Abstract
Microfluidic devices can form monodisperse double emulsions, but the fabrication steps are complicated and require specialized equipment. Recently, a method to convert single emulsions into double emulsions using vortex mixers has been proposed. Using this method, we demonstrate the production of double emulsions using commercially available single-emulsion microfluidic chips. We characterize the effect of vortex speed, vortex duration and the number of vortex/flick cycles on the average diameter and coefficient of variation of the double emulsions. Using fluorescent nanoparticles as tracers, we show that droplet breakup occurs during the second emulsification (using the vortex), but did not observe any fusion between the cores of double emulsion droplets. We also found that some inverted double emulsion droplets containing the outer water phase in their core were produced during vortex emulsification. Finally, while commercial chips only exist with a finite range of channel size that sets the monodispersed emulsion droplet radius, we show that the double-emulsion droplet radius can be adjusted using osmotic pumping. Our method is simple, available and user-friendly for biomedical researchers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Droplet microfluidics has become an important method for the sorting and analysis of biological samples due to its remarkable capacity to confine samples in microscale volumes and with fine control over the reaction conditions (Suea-Ngam et al. 2019; Sohrabi and Moraveji 2020; Shang et al. 2017; Chou et al. 2015). Most biological assays, including antigenic tests and PCR are done in water, and can, therefore, be encapsulated in oil as independent reactors, thereby forming single emulsions of water in oil.
Fluorescence-activated cell sorting (FACS) is an attractive way to sort droplets in microfluidics: FACS instruments are often available in biology laboratories as it is one of the workhorse of biology research, it is highly versatile, has a high throughput and higher sorting speed than droplet sorting instruments (Lagus and Edd 2013; Mutafopulos et al. 2019). However, the FACS is designed for biological samples in water, and cannot work on insulating fluids, such as oil. This means that a FACS cannot sort simple water in oil emulsions that can be generated by commercially available microfluidic chips. Therefore, it is necessary to generate water-in-oil-in-water (W/O/W) double emulsions to sort and analyze cells with FACS (Mastrobattista et al. 2005; Bernath et al. 2004; Shahi et al. 2017; Hai et al. 2004). Such emulsions look like pseudo-cells, where the cell membrane would be replaced by a layer of oil. In the remaining of the paper, the outer liquid phase that contains the droplets (either single or double emulsion) will be called continuous phase, while the liquid phase forming the droplets will be referred to as the dispersed phase.
Microfluidic channel devices are widely used in the generation of double emulsion, because they can produce droplets with a very narrow size distribution (Rotem et al. 2012; Nawar et al. 2020). There are many types of devices used in the generation of double emulsion by microfluidic devices, which can be mainly divided into cross-flow devices and co-flow devices (Romero and Abate 2012; Wang et al. 2019; Vladisavljević et al. 2013). Most cross-flow devices are prepared based on PDMS, such as a two-step T-junction device (Okushima et al. 2004), Y-junction and cross-junction structures are also often used in microfluidic chips. However, fabricating the devices requires to carefully control the wetting properties of the channels to match that of the continuous phase. Indeed, hydrophilic channels can only form oil droplets dispersed in water (O/W), while hydrophobic channels can only generate water droplets dispersed in oil (W/O) (Shui et al. 2009). To generate W/O/W emulsions, a first section of the microfluidic chip must be hydrophobic (to produce the water in oil emulsion) and another section must be hydrophilic (to encapsulate the water in oil droplets into another water phase) (Liao et al. 2018; Romanowsky et al. 2010; Abate et al. 2008, 2010). Spatial control of wetting properties based on plasma treatment can be achieved using metallic electrodes (due to the tip effect) (Al‐Bataineh et al. 2012), or simply by blocking some inlets of the channel while letting others open so that the ionized gas can penetrate selected sections of the microfluidic chip (Kim et al. 2015). Yet, it is difficult to precisely control the location and local power of the plasma. Thus, it is challenging to produce microfluidic chips able to generate double emulsions.
Co-flow devices address the wetting issues using different materials for each step of the production of the double emulsion. However, co-flow devices are made by hand by inserting capillary tips of decreasing radii into each other. They are prone to alignment defects and the production is slow. Therefore, it is necessary to look for simpler ways to generate double emulsions, which can be analyzed and sorted by FACS. Sukovich et al. (Sukovich et al. 2017) dispersed W/O microfluidic emulsions into aqueous carriers by pipetting or vortexing, generating double emulsion droplets that can be analyzed and sorted with FACS. This method is faster and simpler, requiring no specialized devices and it is less sensitive to wettability and interfacial tension. Wang et al. (Wang et al. 2022) have shown that the double emulsion is produced by shear against the tube wall as the fluid is rapidly accelerated by the vortex mixer. They also demonstrated that the droplet size could be adjusted by osmotic shrinking, for instance to generated opalescent supra-particles.
These two works have shown that double emulsions have a tremendous potential for biology and material science. Yet, wide adoption is complicated, because the microfluidic chips to produce the single emulsions were produced by soft lithography, which limits its access to biomedical and material science researchers which may not have easy access to clean room facilities. In this paper, we show that this issue can be addressed using commercially available drop-making chips to produce the single emulsions, and then using the vortex method to produce the double emulsion. We characterize the effect of vortex speed, vortex duration and the number of vortex/flick cycles on the average diameter and coefficient of variation of the double emulsions. We also explore some quality defects that go along using a vortex for double emulsification, including droplet rupturing and fusion. Finally, control over the droplet size is explored.
The paper is structured as follows: after characterizing the single emulsion produced by the commercial microfluidic chips, we use the vortex method to produce a double emulsion (W1/O/W2). We show that a single core double emulsion can be obtained by finely adjusting the vortex speed. By encapsulating fluorescent nanoparticles into the double emulsion, we find that the content of some droplets is spilled into the outer water phase but droplet fusion events were never observed. Finally, the size of the produced droplets is adjusted using osmotic pressure.
2 Materials and methods
2.1 Generation of single emulsions
The single emulsions are prepared using a commercial microfluidic chip (Fluidic 537, Chipshop, Germany). The chips are made of polycarbonate without hydrophilic treatment. The liquids (oil and water) are initially stored in two single-use plastic syringes (1 mL, KDL). The syringes are pressed by two syringe pumps (KDS-210) and the flowing fluid is transported to the chip using polytetrafluoroethylene tubing [inner diameter (ID): 0.5 mm, outer diameter (OD): 2.5 mm,]. The tubing is connected to the chip by insertion into larger silicone tubing sleeves (ID: 0.76 mm, OD: 1.65 mm, Chipshop company) connected to male luer inserts (Chipshop company). The experiments are visualized using a fluorescent microscope (OLYMPUS, CKX53), and the droplet images are analyzed using ImageJ. The setup to generate single emulsions is shown in Fig. 1.
The dispersed phase is deionized (DI) water. In some experiments 100 nm diameter fluorescence polystyrene particles are dispersed in the fluid with a volume fraction of 2% (this yields on average 104 particles per droplet). The particles GF100C (green, G) and RF100C (red, R) were provided by Huge Biotechnology (China).
The continuous phase is HFE-7500 oil containing 1% mass fraction of PEG-Krytox surfactant (Emulseo, France). The surfactant in the continuous phase is critical to prevent droplet coalescence once the emulsion is formed. Optimized volumetric flow rates of the two phases are 10 μL/min for the continuous phase (oil/surfactant) and 3 μL/min for the dispersed phase (water/fluorescent beads). Starting from zero, these flow rates are gradually ramped up to reach the optimized flow rates. It takes several minutes for the pressure in the chip to equilibrate, and this time can be considerably longer if some air bubbles remain trapped in the syringes. The drop-making section of the chip is continuously monitored using the microscope until a steady stream of droplets is observed. The early emulsion produced by the chip is then discarded, and an Eppendorf tube (1.7 mL DNA LoBind Eppendorf) containing some continuous phase is placed at the outlet to collect the single emulsion. 500 μL of emulsion can be produced in less than 1 h.
Before observing these emulsions, samples are diluted in the oil phase. The resulting single emulsion can then be observed with a fluorescent microscope.
2.2 Vortexed double emulsion
Unless specified otherwise, 50 μL of single emulsions are added to 100 μL carrier aqueous phase (10% PEG 35 K, 4% Tween 20, 1% pluronic acid) in 1.7 mL DNA LoBind tube. Similar to single emulsions, the combined surfactants are important to ensure the stability of the produced emulsion. The tube is flicked 5 times before shaking the liquid with the vortex mixer (VWR Analog Vortex Mixer-10153-838). We generate the double emulsions by adjusting vortex speed, every vortexing time is 15 s, and flick/vortex cycle is repeated seven times.
Before observing these emulsions, samples are diluted in a diluent containing 1% PEG35K, 4% Tween and 1% pluronic.
2.3 Double-emulsion droplet size tuning by osmotic pumping
We generate double emulsions as described in “Generation of single emulsions” and “Vortexed double emulsions”, and dilute them 1 time with phosphate-buffered serum (PBS) containing 1% PEG35K and 1% pluronic acid.
3 Results and discussion
3.1 Single emulsions
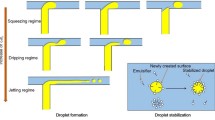
Previous studies indicate that the droplet size produced by microfluidic chips is approximately equal to the channel diameter and is not very sensitive to the flow rate. The flow rate can be increased until a jetting flow regime sets on, where the dispersed phase forms an elongated jet that produces a polydispersed emulsion. Therefore, we restrict the dispersed phase flow rate to 10 μL/min, which was much below the jetting regime of our chips. Next, we adjust the flow rate ratio of the continuous phase (oil/surfactant) to dispersed phase (DI water). When this ratio is too small, long slugs of dispersed phase are produced (squeezing flow regime), which defeats the purpose of microfluidics (making small droplets), whereas when this ratio is too large, the continuous phase is wasted. Using optimized flow rates, 0.5 mL of emulsion can be generated in less than 1 h. Examples of emulsions generated with these chips are shown in Fig. 2. Figure 2a, b shows water droplets containing 100 nm fluorescent red particles (R), and Fig. 2c, d shows water droplets containing 100 nm fluorescent green particles (G). The average diameter of single emulsion droplets is 45.51 μm, with a coefficient of variation (CV) of 4.1%, which is consistent with the good droplet uniformity usually obtained with microfluidic devices. These monodispersed emulsions can then be processed using the vortex mixer to generate double emulsions.
Commercial microfluidic single emulsion droplets: a single emulsions containing red nanoparticles (R) under brightfield, b single emulsions containing red nanoparticles (R) under red fluorescence imaging, c single emulsions containing green nanoparticles (G) under brightfield, d single emulsions containing green nanoparticles (G) under green fluorescence imaging, and e size distribution of single emulsions
3.2 Generation of double emulsions
Converting a single emulsion into a double emulsion using a vortex mixer is simpler than using microfluidic chips. When using a vortex, the water in oil emulsion is dispersed by the shear stress of the flow in the agitated tube. The size of the outer oil droplet depends on the speed of the vortex, and single-core double emulsions are obtained when the oil phase only has room for one water droplet only. The vortex speed can be adjusted at discrete levels set by the manufacturer.
At low vortex speed (speed 6), there are some multicore double emulsions (Fig. 3a). The average diameter of double-emulsion droplets is 45.8 μm, with a CV as high as 23% (Fig. 3e). This size distribution of double emulsions is wider than typical microfluidic emulsions (Fig. 3e). The reasons for this wide distribution are not clear, and it is especially surprising that a large fraction of the droplets tend to be smaller than the size of the single-emulsion droplets (45 μm). One possibility is that the shear stress is not uniform in the vortex, so that some smaller regions (such as the contact line between the agitated liquid and the tube) would experience a higher stress, which could generate double emulsions if small enough droplets are present nearby. Nearly one-fifth of emulsions are multicore small droplets, which may have been encapsulated together on the contact line. Direct observation of the dynamics of the contact line could shed some light of this surprising size distribution and droplet topology.
a–c Generation of double emulsion (50 μL of single emulsions are added to 100 μL of carrier aqueous phase) at vortex speeds of 6, 7.5 and 8, respectively. d average diameter of double emulsion droplets depending on the vortexing speed, e double emulsion droplet diameter distribution obtained at speeds of 6, 7.5 and 8. The blue dash line marks the average diameter of single emulsion. The pie chart in inset shows the ratio of single-core double emulsion at different speed
At the speed of 7.5 (Fig. 3b), nearly all double emulsion droplets have a single core with a narrow size distribution that reflects the original single emulsion (Fig. 3e). The average diameter of double emulsion droplets is 58.69 μm, with a corresponding CV of 8.9% (Fig. 3d).
When setting the vortex speed to 8 (Fig. 3c), the fraction of single-core double emulsion droplets among double-emulsion droplets is lower than when using a speed of 7.5. The resulting double emulsion is also less uniform in size than at lower speed (Fig. 3e), with an average diameter of 45.55 μm and a CV of 17.65% (Fig. 3d). We infer that the excessive shear obtained at high speed may have split inner-phase water droplets.
These results can be discussed in light of Wang et al. postulated mechanism (Wang et al. 2022). In their model, the centrifugal force due to the rotating motion of the vortex drives a lapping motion of the continuous phase against the tube wall. The shear of this lapping motion breaks the drop aggregates and decides the thickness of the double-emulsion droplet shell.
Insufficient vortex speed generates more multi-core droplets, most likely because the lapping shear is not strong enough to break up the outer phase in fragments small enough to accommodate only one water droplet. Reversely, the shear obtained at high vortex speed could disperse the oil in droplets too small to accommodate a water droplet, which would decrease the number of single-core double emulsion droplets.
Based on those experiments, we deduce that, for this experimental setup and set of reagents, a vortex speed of 7.5 is optimum to generate uniform, stable, single-core double emulsion.
In addition to vortex speed, two other adjustable parameters are the number of flick/vortex cycle, the duration of each vortexing step and ratio of single emulsions to carrier phase.
The average diameter of single-core double emulsions (thereby omitting multicore emulsions and oil droplets) is insensitive to the number of vortex/flick cycles, and the coefficient of variation decreases with the number of vortex/flick cycles [Fig. 4b(i) and b(ii)]. When the number of cycles is not sufficient, such as 3 cycles, as shown in Fig. 4a, we observe more multicore emulsion droplets and the emulsion becomes more heterogeneous, as shown by the high CV value (nearly 40%) in these experiments [Fig. 4b(i) and b(ii)]. Reversely, excessive vortex/flick cycles yield an emulsion with abundant small oil droplets (7 cycles of 10 s each, and 10 cycles of 15 s each) or even more complex multicore emulsions (10 cycles of 10 s).
Effect of number of vortex/flick cycles, vortexing duration and single-emulsion to carrier phase ratio on the conversion of single emulsion to double emulsion. Unless otherwise specified, 50 μL of single emulsion are added to 100 μL of carrier aqueous phase and vortexed at a speed of 7.5 for 15 s, repeated 7 times. a Experimental pictures for varying vortex/flick cycles and vortexing duration. Pink pictures indicate insufficient number of double emulsion droplets to obtain an average diameter and CV; b average diameters and coefficient of variation (CV) derived from the pictures shown in (a): (i, ii) average diameter and CV for varying number of cycles, (iii, iv) average diameter and CV for varying vortexing duration. (c): (i, ii) Experimental pictures for single-emulsion to carrier phase ratio of 1:4 and 2:1, respectively. The combined volume is kept at 150 μL. (iii) average diameter at different single-emulsion to carrier phase ratio
The average diameter of single-core double emulsions (thereby omitting multicore emulsions and oil droplets) decreases with the vortexing time and the coefficient of variation follows a U-shaped curve with the vortexing time [Fig. 4c(i) and c(ii)], with a marked minimum obtained at 15 s. When the vortexing time is too short (e.g., 10 s), the single-phase emulsion remains packed together and generates large multi-core emulsions and little single-core emulsions. On the opposite, excessive vortexing time breaks the droplets from the single-phase emulsion (vortexing time of 20 s repeated 7 times, Fig. 4a).
The concentration of single emulsion also influences the generation of double emulsions. The thickness of the oil shell grows with the ratio of single-phase emulsion to carrier phase [Fig. 4c(iii)]. When the concentration is low, the oil shell is thin and W/O/W droplets are rare but oil droplets are abundant [Fig. 4c(i)]. This may suggest that the double emulsions are unstable when using such low concentrations. On the opposite, when using more single-phase emulsion that carrier phase, we observe numerous multi-core droplets [Fig. 4c(ii)].
Based on these experiments, the optimized parameters to generate uniform and stable double emulsion droplets are to add 50 μL of single emulsions to 100 μL of carrier aqueous phase in 1.7 mL centrifugal tubes, flick/vortexing 7 times with a speed of 7.5 for 15 s each time.
With these optimized parameters, we compare the resulting double emulsions to those obtained with different methods in terms of coefficient of variation (Fig. 5). Such methods have been extensively reviewed by Chong et al. (Chong et al. 2015). Techniques based on electrospraying can generate highly monodispersed double emulsions but require the continuous phase to be a dielectric, and the shell phase to be conductive. This is not satisfied by W/O/W emulsions. Membrane emulsification and vertical cross type emulsifications produce less homogeneous emulsions than the method using single-emulsion and vortex. The coefficient of variation measured in our work is slightly higher than gold-standard coaxial microfluidics, but avoid many of challenges related to the difficult and poorly reproducible fabrication of such channels. We note that Wang et al. (Wang et al. 2022) have obtained even lower CV with this method of single-emulsion followed by vortex than coaxial microfluidics.
Although these optimized parameters allow to generate single-core double emulsions, it is not immediately clear if some single-emulsion water droplets ruptured during the vortex double-emulsification process. We next investigate this issue.
3.3 Rupturing and leakage of inner-phase water droplets during vortex double emulsification
In this section, we evaluate double-emulsification defects that could occur during the vortex step (Fig. 6a). Having previously considered multiple core double emulsions, we investigate (i) the rupturing of droplets and (ii) the formation of “empty” droplets that contain the carrier phase (outer water phase).
To evaluate the likelihood of droplet rupturing or leaking during emulsification, we produce single-emulsion droplets containing fluorescent particles. The double emulsions obtained after vortex emulsification are shown in Fig. 6b, c. Some red particles are visible on the outer surface of the oil shell. Recalling that no particles were present originally in the carrier phase (outer water phase), this indicates that some of the single-emulsion droplets have ruptured during the vortex double emulsification.
The possibility for the inner water phase to rupture suggests that the shearing force may sometimes exceed the capillary pressure that holds the droplets together. Reversely, the shearing force may also be strong enough to disperse the carrier phase into smaller water droplets, that could then become double-emulsion droplets. To test this hypothesis, we dispersed green fluorescent particles in the carrier phase, while keeping the single emulsion without particles. Similar to other double emulsification processes, the resulting double emulsion obtained after vortex mixing is diluted in a clean (particle-free) diluent at a volume ratio 1:10. Therefore, the particle concentration in the outer phase is nearly 10 times lower than the particle concentration in the carrier phase before vortex mixing.
The resulting double emulsification is shown in Fig. 6d. Some of the inner cores of the double emulsion droplets are filled with green particles, which indicates that vortex emulsification can generate double emulsion droplets containing the carrier phase. Yet, most of the side-products appear to be oil droplets. Such oil droplets are inert and can be easily distinguished from water droplets based on their size. Therefore, they usually have a very negligible impact on biological experiments. If necessary, they can be removed by sedimentation (the density of water is 1 g/cm3, the density of HEF-7500 is 1.614 g/cm3).
3.4 Absence of merging of inner phase droplets during vortex double emulsification
In microfluidics, droplets are often used as independent micro-reactors. This can only remain true if no droplet merging happens during emulsification. To investigate this possibility, two sets of single emulsion are added to the carrier aqueous phase: one is 25 μL of single emulsion containing red nanoparticles (R), the other is 25 μL of single emulsions containing green nanoparticles (G). Some possible outcomes are shown in Fig. 7a: single-core double emulsion may be produced, but also multicore double emulsions that can be homogeneous (RR, GG) or heterogeneous (RG). Finally, double-emulsion droplets with fused cores could be envisioned, which would be unsuitable as independent microreactors.
Experimentally produced double emulsions are shown in Fig. 7b, c. We found no fused core droplets in any of our experiments, which indicates that coalescence during vortex emulsification is extremely unlikely, and therefore, such emulsions can be used as independent microreactors. A few homogeneous multicore droplets were observed, but no heterogeneous multicore ones.
The absence of heterogeneous multicore droplets has implications on the formation process of multicore double-emulsion droplets when using vortex emulsification. Indeed, if the formation of multicore droplets was a simple random pairwise collision process, statistics suggest that heterogeneous multicore droplets should be as abundant as homogeneous ones (RR and GG taken together). Instead, the absence of heterogeneous multicore droplets suggests that multicore droplets may be formed by the encapsulation of two small droplets stuck together in a larger oil shell. Indeed, the G and R emulsions are initially (prior any mixing) separated, such that homogeneous multicores are considerably more likely than heterogeneous ones.
3.5 Droplet shrinking controlled by osmotic pumping
To a large extent, the size of the droplets generated by a microfluidic device is set by the dimensions of the drop-maker nozzle. When using commercial chips, the channel size cannot be adjusted, which restricts the size of droplets that can be produced. For instance, injection molding is best suited for channels no smaller than 50 μm. To produce smaller droplets, we shrink the double emulsions by osmotic pumping. Water is slightly soluble in fluorinated oils, and can, therefore, diffuse through it. When inner and outer water phase have the same activity (the same osmotic pressure), the fluxes of water between inner and outer phase are balanced. However, when the activity is different (due for instance to different salt concentrations), the diffusion flux is not balanced and a neat flux of water is established across the oil shell. For dilute solutions, water flows from the water-rich regions to the water-poor regions, which allows shrinking droplets by immersing them in salt solutions. This is similar to the strategy used by Wang to generate opalescent supra-particles (Wang et al. 2022).
We demonstrate this shrinking by dispersing the double emulsion in PBS, and taking photographs every hour (Fig. 8a–d). The emulsion remains stable when immersed in PBS, but the droplet size gradually shrinks and we observe visually that the oil shell becomes thicker. The average diameter of the droplets in the double emulsion is shown in Fig. 8e This shows that the droplet size of double emulsions can be adjusted after production.
Shrinking of double emulsions (incubated in PBS): a before incubation, b incubating for 1 h, c incubating for 3 h, d incubating for 4 h, e the area distribution of double emulsions after incubating for different durations. The error bars indicate the standard error. Each datapoint is the average of at least 30 droplets
3.6 Troubleshooting
The above observations are synopsized into a reverse-index relating possible issues with double-emulsion production relatively to the operating parameters. We assume that the single emulsion was produced with a good quality (highly monodisperse).
Problem | Possible cause |
|---|---|
Many multicore droplets | The vortex speed is too slow |
The vortex time is too short | |
Not enough flick cycles | |
The ratio of single emulsion to carrier phase is too high | |
Many oil droplets | The vortex speed is too fast |
The vortex time is too long | |
Too many flick cycles | |
The ratio of single emulsion to carrier phase is too low | |
Droplet content is spilled in the carrier phase | The vortex speed is too fast.* |
*While excessive vortex speed was experimentally shown to trigger droplet leakage, it is likely that any cause listed in “many oil droplets” could be responsible for the droplet leakage.
4 Conclusions
Double emulsions could become an important tool for biomedical and material research in the near future, but their production remains complicated and often requires access to clean room facilities. In this paper, we have demonstrated a simple method using commercial microfluidic chips and vortex mixer to generate single and double emulsions. Furthermore, the resulting droplet size can be adjusted using osmotic pumping.
In addition to producing double emulsions, we have studied the occurrence of adverse events during vortex emulsification, such as high polydispersity (high CV), droplet rupturing and merging. We have found that droplet rupturing occurs during vortex emulsification. Core fusion was never observed in any of our experiments, which suggests that droplets can safely be considered as independent micro-reactors. Interestingly, multicore double emulsion droplets were observed. Statistics suggest that multicore emulsions are formed by single emulsion droplets encapsulated together in the oil shell rather than by the coalescence of two single-core double emulsion droplets. Additional experiments or simulations may be needed to confirm this mechanism.
References
Abate AR, Krummel AT, Lee D, Marquez M, Holtze C, Weitz DA (2008) Photoreactive coating for high-contrast spatial patterning of microfluidic device wettability. Lab Chip 8(12):2157–2160
Abate AR, Thiele J, Weinhart M, Weitz DA (2010) Patterning microfluidic device wettability using flow confinement. Lab Chip 10(14):1774–1776
Al-Bataineh SA, Szili EJ, Gruner PJ, Priest C, Griesser HJ, Voelcker NH et al (2012) Fabrication and operation of a microcavity plasma array device for microscale surface modification. Plasma Processes Polym 9(7):638–646
Bernath K, Hai M, Mastrobattista E, Griffiths AD, Magdassi S, Tawfik DS (2004) In vitro compartmentalization by double emulsions: sorting and gene enrichment by fluorescence activated cell sorting. Anal Biochem 325(1):151–157
Chong D, Liu X, Ma H, Huang G, Han YL, Cui X et al (2015) Advances in fabricating double-emulsion droplets and their biomedical applications. Microfluid Nanofluid 19(5):1071–1090
Chou W-L, Lee P-Y, Yang C-L, Huang W-Y, Lin Y-S (2015) Recent advances in applications of droplet microfluidics. Micromachines 6(9):1249–1271
Hai M, Bernath K, Tawfik D, Magdassi S (2004) Flow cytometry: a new method to investigate the properties of water-in-oil-in-water emulsions. Langmuir 20(6):2081–2085
Kim SC, Sukovich DJ, Abate AR (2015) Patterning microfluidic device wettability with spatially-controlled plasma oxidation. Lab Chip 15(15):3163–3169
Lagus TP, Edd JF (2013) A review of the theory, methods and recent applications of high-throughput single-cell droplet microfluidics. J Phys D Appl Phys 46(11):114005
Liao Q-Q, Zhao S-K, Cai B, He R-X, Rao L, Wu Y et al (2018) Biocompatible fabrication of cell-laden calcium alginate microbeads using microfluidic double flow-focusing device. Sens Actuators, A 279:313–320
Mastrobattista E, Taly V, Chanudet E, Treacy P, Kelly BT, Griffiths AD (2005) High-throughput screening of enzyme libraries: in vitro evolution of a β-galactosidase by fluorescence-activated sorting of double emulsions. Chem Biol 12(12):1291–1300
Mutafopulos K, Spink P, Lofstrom C, Lu P, Lu H, Sharpe J et al (2019) Traveling surface acoustic wave (TSAW) microfluidic fluorescence activated cell sorter (μFACS). Lab Chip 19(14):2435–2443
Nawar S, Stolaroff JK, Ye C, Wu H, Xin F, Weitz DA (2020) Parallelizable microfluidic dropmakers with multilayer geometry for the generation of double emulsions. Lab Chip 20(1):147–154
Okushima S, Nisisako T, Torii T, Higuchi T (2004) Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 20(23):9905–9908
Romanowsky MB, Heymann M, Abate AR, Krummel AT, Fraden S, Weitz DA (2010) Functional patterning of PDMS microfluidic devices using integrated chemo-masks. Lab Chip 10(12):1521–1524
Romero PA, Abate AR (2012) Flow focusing geometry generates droplets through a plug and squeeze mechanism. Lab Chip 12(24):5130–5132
Rotem A, Abate AR, Utada AS, Van Steijn V, Weitz DA (2012) Drop formation in non-planar microfluidic devices. Lab Chip 12(21):4263–4268
Shahi P, Kim SC, Haliburton JR, Gartner ZJ, Abate AR (2017) Abseq: ultrahigh-throughput single cell protein profiling with droplet microfluidic barcoding. Sci Rep 7(1):1–12
Shang L, Cheng Y, Zhao Y (2017) Emerging droplet microfluidics. Chem Rev 117(12):7964–8040
Shui L, Van Den Berg A, Eijkel JC (2009) Interfacial tension controlled W/O and O/W 2-phase flows in microchannel. Lab Chip 9(6):795–801
Sohrabi S, Moraveji MK (2020) Droplet microfluidics: fundamentals and its advanced applications. RSC Adv 10(46):27560–27574
Suea-Ngam A, Howes PD, Srisa-Art M, DeMello AJ (2019) Droplet microfluidics: from proof-of-concept to real-world utility? Chem Commun 55(67):9895–9903
Sukovich DJ, Kim SC, Ahmed N, Abate AR (2017) Bulk double emulsification for flow cytometric analysis of microfluidic droplets. Analyst 142(24):4618–4622
Vladisavljević GT, Khalid N, Neves MA, Kuroiwa T, Nakajima M, Uemura K et al (2013) Industrial lab-on-a-chip: design, applications and scale-up for drug discovery and delivery. Adv Drug Deliv Rev 65(11–12):1626–1663
Wang X, Zhu J, Shao T, Luo X, Zhang L (2019) Production of highly monodisperse millimeter-sized double-emulsion droplets in a coaxial capillary device. Chem Eng Technol 42(6):1330–1340
Wang J, Hahn S, Amstad E, Vogel N (2022) Tailored double emulsions made simple. Adv Mater 34(5):2107338
Acknowledgements
This work was supported by the National Natural Science Foundation of China with Grant Nos. 12004078, 51950410582, and 61874033, the Science and Technology Commission of Shanghai Municipality Nos 22QA1400900 and 22WZ2502200, and the State Key Lab of ASIC and System, Fudan University with Grant No. 2021KF003, 2020KF006, 2021MS001 and 2021MS002. This work was supported by the Fundamental Research Funds for the Central Universities with Grant No. D5000210626. This project was also supported by Institut Pasteur (PTR 393-ZOOFOAMENV) as well as the Chinese Academy of Sciences, a Shanghai Municipal Science and Technology Major Project (Grant No. 2019SHZDZX02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, S., Mao, L., Ying, J. et al. Generation of double emulsions from commercial single-emulsion microfluidic chips: a quality-control study. Microfluid Nanofluid 26, 71 (2022). https://doi.org/10.1007/s10404-022-02575-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-022-02575-7