstrate increasing breaks in the monolayer. Live cell count data averaged from three wells imaged at 20× magnification resulted in 392 ± 42 cells per field of view. There did not appear to be any trends concerning the number of living cells with respect to the strain magnitude applied. Thus we assumed that there was, on average, 783 living cells per field of view in the 10× case and this number was subsequently used to calculate the ratio of injured cells to living shown in Fig. 5. For both collagen and pronectin coating, there are an increasing number of dead cells with increasing strain. In the case of the pronectin-coated wells, the increase in cell injury is significant at the three highest levels of strain, while in the case of collagen-coated wells, the increase is significant at all levels of strain. At 75% and 100% ΔSA, the number of injured cells is significantly higher in the pronectin-coated wells than those grown on collagen.
Fluorescent images (20× objective) of injured cells stained red with ethidium homodimer (top), living cells stained green with calcein AM (middle) and the combination of both images (bottom) for unstretched cells (left), cells stretched at 50% change in surface area (middle), and those stretched at 100% change in surface area (right) on pronectin coated membranes.
Phospholipid Secretion
Figure 6a demonstrates the percentage [3H] PC of cells grown on tissue culture plastic and sham incubated (left) or incubated in ATP for 3 h (right). There is a nearly 3-fold increase in [3H] PC secreted in the cells exposed to ATP suggesting increased surfactant secretion in response to this known secretagogue. Figure 6b shows the PC secretion as a function of ΔSA amplitude for cells grown on pronectin or collagen. There is no correlation between ΔSA amplitude and PC secretion for cells grown on either membrane. Interestingly, in both the unstretched cells and at each level of strain PC secretion is significantly lower on the collagen-coated substrates, suggesting that the underlying matrix protein has a significant effect on surfactant secretion.
Discussion
During breathing or mechanical ventilation the lung undergoes cyclic deformation, which is transmitted to the numerous cell types in the lung. During mechanical ventilation in the setting of ALI, it is quite likely that due to atelectasis, flooding and a host of other factors, the effective lung size is reduced resulting in ventilation of a “baby lung”.4,5 This would presumably result in supra-physiologic tidal volumes being delivered to localized regions within the lung and subsequently supra-physiologic strains would likely be transmitted to the epithelium lining the alveolar walls. To test the effects of large surface area changes on epithelial cells, we have developed a stretching device capable of delivering cyclic strains of up to 100% ΔSA, almost three times that thought to be attained during a maneuver to total lung capacity and well beyond the current capacity of commercially available stretching devices.18 We have also shown that the deformations delivered by this device are equibiaxial, uniform across the surface area in question and repeatable over long durations of stretch protocols. The primary findings obtained with this device are that (1) MLE-12 cells demonstrated increasing cell injury in a dose dependent manner with increasing strain, (2) cells grown on pronectin were significantly more susceptible to injury than those cultured on type I collagen and (3) while these cells demonstrated increased PC secretion in response to ATP no relationship was found between strain amplitude and PC secretion. Interestingly, there was a significantly less PC secretion by cells grown on type I collagen than those grown on pronectin for both unstretched control cells and at all levels of strain.
Device Characteristics
Our new device was designed to utilize commercially available bioflex cell culture plates (Flexcell Int, Hillsboro, NC) to apply uniform, equibiaxial strains to cells grown in culture. In contrast to commercially-available stretching devices, this device uses a computer-controlled linear actuator to pull a platform containing up to two bioflex plates firmly affixed over lubricated indentor posts situated directly below each well. Each indentor post is removable allowing unstretched control cells to be used within the same sample. We have shown that this device delivers uniform strain in all radial directions as well as reproducible changes in membrane surface area as a function of indentor depth above the membrane. The characterization of indentor depth to change in surface area also allows us considerable freedom to apply virtually any dynamic stretch pattern, in terms of change in surface area, that would be of interest in cell culture studies and can apply larger magnitudes of stretch than any cell stretching device currently available. Additionally, since the indentors are stationary, the cells remain in the same horizontal plane during stretch which may allow future direct visualization of the stretched cells. Finally, the fact that this device fits within the controlled environment of an incubator ensures that the stretching can be done in a physiologic environment allowing the utilization of long-term stretch protocols.
Cell Injury
We found that the MLE-12 cell line demonstrated a dose-dependent elevation in cell injury with increasing levels of strain. While the increase in cell injury quickly reached significance with respect to unstretched control cells, the overall ratio of injured cells to living cells remained remarkably low. We estimated that in each field of view there were approximately 780 living cells per image corresponding to less than 5% cell injury in the most extreme case. This is in stark contrast to results reported in primary cell culture where Tschumperlin and Margulies19 reported a 50% rate of cell death in primary AEII cells stretched to 50% ΔSA 5 days following isolation. Waters et al.21 utilized the MLE-12 cell line to determine the effects of strain on Na+–K+-ATPase activity and found that using a maximum linear strain of 20% (an approximate 45% ΔSA) these cells developed increased Na+–K+-ATPase activity when cultured on type I collagen coated bioflex plates. This study reported no apparent cell injury and the fact that cell function increased with strain in these cell populations suggests that a majority of these cells were uninjured and could easily tolerate these levels of strain.18 The apparent discrepancy between the MLE-12 and primary cell culture likely lies in the fact that primary cells proliferate very little and must spread in order to cover additional surface area. The MLE-12 cells are capable of proliferating and can adapt in the form of additional cells reducing the effective surface area per cell and hence likelihood of injury. Another possibility, as the cell line creators pointed out,22 is that this cell line was immortalized at a different state of development and may represent a subtype of respiratory epithelial cells that has a higher tolerance for mechanical strain than “true” AEII cells.
It has been demonstrated that the extracellular matrix to which the cells adhere not only forms a scaffold for growth, but can also play a role in the modulation of a number of key cell functions.15 For example, Sugahara et al.16 found that AEII primary cells adhere much more strongly to fibronectin than other substrata and that adhesion to fibronectin resulted in more defined and larger bundles of actin stress fibers within the cytoskeleton. These cells also formed a tighter, better organized and more polarized monolayer than cells grown on collagen. This could explain the increased cellular injury in response to stretch that we found on pronectin, a protein biochemically similar to fibronectin, than those cultured on type I collagen. Stiffer cells with stronger attachments to both the underlying matrix as well as neighboring cells would likely be less able to tolerate excessive cyclic strains and more prone to cellular injury or membrane rupture. In contrast, the cells coating the collagen membrane may be more flexible with less rigid attachments to the underlying substrata with a higher likelihood of breaking attachments or slipping rather than transmitting the force directly to the cell membrane and cytoskeleton resulting in injury.
PC Secretion
While we demonstrated a significant increase in PC secretion in response to ATP, stretch elicited no response in surfactant secretion at any level of strain. This observation is in contrast to what is thought to occur within the lung in-vivo 9,20 and what has been shown in primary cell cultures elsewhere.12, 13, 23 The lack of stretch-induced PC secretion may not be unexpected considering these cells secrete PC in response to ATP, however will not do so when exposed to forskolin.21 Forskolin is known to activate adenylate cyclase which is thought to be involved in one of the signaling pathways that ultimately leads to surfactant secretion. ATP is thought to activate a separate protein kinase C mediated pathway,12 however the mechanism of stretch-induced surfactant secretion is not completely clear beyond the observation that it occurs in conjunction with a rise in intracellular Ca2+ concentration.12 It is likely that, while some pathways between signal transduction and PC secretion are intact in this cell line, the pathways that involve mechanotransduction of strain that result in PC secretion in primary alveolar type II cells is not completely functional as is the case with forskolin induced secretion, but not ATP. This suggests that the MLE-12 cell line is an inadequate model for experiments that wish to investigate the relationship between mechanical strain and stretch induced surfactant secretion in cell culture. Therefore, it is necessary to utilize primary alveolar type II epithelial cells while they still retain their type II characteristics when conducting experiments concerning stretch-induced surfactant secretion.
It should be noted that Edwards et al.6 exposed rat type II alveolar epithelial cells to 30 min of cyclic strain at 50% change in surface area at three cycles per minute and demonstrated enhanced PC secretion. There was, however, a significant delay in secretion with a vast majority found to occur between 1 and 4 h post-stretch exposure. Our media was examined 1 h post-stretch exposure based on the data of Wirtz and Dobbs23 where they found a majority of PC secretion due to a single applied stretch occurred within 1 h of stretch. This raises the possibility that our lack of stretch-induced PC secretion was due to an inadequate incubation time post-exposure. We do not feel this is the case based on preliminary experiments where these cells exposed to similar stretch protocols and extended incubation periods resulted in no observable changes in surfactant secretion (unpublished observation).
It is interesting to note, however, that in all cases PC secretion was enhanced in cells cultured on pronectin in comparison with those grown on collagen. This observation is unexpected considering that primary AEII cells cultured on fibronectin flatten and lose their lamellar bodies while those cultured on type I collagen are more steadfast in retaining their cuboidal appearance,1 suggesting a retention of AEII cell characteristics. However, due to its nature this cell line does not appear to differentiate significantly over time in culture so this aspect may not play a role in our case. It is unknown whether the increased secretion from a fibronectin-like substrate would translate to primary cells or living cells in-vivo, however this observation warrants more investigation.
The mechanism behind the different PC secretion on the two substrata is unclear, but maybe related to differences in the chemical composition and/or stiffness of the membranes.17 It is known that membrane pre-stress can considerably alter cell function under a number of circumstances.14 In order to determine if the stiffness was considerably different between membranes coated with type I collagen or pronectin and if this may have contributed to the variations we saw in cell viability and PC secretion, we measured the stress–strain curves of the elastic membranes under both conditions using a method described in detail elsewhere.2 We found that in the case of both matrix proteins, the stress–strain characteristics were virtually identical suggesting that membrane stiffness could not explain our results. Therefore it is likely that the variations in behavior between the groups is due to changes in cell characteristics that arises from the utilization of different integrin sites for cell adhesion or the density of these adhesions.
Summary
We have presented a new cell stretching device that can be used for long-term experiments delivering large mechanical strains to living tissue in a physiologic environment. This device was used to characterize the viability and surfactant secretion properties of the MLE-12 cells when exposed to 30 min of continuous, cyclic strains. We found that these cells demonstrated a minor, but dose-dependent increase in cell death with respect to increasing strain and a higher susceptibility to injury when cultured on pronectin as opposed to type I collagen. While ATP induced enhanced PC secretion in these cells mechanical strain had no effect on PC secretion. Additionally, the composition of the extracellular matrix significantly influenced PC secretion. This suggests that the underlying extracellular matrix plays a factor in several cell functions including cellular injury and surfactant secretion.
References
Adamson I. Y., G. M. King, L. Young. Influence of extracellular matrix and collagen components on alveolar type 2 cell morphology and function. In Vitro Cell. Dev. Biol. 25:494–502, 1989.
Cavalcante F. S., S. Ito, K. Brewer, H. Sakai, A. M. Alencar, M. P. Almeida, J. S. Jr. Andrade, A. Majumdar, E. P. Ingenito, B Suki. Mechanical interactions between collagen and proteoglycans: Implications for the stability of lung tissue. J. Appl. Physiol. 98:672–679, 2005.
Crapo J. D., B. E. Barry, P. Gehr, M. Bachofen, E. R. Weibel. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 125:740–745, 1982.
Dreyfuss D., G. Basset, P. Soler, G. Saumon. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am. Rev. Respir. Dis. 132:880–884, 1985.
Dreyfuss D., G. Saumon. Ventilator-induced lung injury: Lessons from experimental studies. Am. J. Respir. Crit. Care Med. 157:294–323, 1998.
Edwards Y. S., L. M. Sutherland,J. H. Power,T. E. Nicholas, A. W Murray. Cyclic stretch induces both apoptosis and secretion in rat alveolar type II cells. FEBS Lett. 448:127–130, 1999.
Fisher J. L, S. S. Margulies. Na(+)-K(+)-ATPase activity in alveolar epithelial cells increases with cyclic stretch. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L737–746, 2002.
Frick M., C. Bertocchi, P. Jennings, T. Haller, N. Mair, W. Singer, W. Pfaller, M. Ritsch-Marte, P. Dietl. Ca2+ entry is essential for cell strain-induced lamellar body fusion in isolated rat type II pneumocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 286:L210–220, 2004.
Mason R. J., D. R. Voelker. Regulatory mechanisms of surfactant secretion. Biochim. Biophys. Acta 1408:226–240, 1998.
Ranieri V. M., P. M Suter, C. Tortorella, R. De Tullio, J. M. Dayer, A. Brienza, F. Bruno, A. S. Slutsky. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA 282:54–61, 1999.
Rice W. R., F. M. Singleton. Regulation of surfactant secretion from isolated Type II pneumocytes by substance P. Biochim. Biophys. Acta 889:123–127, 1986.
Rooney S. A.. Regulation of surfactant secretion. Comp. Biochem. Physiol. A 129:233–243, 2001.
Rose F., K. Zwick, H. A. Ghofrani, U. Sibelius, W. Seeger, D Walmrath, F. Grimminger. Prostacyclin enhances stretch-induced surfactant secretion in alveolar epithelial type II cells. Am. J. Respir. Crit. Care Med. 160:846–851, 1999.
Rosenblatt N., S. Hu, J. Chen, N. Wang, D. Stamenovic. Distending stress of the cytoskeleton is a key determinant of cell rheological behavior. Biochem. Biophys. Res. Commun. 321:617–622, 2004.
Schnaper H. W., H. K. Kleinman. Regulation of cell function by extracellular matrix. Pediatr. Nephrol. 7:96–104, 1993.
Sugahara K., T. Kiyota, R. A. Clark, R. J. Mason. The effect of fibronectin on cytoskeleton structure and transepithelial resistance of alveolar type II cells in primary culture. Virchows Arch B 64:115–122, 1993.
Suki B., S. Ito, D. Stamenovic, K. R. Lutchen, E. P. Ingenito Biomechanics of the lung parenchyma: Critical roles of collagen and mechanical forces. J. Appl. Physiol. 98:1892–1899, 2005.
Tschumperlin D. J, S. S. Margulies. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J. Appl. Physiol. 86:2026–2033, 1999.
Tschumperlin D. J., S. S. Margulies. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am. J. Physiol. 275:L1173–1183, 1998.
Van Golde L. M., J. J. Batenburg, B. Robertson. The pulmonary surfactant system: Biochemical aspects and functional significance. Physiol. Rev. 68:374–455, 1988.
Waters C. M, K. M. Ridge, G. Sunio, K. Venetsanou, J. I. Sznajder. Mechanical stretching of alveolar epithelial cells increases Na(+)-K(+)-ATPase activity. J. Appl. Physiol. 87:715–721, 1999.
Wikenheiser K. A., D. K. Vorbroker, W. R. Rice, J. C. Clark, C. J. Bachurski, H. K. Oie, J. A. Whitsett. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc. Natl. Acad. Sci. USA 90:11029–11033, 1993.
Wirtz H. R., L. G Dobbs. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250:1266–1269, 1990.
Zupancich E., D. Paparella, F. Turani, C. Munch, A. Rossi, S Massaccesi, V. M. Ranieri. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 130:378–383, 2005.
Acknowledgments
This work was supported by NIH HL076372.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arold, S.P., Wong, J.Y. & Suki, B. Design of a New Stretching Apparatus and the Effects of Cyclic Strain and Substratum on Mouse Lung Epithelial-12 Cells. Ann Biomed Eng 35, 1156–1164 (2007). https://doi.org/10.1007/s10439-007-9262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9262-5