Abstract
Double-emulsion droplets have found widespread applications in various engineering and biomedical fields because of their capability in encapsulating different components in each layer. The conventional double-emulsion method is the two-stage stirring emulsification method, which suffers from poor monodispersity and low encapsulation efficiency. With recent advances in microfabrication, some novel methods for fabricating double-emulsion droplets have been developed, including microfluidic emulsification (double-T-junction microchannel, double-cross-shaped microchannel and several three-dimensional microchannels), membrane emulsification and coaxial electrospraying. These methods have shown significantly improved droplet features (e.g., size, size uniformity, thickness of each layer, generation throughput capability). Herein, this paper first reviews the state-of-art approaches for fabricating double-emulsion droplets and discusses their advantages and disadvantages. The applications of double-emulsion droplets in biomedical fields, including cell encapsulation, drug delivery and controlled release, and synthetic biology are also discussed. In conclusion, future perspectives are given.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Double-emulsion droplets have found widespread applications in both scientific and industrial fields, due to their capability of encapsulating multiple components simultaneously. These droplets can be used as templates to fabricate microcapsules and microparticles with core–shell structure (Gong et al. 2014; Jaganathan et al. 2014; Zhang et al. 2012), which are highly desirable for applications ranging from pharmacy (Ahmad et al. 2014; Gonçalves et al. 2014; Palamoor and Jablonski 2014), biology (Harada and Discher 2011), to food science (Perez-Moral et al. 2014). Generally, the shell layer of the double-emulsion droplets is used to encapsulate specific compounds (e.g., nutrient composition, drug, cell), which are later released at proper conditions in these applications. For example, microparticles solidified from double-emulsion droplets using gelatinized sweet potato starch (SPS) as shell layer are used to carry and release drugs (Park et al. 2014). The drugs are encapsulated effectively and released in small intestine with the degradation of SPS through enzymatic hydrolysis. In biological evolution study, microparticles solidified from double-emulsion droplets are used to encapsulate single cells, which provides well-controlled cell environments for directed evolution (Zinchenko et al. 2014). For these applications, it is of great importance to control the structure (e.g., droplet size, size distribution and thickness of each layer) (Yuan and Williams 2014), fabrication capacity (Zinchenko et al. 2014) and efficiency of encapsulating functional particles (Sun et al. 2010) (e.g., drugs, cells) of double-emulsion droplets.
However, the conventional double-emulsion method (e.g., two-stage stirring emulsification method) has poor monodispersity and encapsulation efficiency (the ratio of the actual amount of encapsulated compounds to the theory amount of encapsulated compounds), which have significantly limited its application (Okochi Hideaki 1997). With recent advances in micro- and nanotechnologies, various novel methods have emerged for fabricating double-emulsion droplets with controlled features (e.g., size, size uniformity, thickness of each layer, generation throughput capability) (Shao et al. 2013; Wang et al. 2014; Zhao et al. 2014). For instance, a microfluidic device consisting of a series of flow-focus junctions has been developed to fabricate double-emulsion droplets with controlled shell thickness (Abate et al. 2011). A coaxial electrospraying technology has been developed to fabricate double-emulsion droplets with improved encapsulation efficiency and controllable release of the encapsulated protein (Zamani et al. 2014).
Although there are several good reviews on the topic of double-emulsion droplets (Jaworek 2008; Vandergraaf et al. 2005; Wang et al. 2014; Yuan and Williams 2014), these reviews focus only on one or a few technologies and do not pay attention to the comparison of different technologies. This paper reports the integration of emerging micro- and nanotechnologies with double emulsions and reviews their advantages and limitations to help investigators select suitable method according to different requirements. We first present state-of-the-art methods for fabricating double-emulsion droplets, including microfluidic technology, membrane emulsification technology and coaxial electrospraying technology, and discuss both advantages and limitations of these methods. Then, we discuss the applications of double-emulsion in biomedical fields. Finally, conclusions and future perspectives are given.
2 Methods for generating double-emulsion droplets
2.1 Conventional double-emulsification method
The conventional double-emulsion method, i.e., the two-stage stirring emulsification method, has been applied used for a long time (Okochi Hideaki 1997). In this method, the first step is to get water in oil (W/O) emulsions by hydrophobic emulsifiers or oil in water (O/W) emulsions by hydrophilic emulsifiers. In the second step, the prepared single emulsions are re-emulsified in an aqueous or an oil phase by emulsifiers to fabricate double-emulsion droplets. Thompson et al. (2015) used a judicious combination of hydrophilic and hydrophobic block copolymer as highly anisotropic emulsifiers to fabricate W/O/W droplets with diameter ranging from 30 to 80 μm via the two-stage stirring emulsification method, which stayed stable at 20 °C for 10 weeks.
In conventional double-emulsification method, the disperse phase breaks up in the continue phase to form droplets due to shear or impact stresses induced by stirring (Shah et al. 2008). Droplet formation is relevant to the stress region, the physical properties of the liquids and the interface, and so on. However, the liquid flow is chaotic in the stirring region resulting in spatially non-uniform and not well-controlled stress, which induces the poor monodispersity and low encapsulation efficiency (Yuan and Williams 2014).
2.2 Microfluidic emulsification technology
In recent years, microfluidics, as a novel technology, has shown great potential to address the challenges in the fields of chemical and biological analysis, in which the miniaturization of reaction volume is preferred. The integration of microfluidic technology in emulsifying process has resulted in the emergence of microfluidic emulsification approaches (Han et al. 2013; Wang et al. 2014; Zhao and Middelberg 2011), which offer great control over the emulsification progress and the fabricated droplets. In this technology, the dispersed phase is pressed into another immiscible continuous phase using specially designed microchannel, and thus monodisperse emulsion is generated. Several types of microchannels have been developed to fabricate double-emulsion droplets including double-T-junction microchannel, double-cross-shaped microchannel and several three-dimensional (3D) microchannels.
2.2.1 Vertical-cross-type microchannels
Vertical-cross-type microchannels are the assembly of several vertically crossed microchannels. Droplets can be formed by shearing one liquid (dispersed phase) into the second immiscible one (continuous phase). The structure of the droplets is mainly determined by the interfacial tension and shear force, and the continuous phase can entrain the generated droplets downward (Li et al. 2012, 2015). The most common structures for the fabrication of monodisperse double-emulsion droplets are double-T-junction microchannels (Fig. 1a) and double-cross-shaped microchannels (Fig. 1b). In T-junction microchannels, the size and fabrication frequency of the droplets can be precisely controlled by regulating the flow rates of the dispersed and continuous fluids, respectively (Garstecki et al. 2006; Li et al. 2012). Double-T-junction microchannel is an improved device based on the T-junction microchannel, which consists of two T-junction and involves a two-step emulsification process to fabricate double-emulsion droplets (Fig. 1a) (Okushima et al. 2004). Based on the principle of adjusting the ratio of the breakup rates in the two junctions, the number of enclosed droplets can be controlled.
Schematic of microfluidic emulsification technologies for fabricating double-emulsion droplets and their production images. a The double-T-junction microchannel where dripping instabilities are present in both junctions to fabricate double-emulsion droplets in a two-step process (I) and the fabricated O/W/O double-emulsion droplets with the diameters of internal and external droplets being 68 and 170 μm (II) (Okushima et al. 2004). b The double-cross-shaped microchannel (I) and dripping instabilities are present in both junctions to fabricate double-emulsion droplets in a two-step process (II) (Abate et al. 2011). c The moving-wall chopping microchannel with a controllable moving-wall structure (CMW), a flow-focusing structure (FFS) and a pneumatic liquid-cutting chopper (PLCC) (I) and the images of W/O/W double-emulsion droplets with the CV of inner droplets being <5.52 % and that of W/O/W droplets being <4.95 % (II) (Lin et al. 2008). d The air-bubble-triggered microchannel (I) and the fabricated double-emulsion droplets (II) (Abate and Weitz 2011). e The parallel-flow configuration flow-focusing microchannel (I) and the polymerized microcapsules solidified from double-emulsion droplets (II) (Oh et al. 2006). f The reverse-flow configuration flow-focusing microchannel (I) and the W/O/W double-emulsion droplets with a single inner droplet (II) (Utada et al. 2005). g The small-junction-to-large-junction microchannel that a small-cross-sectional-area focusing microchannel connects with a large-cross-sectional-area focusing microchannel (I) and the fabricated double-emulsion droplets (II) (Chang and Su 2008). h The sudden expansion microchannel consists of two junctions for injecting water droplets into oil droplets to form W/O/W double-emulsion droplets (I) and the fabricated double-emulsion droplets (II) (Kim and Kim 2014)
Double-cross-shaped microchannel is similar to that in T-junction microchannel (Fig. 1b). However, comparing with T-junction microchannel, cross-shaped microchannel shows better controllability in the fabrication of double-emulsion droplets because two morphologies of double-emulsion droplets including equilibrium structures and non-equilibrium structures can be obtained by control of microfluidics, where equilibrium and non-equilibrium structures mean complete or partial engulfment of the disperse phase by the continuous phase (Pannacci et al. 2008).
In vertical-cross-type microchannels, the types of double-emulsion droplets are controlled via adjusting the wetting property of microchannel inner surface (Abate et al. 2008). The inner surface should be hydrophobic while the water phase is dispersed in the oil phase, and hydrophilic while the oil phase is dispersed in the water phase (Hwang et al. 2012). Dreyfus et al. (2003) studied the effect of the surface wetting property on the droplet formation and shown that droplets continuously formed when the microchannel surface was wetted by the fluid. The geometry and wettability of the microchannel have a significant influence on the monodispersity and stability of the double-emulsion droplets (Okushima et al. 2004). Vertical-cross-type microchannel method can provide double-emulsion droplets with size <920.7 μm, size coefficient of variation (CV) in 2.6–19 %, fabrication frequency up to 2500 drops/s and encapsulation efficiency more than 91 % (Table 1). Compared with other microfluidic emulsification methods, vertical-cross-type microchannels are simple and easy to produce. But the droplets encapsulation efficiency is lower than that of 3D flow-focusing microfluidics.
2.2.2 Moving-wall chopping microchannels
Microfluidics can also be combined with other technologies (e.g., liquid-chopping technology) to fabricate monodisperse droplets (Hsiung et al. 2006). The fabrication device includes a T-junction channel with a moving-wall structure, a flow-focusing structure and a pneumatic liquid-cutting chopper with a pair of moving-wall structure downstream (Fig. 1c).
In the platform, double-emulsion droplets are fabricated in three steps, Fig. 1c (Lao et al. 2009; Lin et al. 2008). Firstly, the dispersed phase is focused into a stream by using hydrodynamic focusing and is chopped by the moving-wall structure to fabricate single-emulsion droplets (inner droplets). Secondly, the inner droplets are injected into a continuous phase in the flow-focusing structure, and a narrow stream is formed. Finally, the prefocused single emulsions are cut to form double-emulsion droplets with well-controlled sizes. Perpendicularly deflecting the compliant side using injected air drives the moving-wall, which is the most important characteristic of this technology.
This platform combines the advantages of both microfluidic technology and the moving-wall configuration, and its adjustable property makes it a promising double-emulsion fabrication device. The internal and external droplet size can be well adjusted through tuning the moving-wall and the pneumatic liquid-cutting chopper without changing the flow rate. The droplets from moving-wall chopping microchannels have a size from 138 to 165 μm, CV <6 %, Table 1. But the integration of a moving wall to microfluidic devices increases the assembly difficulty, thus limiting its mass production capability.
2.2.3 Air-bubble-triggered microchannels
A method based on air-bubble-triggered droplet formation has been developed (Abate and Weitz 2011), which has similar structure to the T-junction. The difference is that the air is compressed to the microchannel regularly to block the continuous phase.
The whole emulsion process can be operated easily as follows: Firstly, a cross-channel is used to form a coaxial jet. The dispersed phase flows into the central channel, and the continuous phase can be injected into the two side channels. Thus the coaxial jet, including the dispersed phase surrounded by the continuous phase, is formed. Without the effects of other forces, the jet keeps steady and will not break into droplets (Guillot et al. 2007). Secondly, air bubbles are forced into the continuous phase flowing in another microchannel alongside the jet. Finally, the compound fluid is mixed with the dispersed phase in the cross-region, and the fluid between the consecutive bubbles forms the droplets, Fig. 1d.
Because the droplets are fabricated through cutting off the fluid using air flow, the fabrication frequency can be increased greatly by increasing the air fluid rate. The air-bubble-triggered droplet formation microfluidics enables the fabrication of monodisperse droplets with size range from 23 to 32 μm, and CV <4 % in a high throughput manner up to 6000 drops/s (far above that of other planar microfluidics methods), Table 1. Regulating the bubble frequency and bubble spacing can control the droplet size, while the fabrication frequency can also be adjusted via regulating the flow rate of all the fluids. In addition, there exists a required minimum amount of outer fluid for forming droplets. Besides, there exist a number of air bubbles in the emulsion, which may block the microchannels and thus need to be removed, depending on the application.
2.2.4 3D flow-focusing microchannels
Flow-focusing microchannel is another commonly used type of microchannels to fabricate double-emulsion droplets. Two immiscible fluids are pressed into the flow-focusing microchannel through the central channel and side channel separately. The flow condition changes simultaneously with decreasing sectional area in the focusing section. Due to the Rayleigh–Plateau instability, the drag force applied by the continuous phase and the interfacial tension applied by disperse phase, the coaxial mixed flow breaks up into uniform droplets at the end of the focusing section, and double-emulsion droplets are subsequently formed after they are dispersed into the continuous phase (Kim et al. 2011).
Two different flow configurations may be used in flow-focusing microchannels, i.e., parallel-flow configuration (Fig. 1e) and reverse-flow configuration (Fig. 1f), depending on the flow directions of the outer fluid and the middle fluid. In the parallel-flow configuration, the apparatus is constructed by arranging three kinds of capillaries in a PDMS block where a center hole is prepunched coaxially. The inner and middle fluids are injected into each channel entrance and travel through the intermediate capillary to form the coaxial jet. The coaxial jet flow is focused at the exit of the capillary and subsequently split into double-emulsion droplets in the continuous phase (Oh et al. 2006). In the reverse-flow configuration, the inner fluid is injected into the conical capillary, while the middle fluid is injected into the channel surrounding the inner fluid, forming the coaxial stream at the tip of the conical capillary. The outer fluid is injected into the outer region from the reverse direction, and all fluids pass through the outlet of the capillary and consequently break up to form droplets.
A small-junction-to-large-junction microfluidic emulsification device has also been developed to fabricate double-emulsion droplets (Fig. 1g) (Chang and Su 2008), where two focusing junctions are connected by an embedded orifice. Varying flow rates can separately drive the inner, middle and outer fluids into the microchannels. Similar to the parallel-flow-focusing microchannels, the inner water fluid and the middle oil fluid are injected into the first focusing junction and shaped into a W/O coaxial stream. According to the prior 3D flow-focusing work (Takeuchi et al. 2005), the embedded orifice is used to prevent the W/O coaxial stream touching with the channel wall in the downstream. Since the cross-sectional area of orifice is smaller than that of the collection channel, the dispersed phase can be surrounded fully by the continuous phase (Rotem et al. 2012). A spherical cap formed by the W/O coaxial stream appears on the center of the orifice, where the cap is fully surrounded and wetted by the outer water phase. The shear as induced by outer water phase increases with the growth of the cap. When this shear is large enough to restrain the cap growth, the cap breaks away from the orifice forming a droplet. Furthermore, the width of downstream channel connecting with the exit of the embedded orifice is significantly reduced, which results in the accelerated flow rate and increased transverse perturbation near to the exit, making it easier for fabricating droplets (Chang and Su 2008). In this method, droplets are fabricated with mean diameter of 150–470 μm and CV <7.5 %.
Compared with vertical-cross-type microchannels, parallel-flow configuration microchannels and reverse-flow configuration microchannels, the small-junction-to-large-junction microchannels have some unique advantages. For instance, the production of double emulsions is limited by the wettability of the channel surface in planar microchannels, where W/O single-emulsion droplets are fabricated in hydrophobic channels and O/W single-emulsion droplets are fabricated in hydrophilic channels (Seo et al. 2007). In other words, the wettability of the channel surface has to be opposite between the two junctions (~microns) (Abate et al. 2010a), which is technically challenging to achieve. But in small-junction-to-large-junction microchannels, the orifice sectional area is smaller than that of the collection channel, where the disperse phase is fully surrounded and wetted by the continuous phase, avoiding the need for surface modification (Sim et al. 2010). But different from the planar microchannels manufactured by a single stamping or lithographic techniques, the small-junction-to-large-junction microchannel consists of a small junction and a large junction. Hence, the two models are manufactured, respectively, and then aligned to connect into a whole, which is not easy from production perspectives (Rotem et al. 2012). Meanwhile, the production of double-emulsion droplets strictly relies on the key parameters such as flow rate and microchannels geometry (Rotem et al. 2012). It requires a delicate design to execute productions regardless of the wetting conditions of the channel wall. In 3D flow-focusing microchannels, the size, diameter ratio and fabrication frequency of double-emulsion droplets are controlled via adjusting the diameter of the configuration and flow rate of three fluids. By optimizing these parameters, uniform droplet size (diameter 20–470 μm, CV 1–7.5 %), great encapsulation efficiency (near 100 %) and good fabrication frequency (up to 10,000 drops/s) can be achieved, Table 1.
2.2.5 Sudden expansion channels
Kim et al. introduced a microfluidic method to fabricate double-emulsion droplets by a device consisting of a narrow channel connected with a sudden expansion channel (Kim and Kim 2014). The device is composed of two junctions: One is for fabricating oil drops and the other is for making double-emulsion droplets by oil drops (Fig. 1h). There are three conical capillaries coaxially embedded in two square capillaries. The middle capillary is tapered at both sides, but the left and the right capillaries are tapered just at one side. The left capillary has a 20-μm-diameter tapered orifice. The left side of the middle capillary is a tapered 190-μm-diameter orifice, while the right side is a tapered 68-μm-diameter orifice. The right capillary has a tapered 306-μm-diameter orifice. These diameters are carefully selected to ensure the smooth process. The left capillary is treated using n-octadecyltrimethoxyl silane (Sigma-Aldrich) to make it hydrophobic, while the middle and right capillaries are treated with 2-[methoxy(polyethyleneoxy)propyl] trimethoxy silane to make them hydrophilic. The junction for fabricating oil drops is similar to flow-focusing microchannels. Due to the drag force of the continuous phase and the interfacial tension of disperse phase, the coaxial mixed flow breaks up into uniform droplets at the left inlet of the middle capillary. Oil droplets accelerate through the middle capillary with high inertia force. The droplets are deformed by high inertia force and breaks up when the back interface of the droplets reaches the sudden expansion channel. Through the breakup, water droplets are injected into the oil droplets and double-emulsion droplets with mean diameter from 278 to 410 μm are fabricated.
Moreover, the water droplets can also be injected to fabricate W/O/W double-emulsion droplets, where two distinct inner droplets will exist. These droplets can be transformed into bilayer membranes of polymersomes and then segregate the inner water phase to show its potential on sampling the fluids and further studying.
2.2.6 Advantages and disadvantages of microfluidic emulsification technology
The effective structure in microchannel system (e.g., microchannel, microreactor) is at microscale in diameter, which offers a much higher area/volume ratio compared to the macroscale device. Consequently, some special effects related to the flow condition appear, such as the laminar effect, interfacial tension and capillary effect, which provide a better control over the emulsion fabrication process.
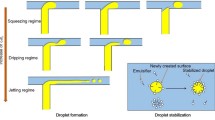
By adjusting the diameter of the microchannels, the flow rate of all fluids, the capillary number and other related parameters, the fabrication characteristics can also be tuned (Erb et al. 2011; Kim et al. 2011; Okushima et al. 2004; Whitesides 2006). Generally, the droplet size is similar to the dimension of channels, and thus the droplet size can be controlled via adjusting the dimension of channels. There are two mechanisms of droplet generation using microfluidics depending on the flow rate, i.e., dripping and jetting (Utada et al. 2005). Dripping fabricates droplets with high monodispersity close to the orifice, similar to a dripping faucet. In contrast, jetting forms a long jet that is over three times as long as the diameter of orifice downstream, where it breakups and forms droplets with low monodispersity. The breakup mechanism dictates the droplet size distribution, and the rate between the inner fluid and the middle fluid dictates the number of inner droplets. When the flow rates of the inner and middle fluids are the same, the middle and inner fluids break simultaneously, and droplets with an inner droplet can be fabricated in both dripping and jetting. The outer fluid flow rate dictates the mutual transition of dripping and jetting. When the flow rate of outer fluid is lower, the dripping regime occurs. With increasing flow rate, the coaxial jet is stronger and the inner fluid is thinner, then smaller double-emulsion droplets are fabricated. With increasing flow rate, the droplets diameter linearly decreases. With the flow rate of the outer fluid increasing over a threshold value, the jet rapidly lengthens and the jetting regime displaces the dripping regime, resulting in an increased droplet diameter. But the diameter of the coaxial jet near to the exit orifice decreases, which results in the decreased fabrication frequency.
Moreover, the dimensionless capillary number, Ca, is very important in droplet formation (Teh et al. 2008), which is defined as: Ca = ην/γ, where η is the viscosity of the continuous phase, ν is the velocity of the continuous phase, and γ is the interfacial tension between the oil and water phases. Droplet breakup occurs when Ca is over a certain value that depends on the device geometries. The capillary number guides us to select suitable materials considering the relative viscosity between the discrete and continuous phases. For instance, selecting a more viscous continuous phase can accelerate the droplet formation.
Compared to conventional devices fabricating double-emulsion droplets, the volume of the microfluidic reaction equipment is ultrasmall, significantly increasing the emulsification efficiency and thus reducing the consumption of the reagents. Besides the emulsification efficiency, the controllability and flexibility of microfluidic devices are good. Therefore, the microfluidic emulsification technology is of great importance in both research fields and practical applications.
But there also exist some disadvantages on the microfluidic emulsifications. As shown in Table 1, the double-emulsion droplets fabricated by microfluidics have larger sizes compared with membrane emulsification and coaxial electrospray. In general, microfluidics are used to fabricate droplets with diameter larger than 10 μm (Parhizkar et al. 2014). The double emulsion fabricated by microfluidic emulsification technology is always in metastable state. To maintain the stability of the double emulsion, surfactants that provide a stabilizing interfacial repulsion are usually required to inhibit droplet coalescence (Bremond et al. 2008). However, the surfactants prevent reagents from entering the droplets. So it is difficult to add new reagents into stable double emulsions and perform multistep reactions, which significantly restricts the application of the double-emulsion droplets (Abate et al. 2010b). Meanwhile, except small-junction-to-large-junction microchannels, most microchannels need surface treatment. For example, the hydrophobic channels fabricate W/O droplets and the hydrophilic channels fabricate O/W droplets. The treatment increases the complexity of the process. Another limitation of microfluidic emulsification is the low production rate resulting from the dripping-to-jetting transition (Ambravaneswaran et al. 2004; Utada et al. 2008). At the jetting region, the dripping forming uniform droplets may transform to the jetting forming droplets with a broad size distribution. The droplets are almost formed under slow, controlled flow conditions to minimize the polydispersity of the droplets, and these low flow rates seriously limit the production frequency of the droplets (Abate and Weitz 2011). Besides, the pumps used in microfluidics may cause greater resistance in microchannels, clogging by bubbles and particles at constrictions and valves (Rife et al. 2000).
2.3 Coaxial electrospray technology
The electrospray technology is one of the few technologies to fabricate submicron scale droplets with maintained monodispersity (Jaworek 2007). In this method, a conductive fluid slowly flows through an electrified needle in an outer medium (gas or a dielectric liquid). When the flow rate and the potential difference between the needle and the surroundings are appropriate, a conical liquid (Taylor cone) is generated at the needle exit. Finally, the conical liquid will breakup and the droplets are sprayed from the needle exit, Fig. 2a.
Schematic of coaxial electrospray technology for fabricating double-emulsion droplets and the production images. a The typical coaxial electrospray structure fabricating double-emulsion droplets where the outer fluid is air (I) (Loscertales et al. 2002), the structured Taylor cone where the outer fluid is insulating host liquid (II) (Marín et al. 2007) and the fabricated double-emulsion droplets (III) (Marín et al. 2007). b The combine T-junction with coaxial electrospray method (I) and the microbubbles at the tip of the outlet at supplied voltages of 0, 6 and 12 kV (II) (Parhizkar et al. 2014)
Many devices have been designed to fabricate double-emulsion droplets based on electrospray technology, which can be divided in two typical kinds according to the external media (i.e., air and dielectric liquid). Loscertales et al. (2002) reported a method to fabricate double-emulsion droplets, by injecting the inner and outer fluid with proper flow rates into the two coaxially located needles, where the outer needle is connected to an electrical voltage of several kilovolts (Fig. 2a). The conical liquid is generated at the tip of the needles, and at last the liquid breaks up into an aerosol of uniform droplets. The droplets are sprayed on a plate of water, forming double-emulsion droplets. The fabrication process and collection process can be combined by replacing the external media (i.e., air) by dielectric liquid (Marín et al. 2007), which has proved its ability to fabricate micro- and nano-double-emulsion droplets just in one step. The size and diameter ratio of the double-emulsion droplets fabricated in both methods can be controlled via regulating the flow rates of the inner and outer liquid, as well as the applied electrical voltage. In this method, the droplets are fabricated with size low to 0.289 μm, CV in 15–44 %, fabrication frequency above 4.6 × 106 drops/s and encapsulation efficiency from 51 to 61 % (Table 1).
Due to the solid theoretical basis and simple configuration, electrospray technology offers several advantages in fabricating double-emulsion droplets, including broader droplet size range, great fabrication frequency and its expandability, Table 1. The droplet size is broader than those obtained by other methods, which is not restricted by the internal diameter of the needles. Electrospray technology provides a precise control of the ratio of the inner and outer fluid radius via changing their flow rates and the applied voltage, and the resulting size could range from dozens of nanometers to hundreds of micrometers. The fabrication frequency can be above 4.6 × 106 drops/s. Besides, this technology can be easily expanded. For instance, if the number of the coaxially arranged needles adds up to three, the resulting electrohydrodynamic flow allows preparing monodisperse droplets with three layers, double-layered bubbles or porous encapsulated threads at nano- and microscales (Ahmad et al. 2008).
However, the liquids that can be used in this technology are limited, which need to satisfy certain requirements. The size distribution in pure electrospray is wide, and the encapsulation efficiency is low (Table 1). Compared with the electric field force, the effect of gravity is so small that it can be ignored because the influence zone has only a size of several micrometers (Suvorov and Litvinov 2000). The steady Taylor cone could only be formed with the action of the electric field force, and thus at least one liquid (either inner or outer) in the electrospraying configuration should be electrical conductive (called driving liquid) (Loscertales et al. 2002). Also, the viscosity of the outermost liquid should not be too high to fragment the Taylor cone (Farook et al. 2007). The requirement of high voltage may also involve the issue of adverse impact on the particles encapsulated in the droplets such as cells and proteins.
Furthermore, the electrospray technology can also be combined with microfluids to fabricate double-emulsion droplets. A method combining T-junction with coaxial electrospray was reported to fabricate droplet with diameter much smaller than that of the channels (Parhizkar et al. 2014), Fig. 2b. There exist three capillaries: two being Teflon FEP and one being stainless steel. The two Teflon FEP capillaries are inserted vertically into a PDMS block as inlet channels for a dielectric fluid (air) and a conductive liquid (glycerol–water mixtures). The third capillary is inserted into the block coaxially separated by a certain distance with the air channel to compose a T-junction. Air supplied by a pressurized air tank connected with the air channel is inserted into the junction at a constant pressure. Meanwhile, the liquid phase is inserted by a stainless steel syringe at a constant flow rate. The steel capillary channel is connected to a high voltage relative to the ground electrode placed a certain distance below the bottom of the outlet channel. Because of high resistance caused by the small channel cross-sectional area, the fluid has a low current under the applied voltage, which can break up the fluid to form droplets. Normally, droplets fabricated through microchannels have a similar size to the dimension of channels. The composite microfluidic and electrohydrodynamic device has been used to fabricate monodisperse droplets with diameter at 25 μm (much smaller than the channel diameter), CV <1 % and fabrication frequency up to several thousand drops/s (Parhizkar et al. 2014).
2.4 Membrane emulsification technology
Membrane emulsification technology is another method for fabricating double-emulsion droplets, where the pure dispersed phase passes through the micropores of a porous membrane with the continuous phase flowing on the other side of the membrane surface (Vandergraaf et al. 2005). The droplets form and grow at the pore outlet, and they eventually detach from the membrane until reaching a certain droplet size, taking away by the continuous fluid.
The formation and detachment of the droplets are mainly determined by the force balance including the drag force from the continuous phase, the interfacial tension force, the droplet buoyancy and the applied driving pressure (Gomaa et al. 2014). The size of the droplets can be tailored by adjusting the parameters related to these forces (Candéa et al. 2014). Moreover, the morphology of the membrane (e.g., the pore size, porosity) also determines droplet size and droplet size distribution. Generally, the average droplet size linearly depends on the membrane micropore size (Wu et al. 2015). To obtain monodisperse droplets with high efficiency, the porosity should be maintained in a specific range, since too high porosity results in adhesion of droplets and too low porosity results in decreased throughput (Zhao et al. 2014).
The wetting property also has influence on the droplet formation. To ensure that the membrane pores are not wetted by the disperse phase, the membrane should be always repellant to the disperse phase. For examples, the membrane should be hydrophobic for fabricating O/W droplets and hydrophilic for fabricating W/O droplets. The double-emulsion droplets can be generated by pumping single-emulsion droplet through another membrane, Fig. 3a. A premix membrane emulsification method was introduced with a better size distribution and higher transmembrane fluxes than that in direct membrane emulsification method, Fig. 3b. Compared with direct membrane emulsification, premix membrane emulsification passes an inferior W/O/W emulsion through several membranes by applying appropriate pressure instead of directly pressing the dispersed phase through a single membrane, which gives a narrower size distribution.
Schematic of membrane emulsification technology for fabricating double-emulsion droplets and the production images. a Fabricating W/O/W double-emulsion droplets by direct membrane emulsification. The fluid flows along the arrow direction (Vandergraaf et al. 2005). b The premix membrane emulsification pressing an inferior double emulsion through the membrane (Vandergraaf et al. 2005). c Premix W/O/W double emulsion (I), the emulsion through one-SPG membrane (II) and the emulsion through six-SPG membrane (III) (Vladisavljević et al. 2004)
The most widely used membrane in this technology is Shirasu-porous-glass (SPG) membrane, but the disperse phase flux in the membrane is small, and the membrane is relatively expensive. The development of a new type of membranes with improved properties will greatly accelerate the application of the membrane emulsification technology (Charcosset et al. 2004). Microsieves fabricated by semiconductor fabrication methods have great application in membrane emulsification with high disperse phase flux at low operating pressure (Wagdare et al. 2010), due to extremely low flow resistance and uniform pore size (Kazazi-Hyseni et al. 2014).
The membrane emulsification technology offers several advantages for generating double-emulsion droplets. The energy consumption of the technology is lower than conventional methods, in which severe process conditions are necessary for the fracture of the double-emulsion droplets, and the high shear force is energy intensive, especially for small droplets (<3 μm). In comparison, the energy requirement is much lower in membrane emulsification process because it needs a lower operating pressure (Dragosavac et al. 2012). Besides, the size of the double-emulsion droplets can be tailored with good monodispersion (Pawlik and Norton 2012). The configuration used in the technology is relatively simple and reliable, and less surfactant is required than in conventional methods (e.g., two-stage stirring emulsification method). In the membrane method, the droplets are fabricated with size <300 μm, CV in 4.8–20 %, fabrication frequency up to 2 × 105 drops/s and encapsulation efficiency from 54.94 to 91 % (Table 1).
However, there are also several limitations associated with membrane emulsification technology for fabricating double-emulsion droplets. For instance, the high porosity is beneficial for the throughput, but not for the droplet size, since the droplets may get in contact and coalesce during the droplet formation. To avoid coalesce, the porosity of the membrane surface should ideally be <1.5 % (Abrahamse et al. 2002), significantly limiting the fabrication efficiency. Besides, the monodispersity and encapsulation efficiency of the double-emulsion droplets fabricated by the membrane emulsification technology are much lower than the microfluidic emulsification method, Table 1.
2.5 From a bouncing compound droplet to a double emulsion
Recently, Terwagne and Gilet proposed a method to fabricate double-emulsion droplets (O/W/O), starting from a single compound droplet (Fig. 4) (Terwagne et al. 2010). This method is based on the repeated impacts and rebounds of the compound droplet on a liquid interface. Two containers are used in their method, i.e., one is filled with silicone oil, and the other contains water with anionic surfactant and sodium dodecyl sulfate (SDS). Two syringes (one for each liquid) are used, and the nozzles are connected by a thin copper wire curved downward (Gilet et al. 2009). The left container is vertically vibrated utilizing an electromagnetic shaker. When the amplitude reaches a threshold value, the silicone oil droplet flows downward along the copper wire and merges with the surfactant solution droplet. The mixture droplet stops at the slowest point, and a compound droplet is formed (Lorenceau et al. 2004). The compound droplet is sufficiently large to detach from the wire and bounces on a vertically vibrated liquid surface afterward. As long as the amplitude of the vibration is greater than the threshold, the inner water droplet is penetrated by the oil layer and a tiny oil droplet is left within due to the convergence of capillary waves to the top of the compound droplet (Bartolo et al. 2006). With the vigorous impact going on, a double-emulsion droplet can be formed.
Schematic of bouncing compound drop method for fabricating double-emulsion droplets and the production images (Terwagne et al. 2010). a The bouncing compound drop device. b Through repeatedly impacting the bath at high speed, additional oil droplets enter into the inner water droplets. The four images show the same droplets after 5, 20, 34 and 51 bounces
The droplet dimensions can be tuned from 400 to 1200 μm by adjusting the vibration parameters of the container (e.g., frequency and amplitude). The maximal size of the double-emulsion droplet is a function of the acceleration and the frequency of the bath vibration if the droplet properties are constant (Gilet et al. 2008, 2007; Couder et al. 2005). Double-emulsion droplets fabricated by this method are separated from each other, which offer unique advantages. These droplets can be set into a self-propelled mode which is characterized by an internal rotation of the fluid inside the droplet (Dorbolo et al. 2008), and they can also be merged together to form the traditional double emulsion (Gilet et al. 2007). In short, this spontaneous emulsification technology may be a potentially promising to prepare double-emulsion droplets.
2.6 Materials usable for fabricating double-emulsion droplets
In the sections above, the state-of-art approaches for fabricating double-emulsion droplets are discussed and there exist many good examples for fabricating droplets via different methods. The materials of chemicals and devices depend on the types of the double-emulsion droplets. PDMS and glass are the most common materials to manufacture microchannels, and the SPG membranes are widely used in membrane emulsification. Meanwhile, the water phase is often obtained via dissolving encapsulated components and hydrophilic emulsifier in the deionized water. And the viscosity can affect the formation of the droplets. So in this section, these good examples are listed in Table 2 for the reference of researchers.
3 Applications of double-emulsion droplets in biomedical engineering
The double-emulsion droplets have been used in many fields, including food science (Aditya et al. 2015; Schroën et al. 2015), cosmetic industry (Tal-Figiel 2007), agriculture (Gao et al. 2009) and biomedicine (Fraker et al. 2012). Here, we discuss their applications in biomedical fields such as drug delivery and controlled drug release, cell encapsulation and synthetic biology.
3.1 Applications in drug delivery and controlled release
The encapsulation and controlled release of chemical compounds, especially drugs, is of great importance for the pharmaceutical industry. Among the numerous means of encapsulating and releasing drugs (especially water-soluble pharmaceuticals) (Lensen 2008), double-emulsion technology offers advantages due to the bilayer structure of double-emulsion droplets and tunable properties of each layer (e.g., thickness Jaimes-Lizcano et al. 2013, mechanical properties Hennequin et al. 2009, selectivity Lee and Weitz 2008 and permeability Shum et al. 2008). Microparticles (or nanoparticles) solidified from double-emulsion droplets can preserve their morphology, encapsulate drugs effectively and release the drugs in the target region. Various methods have been utilized to polymerize double-emulsion droplets, such as solvent evaporation (Fig. 5a) (Staff et al. 2013) and UV irradiation (Fig. 5b) (Chen et al. 2013).
Solidifying double-emulsion droplets. a Schematic of solvent evaporation. The polymer solvent is evaporated to induce polymer precipitation and drug-loaded microparticles are formed (I) (Staff et al. 2013). Image of ALN-loaded NP prepared by the double-emulsion solvent evaporation (II) (Cohen-Sela et al. 2009). b Fabricating double-emulsion droplets and solidifying the droplets in the UV irradiation (I). Microparticles were templated from the double-emulsion droplets (II) (Wu and Gong 2012)
In the solvent evaporation method, the double-emulsion droplets encapsulating drugs are dissolved in a water phase, and the polymer is dissolved in an oil phase. The solvent is stirred continuously and evaporates to induce polymer precipitation, and then drug-loaded microparticles are formed (Couvreur et al. 1997). In the shrinking progress, because of the incompressibility of the inner microdroplets, the precipitating polymer shell may break up and form small pores, where the entrapped substance could be released (Rosca et al. 2004). As a kind of photopolymerization technology, UV irradiation is also a commonly used method to solidify double-emulsion droplets, especially for those fabricated from microfluidic devices (Seiffert et al. 2010). A polymer droplet can be rapidly transformed into a solid polymer microparticle via UV irradiation, and thus quasi-instantaneous polymerization can be achieved. A photoinitiator contained in the droplet absorbs the UV light effectively, and reactive free radicals (or ions) are fabricated. By reaction between the initiating radicals (or ions) and the monomer functional groups, the polymerization can be achieved (Chen et al. 2013). While the monomer double-emulsion droplets flow through the downstream UV irradiation area, the shells of the droplets photopolymerize in situ simultaneously, and polymeric microparticles are obtained as well. The shell of microparticles via UV irradiation is porous and cannot trap small compounds efficiently (Giovagnoli et al. 2007). But in some cases, the porous microparticles have their unique advantages. For instance, in inhalable dry-powder systems, the microparticles loaded with drug are directly sucked into the lung and have to avoid phagocytosis of alveolar macrophages. The macrophages can rapidly engulf smooth small microparticles, but the highly porous large microparticles can avoid this phagocytosis and reach to the intended target (Yang et al. 2009).
The release rate of drugs should be controlled after the microparticles have been delivered to a specific body site. The idea of delivering drugs through the employment of degradable polymer coating so as to evaluate and control the release process was reported as early as in 1970s (Mason et al. 1976). In general, the release can be pH or temperature triggered. The microparticles with the incorporation of pH-sensitive or thermo-sensitive groups are firstly targeted to specific regions, and then the release of the encapsulated drugs can be initiated (Geng et al. 2011; Liu et al. 2011). It has been showed that drug release from a microparticle was usually initiated at the drug lixiviating from the polymer coating or the disintegration of polymer shell. The release dynamics depends on many factors, such as molecular weight, mixed microparticle, crystalline degree, drug distribution, poriness, particle size distribution. But in these factors, the size of the microparticles is one of primary factors for drug delivery and release control since it determines the delivery path and release rate. In general, microparticles with smaller size release faster than those with larger size because of their greater surface area/volume ratio. Too small microparticles may exhibit low encapsulation efficiency and undesired rapid release. On the other hand, too large microparticles may not easily reach certain organs and release drug slowly. A controlled drug release from the microparticles can be achieved by controlling the size and size distribution of microparticles. The uniform microparticles have a uniform release rate, and mixing microparticles with different designed size can achieve controlled drug release rate such as zero-order and linear release (Liu et al. 2005b). Obviously, the microparticles with a narrow size distribution can achieve this goal easier compared with those with a broad size distribution. Regarding to the delivery path, there exists an ideal size providing a delivery route and proper release rate (Berkland et al. 2001), where microparticles with diameter approximately in 1–5 μm are good passive targeting of professional antigen-presenting cells, while those in the range of 10–20 μm are used to deliver drug to the tortuous capillary bed. Microparticles with diameter below 5 μm can be taken up preferentially by the cells and deliver DNA vaccines into cells (Wang et al. 2004). The microparticle size from the microfluidics often changes from tens of micrometers to hundreds of micrometers (Table 1). So for microparticles with hundreds of nanometers, membrane methods are more suitable (Table 1). The uniform double-emulsion droplets were fabricated through a SPG membrane and solidified to PELA microcapsules with CV <15 % by evaporating the solvent (Wei et al. 2011). For microparticles with tens of micrometers, microfluidic methods are more suitable because of their good control of droplet and encapsulation efficiency.
With the use of microparticles made by double-emulsion droplets, the controlled release of drug has been widely studied both in vitro and in vivo. Wu et al. (2013) fabricated monodisperse PLGA–alginate core–shell microspheres through microfluidic devices and studied the drug release kinetics. The experiments of release in vitro showed that smaller PLGA cores had a higher release rate. Patel et al. (2012) evaluated the feasibility of using PEG–PLGA copolymers encapsulated a highly soluble drug to release at lung, and got promising results. The microparticles showed well-controlled release with promising treatment effects on brain tumors (Kaimainen et al. 2015). Due to their high efficiency, controllable drug release, and increased systemic bioavailability, microparticles fabricated from double-emulsion droplets hold great promise for the development of drugs and their clinical applications.
3.2 Applications in cell encapsulation
The idea of encapsulating cells was firstly proposed in 1964 for the immune protection of transplanted cells (Chang 1964) and was implemented successfully in immobilizing xenograft islet cells 20 years later. Cell encapsulation aims to provide cells an insulated microenvironment (Chan et al. 2013). The typical W/O droplets, aqueous droplets dispersed in an oil phase, were used to encapsulate single lymph node cells, which can be maintained for a few hours (Nossal 1958). But there also exist some disadvantages of the W/O droplets. When applied in cell culture, the W/O droplets cannot continuously supply nutrients and will desiccate because the oil phase will evaporate (Zhang et al. 2013). Moreover, the cells encapsulated in the W/O droplets cannot be analyzed by the aqueous phase-based flow cytometry. The W/O/W droplets can address these problems. The outer aqueous phase surrounding the droplets can efficiently keep the droplets wet and reduce the oil phase amount. Meanwhile, the oil shell of the droplets acts as a selective membrane and allows the nutrients of outer aqueous phase to diffuse into the inner droplets for long-term cell culture. The shell is very important in the microencapsulation: (1) supporting the living cells as a rigid scaffold and also providing a protecting environment; (2) preventing excessive cell growth for removing the cells when problems arise (Rabanel and Hildgen 2004); (3) enabling free inward diffusion of gases and outward diffusion of therapeutic products and metabolites (Yow and Routh 2006). The final properties of cell-entrapped capsule should be variable to suit the particular application conditions. Cell encapsulation has recently exhibited a great potential in therapeutic treatments of various diseases, such as diabetes (Zou et al. 2012), anemia (Hosny et al. 2015), hemophilia (Fargnoli et al. 2015), cancer (Sun et al. 2012), renal failure (Liu et al. 2012), central nervous system (CNS) insufficiencies (Prajapati et al. 2012). More recently, cell-entrapped microparticles have been explored in applications of tissue engineering (Nicodemus and Bryant 2008) and biosensors (Koh and Pishko 2006).
As an emerging encapsulating medium, double-emulsion droplets show great potential in encapsulating cells. Due to the controllability and the flexibility of the bilayered structure, cells cultured in these droplets (or microcapsules) exhibit improved cellular functions over those cultured in monolayered structures. Oh fabricated yeast cell encapsulated double-emulsion droplets utilizing co-flow configuration and cells remained alive for 2 days after polymerization of the double-emulsion droplets (Oh et al. 2006). Due to the polymer shell, cells could avoid the influences from the outside environment, suggesting potential application of double-emulsion droplets for storing and delivering cells. Gonzalez encapsulated Lactobacillus rhamnosus in double-emulsion droplets and found improved cell viability compared to those non-entrapped cells in an environment with bile salt and low pH (Pimentel-González et al. 2009). Monodisperse double-emulsion droplets were fabricated via microfluidics to provide a microenvironment for biofilm growth (Chang et al. 2015). These results show that double-emulsion droplets can be used to protect bacteria and provide them with an appropriate environment to grow within (Fig. 6) (Rakszewska et al. 2014).
The electrospray technology could be applied in the cell bioprinting directly. In the cell electrospray device, the highly concentrated cellular suspension liquid is injected as an inner fluid. And the viscoelasticity medical-grade medium is injected as an outer fluid (Jayasinghe et al. 2007). While the external medium is gas, the double-layered droplets containing living cells fabricated by electrospray technology do not need to be transported in the continuous phase; consequently, they could be positioned in the gel matrix or the cells scaffold without the need of separating or processing. Various cells have been jetted by the use of bioelectrospray, and the results demonstrate that the cells capsulated via electrospray technology can survive for a long time (Abeyewickreme et al. 2009; Arumuganathar et al. 2008; Odenwälder et al. 2007; Patel et al. 2008).
Encapsulating cells via double emulsions requires a good control of droplet size, size distribution, encapsulation efficiency and throughput because the cell culture should be repeatable and well controlled. Since cell size is normally tens of micrometers, the microfluidic methods are more suitable because of their nearly perfect control of droplets (Table 1). Though the double-emulsion droplets show unique advantages in cell encapsulation, some theoretical and technological limitations still exist in fabrication and encapsulation processes. For instance, the biomaterial effects on capsule properties and the control of capsule properties need to be further investigated (Chan et al. 2013). However, related research work is being carried on by various institutions (Choi et al. 2009; Shah et al. 2008) and clinical applications of cell-laden double-emulsion droplets are expected.
3.3 Applications in synthetic biology
Over the past few years, synthetic biology has attracted a lot of attention as an emerging field due to its advantage in creating novel, functional biological parts and systems using a bottom-up approach (Purnick and Weiss 2009). Conventional genetic engineering focuses on natural biological systems and changes one or a few genes for special purposes. But the advance of bioinformatics and functional genomics makes it possible to understand and construct artificial biological parts and systems for creating valuable biomaterials (e.g., drugs, green fuels). Synthetic biologists develop a new series of theories, models, simulations and tools to understand the biological behavior of the natural cell. Aiming at an artificial cell (Fig. 7), characterizing the variation of gene expression in different cell environments is the basis for ascertaining the principles of gene regulation (Young and Alper 2010). A controllable and reproducible cell environment can accelerate this process. Moreover, the cell environment can also contain the machinery for synthesizing biological components (e.g., proteins and peptides). The artificial cell-like structures, which have a robust shell and an aqueous interior and exterior, are widely applied in synthetic biology as microcompartments for providing a cell environment (Martino et al. 2012). Though the W/O/W droplets cannot be directly used as artificial cell-like structures because of their low membrane permeability, the giant unilamellar phospholipid vesicles (GUVs) and polymersomes templated from the W/O/W droplets are the major tools as artificial shells. The GUV shell consists of a phospholipid bilayer, and the polymersomes are vesicles formed from block copolymers (Discher and Ahmed 2006). Compared with polymersomes, the GUVs are more biocompatible because the phospholipid bilayers are same to the natural cell membranes. So in synthetic biology, they are mostly used to mimic cell membranes and research the biological behaviors in cell. But the phospholipid bilayers are more unstable compared with polymer membranes of polymersomes. Though the polymer membranes have a lower penetrability, their increased stability can enhance the encapsulation efficiency of biological components (Rodríguez-García et al. 2011). So the polymersomes are more suitable for producing biological components in synthetic biology.
The conventional methods for the GUVs are electroformation, reverse emulsification (Arriaga et al. 2014). The electroformation includes two steps. Firstly, some phospholipids are deposited on a substrate. Secondly, after adding pure water, the dry phospholipid deposit self-swells and forms the GUVs by an applied AC electric field (Pott et al. 2008). The reverse emulsification includes three steps (Pautot et al. 2003). The aqueous solution is firstly mixed with the oil phase containing surfactant and forms water droplets. The surfactant adsorbs at the surface of the droplets to stabilize the droplets. Then, an oil phase with low density containing surfactant remains above the aqueous phase and the surfactant covers the interface of water and oil. Thirdly, the firstly fabricated W/O single emulsion is poured into the secondly fabricated solution and passes through the interface of water and oil. After passing the surfactant interface, the droplets obtain a second phospholipid layer and the GUVs are formed. But the conventional methods fabricate the GUV with a broad size distribution. Double-emulsion droplets have a good control in droplet size, shell thickness, encapsulation efficiency and so on. So the GUVs templated from double-emulsion droplets also have these advantages. Moreover, the GUV membranes with spatial heterogeneities are desired, where the spatial heterogeneities play a key role in cross-membrane transport (Arriaga et al. 2014). The phospholipids coming into the microdomains consisting of different compositions and structures, which is similar to the rafts in cell membranes (Lingwood and Simons 2010), exhibit good capacities of assembling and regulating the key proteins and tuning the physical characteristics of the membranes. The microdomains are affected by many factors, e.g., the phospholipid constituents of the shell, the residual solvents and the vesicle size. A novel microfluidic method can fabricate the GUVs with uniform sizes, controlled phospholipid compositions and formation of microdomains (Arriaga et al. 2014). A 3D parallel-flow microchannel device, where a conical capillary inserts into the opposite ends of a conical diffusion capillary, is used to fabricate ultrathin-shell double-emulsion droplets. The inner fluid contains an aqueous solution of PEG and PVA, where the PEG increases the aqueous phase viscosity and the PVA enhances the stability of the inner droplets. A phospholipid mixture of chloroform and hexane containing DOPC, DPPC and cholesterol is used as the middle fluid. And the outer fluid was a PVA solution. The W/O/W droplets are fabricated with shell thickness <1 μm, so there exists minimal residual solvent in the phospholipid membranes. And the middle oil shell contains a highly volatile good solvent chloroform in phospholipid and a nonvolatile insoluble hexane. With the evaporation of the chloroform from the middle oil phase, the hexane dewets from the double-emulsion droplets and the GUVs are formed. Via controlling the phospholipid composition, the dynamic and fuse microdomains are formed, including liquid-disordered microdomains appeared in DOPC and liquid-ordered microdomains appeared in DPPC.
The polymersomes are often used in producing biological components due to their better stability and functionality than the GUVs (Martino et al. 2012). The conventional methods for the polymersomes (e.g., electroformation) are similar to the GUVs because their constructions are so similar. But the same problems of polydisperse droplets low encapsulation efficiency also exist in polymersomes. The polymersomes templated from double-emulsion droplets via microfluidic methods overcome these problems perfectly because of their good control of size and high encapsulation efficiency, shown in Table 1. In the process of fabricating W/O/W droplets, a mixer of chloroform, hexane and block copolymers are added into the oil as the middle fluid similar to the process of fabricating the GUVs. The chloroform evaporates from the middle oil phase and drives the hexane to dewet from the droplets. Then, the oil layer is expelled from the two-block copolymer monolayers at the interfaces of water and oil and then is separated from the water phase by the block copolymer monolayers. At last the polymersomes are fabricated. For instance, Martino et al. (2012) used the microfluidic methods to fabricate monodisperse polymersomes efficiently encapsulating a whole set of biological machinery for producing protein MreB in a high throughput.
Similar to the cell encapsulation, the droplets applied in synthetic biology also require a good control of droplet size, size distribution and high encapsulation efficiency. For instance, while researching the gene expression in artificial cell, there needs many nearly identical artificial cells as samples. These samples can be fabricated via microfluidic methods because of their good control of droplets, shown in Table 1.
4 Conclusions and future perspectives
In this paper, we review the methods for fabricating double-emulsion droplets, including microfluidic emulsification, electrospray technology and membrane emulsification. These methods show their capability to fabricate microscale double-emulsion droplets and are widely applied in biomedical engineering. However, there are still some areas where further research is needed. Most of all, the industrialization of these methods has not been realized and more attentions should be paid in realization of large-scale production. Meanwhile, these methods have their own disadvantages. The application range is restricted due to the use of surfactants, and the control on the morphology of double-emulsion droplets is limited in microfluidic emulsification technology. The liquids that can be used in these methods are limited, while the influence of high electrical voltage is uncertain in electrospray technology. In membrane emulsification technology, the poor monodispersity and the relative high cost hinder its widespread application. All these problems are worth further study. Embedded capillary T-junction with electrohydrodynamic focusing combines microfluidic emulsification technology with electrospray technology and fabricates monodisperse droplets successfully. This method enlightens that combining different methods together may address either method’s disadvantages. Moreover, double-emulsion droplets fabricated through the repeated impacts and rebounds of the compound droplet on to a liquid interface offer unique advantages and are worthwhile of further research. Double-emulsion droplets are widely used in synthetic biology, controlled drug release and cell encapsulation technology due to their capability of encapsulating multiple components. So further research should also focus on improving existing methods and developing new ways to strengthen the capability of double-emulsion droplets to encapsulate multiple components.
References
Abate AR, Weitz DA (2011) Air-bubble-triggered drop formation in microfluidics. Lab Chip 11:1713–1716
Abate AR, Krummel AT, Lee D, Marquez M, Holtze C, Weitz DA (2008) Photoreactive coating for high-contrast spatial patterning of microfluidic device wettability. Lab Chip 8:2157–2160
Abate AR, Thiele J, Weinhart M, Weitz DA (2010a) Patterning microfluidic device wettability using flow confinement. Lab Chip 10:1774–1776
Abate AR, Hung T, Mary P, Agresti JJ, Weitz DA (2010b) High-throughput injection with microfluidics using picoinjectors. Proc Natl Acad Sci 107:19163–19166. doi:10.1073/pnas.1006888107
Abate AR, Thiele J, Weitz DA (2011) One-step formation of multiple emulsions in microfluidics. Lab Chip 11:253–258
Abeyewickreme A, Kwok A, McEwan JR, Jayasinghe SN (2009) Bio-electrospraying embryonic stem cells: interrogating cellular viability and pluripotency. Integr Biol 1:260–266
Abrahamse AJ, van Lierop R, van der Sman RGM, van der Padt A, Boom RM (2002) Analysis of droplet formation and interactions during cross-flow membrane emulsification. J Membr Sci 204:125–137. doi:10.1016/s0376-7388(02)00028-5
Aditya NP, Aditya S, Yang H, Kim HW, Park SO, Ko S (2015) Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem 173:7–13
Ahmad Z, Zhang HB, Farook U, Edirisinghe M, Stride E, Colombo P (2008) Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J R Soc Interface 5:1255–1261. doi:10.1098/rsif.2008.0247
Ahmad N, Ramsch R, Llinàs M, Solans C, Hashim R, Tajuddin HA (2014) Influence of nonionic branched-chain alkyl glycosides on a model nano-emulsion for drug delivery systems. Colloids Surf B 115:267–274. doi:10.1016/j.colsurfb.2013.12.013
Ambravaneswaran B, Subramani HJ, Phillips SD, Basaran OA (2004) Dripping-jetting transitions in a dripping faucet. Phys Rev Lett 93:034501
Arriaga LR, Datta SS, Kim SH, Amstad E, Kodger TE, Monroy F, Weitz DA (2014) Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation. Small 10:950–956
Arumuganathar S, Irvine S, McEwan JR, Jayasinghe SN (2008) A novel direct aerodynamically assisted threading methodology for generating biologically viable microthreads encapsulating living primary cells. J Appl Polym Sci 107:1215–1225. doi:10.1002/app.27190
Bartolo D, Josserand C, Bonn D (2006) Nonlinear dynamics, fluid dynamics, classical optics, etc: singular jets and bubbles in drop impact. Phys Rev Lett 96:124501
Berkland C, Kim K, Pack DW (2001) Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release 73:59–74
Bremond N, Thiam AR, Bibette J (2008) Decompressing emulsion droplets favors coalescence. Phys Rev Lett 100:024501
Candéa TV, Monteiro FS, Tonon RV, Cabral LMC (2014) Effect of process variables on the production of flaxseed oil emulsions by cross-flow membrane emulsification. Food Eng Rev 7:258–264
Chan HF, Zhang Y, Ho Y-P, Chiu Y-L, Jung Y, Leong KW (2013) Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci Rep 3. Art. ID 3462
Chang TMS (1964) Semipermeable microcapsules. Science 146:524–525
Chang F-C, Su Y-C (2008) Controlled double emulsification utilizing 3D PDMS microchannels. J Micromech Microeng 18:065018. doi:10.1088/0960-1317/18/6/065018
Chang CB, Wilking JN, Kim SH, Shum HC, Weitz DA (2015) Monodisperse emulsion drop microenvironments for bacterial biofilm growth. Small. doi:10.1002/smll.201403125
Charcosset C, Limayem I, Fessi H (2004) The membrane emulsification process—a review. J Chem Technol Biotechnol 79:209–218. doi:10.1002/jctb.969
Chen H, Li J, Wan J, Weitz DA, Stone HA (2013) Gas-core triple emulsions for ultrasound triggered release. Soft Matter 9:38–42
Chiarabelli C, Stano P, Luisi PL (2009) Chemical approaches to synthetic biology. Curr Opin Biotechnol 20:492–497. doi:10.1016/j.copbio.2009.08.004
Choi SW, Zhang Y, Xia Y (2009) Fabrication of microbeads with a controllable hollow interior and porous wall using a capillary fluidic device. Adv Funct Mater 19:2943–2949
Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G (2009) A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J Control Release 133:90–95
Couder Y, Fort E, Gautier C-H, Boudaoud A (2005) From bouncing to floating: noncoalescence of drops on a fluid bath. Phys Rev Lett 94:177801
Couvreur P, Blanco-Prieto MJ, Puisieux F, Roques B, Fattal E (1997) Multiple emulsion technology for the design of microspheres containing peptides and oligopeptides. Adv Drug Deliv Rev 28:85–96. doi:10.1016/S0169-409X(97)00052-5
Discher DE, Ahmed F (2006) Polymersomes. Annu Rev Biomed Eng 8:323–341
Dorbolo S, Terwagne d, Vandewalle N, Gilet T (2008) Resonant and rolling droplet. New J Phys 10:113021
Dragosavac MM, Holdich RG, Vladisavljević GT, Sovilj MN (2012) Stirred cell membrane emulsification for multiple emulsions containing unrefined pumpkin seed oil with uniform droplet size. J Membr Sci 392:122–129
Dreyfus R, Tabeling P, Willaime H (2003) Ordered and disordered patterns in two-phase flows in microchannels. Phys Rev Lett. doi:10.1103/PhysRevLett.90.144505
Erb RM, Obrist D, Chen PW, Studer J, Studart AR (2011) Predicting sizes of droplets made by microfluidic flow-induced dripping. Soft Matter 7:8757–8761
Fargnoli AS, Mu A, Katz MG et al (2014) Anti-inflammatory loaded poly-lactic glycolic acid nanoparticle formulations to enhance myocardial gene transfer: an in vitro assessment of a drug/gene combination therapeutic approach for direct injection. J Transl Med 12:171
Farook U, Stride E, Edirisinghe M, Moaleji R (2007) Microbubbling by co-axial electrohydrodynamic atomization. Med Biol Eng Comput 45:781–789. doi:10.1007/s11517-007-0210-1
Fraker CA, Mendez AJ, Inverardi L, Ricordi C, Stabler CL (2012) Optimization of perfluoro nano-scale emulsions: the importance of particle size for enhanced oxygen transfer in biomedical applications. Colloids Surf B 98:26–35. doi:10.1016/j.colsurfb.2012.04.011
Gao F, Su Z-G, Wang P, Ma G-H (2009) Double emulsion templated microcapsules with single hollow cavities and thickness-controllable shells. Langmuir 25:3832–3838
Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM (2006) Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab Chip 6:437–446
Geng H, Song H, Qi J, Cui D (2011) Sustained release of VEGF from PLGA nanoparticles embedded thermo-sensitive hydrogel in full-thickness porcine bladder acellular matrix. Nanoscale Res Lett 6:1–8
Gilet T, Vandewalle N, Dorbolo S (2007) Controlling the partial coalescence of a droplet on a vertically vibrated bath. Phys Rev E 76:035302
Gilet T, Terwagne D, Vandewalle N, Dorbolo S (2008) Dynamics of a bouncing droplet onto a vertically vibrated interface. Phys Rev Lett 100:167802
Gilet T, Terwagne D, Vandewalle N (2009) Digital microfluidics on a wire. Appl Phys Lett 95:014106
Giovagnoli S, Blasi P, Schoubben A, Rossi C, Ricci M (2007) Preparation of large porous biodegradable microspheres by using a simple double-emulsion method for capreomycin sulfate pulmonary delivery. Int J Pharm 333:103–111. doi:10.1016/j.ijpharm.2006.10.005
Gomaa HG, Liu J, Sabouni R, Zhu J (2014) Experimental and theoretical analysis of emulsification characteristics using a high porosity microscreen under oscillatory shear conditions. Colloids Surf A 456:160–168. doi:10.1016/j.colsurfa.2014.05.020
Gonçalves VSS, Rodríguez-Rojo S, Matias AA et al (2014) Development of multicore hybrid particles for drug delivery through the precipitation of CO2 saturated emulsions. Int J Pharm 478:9–18. doi:10.1016/j.ijpharm.2014.11.003
Gong A, Ma X, Xiang L, Ren W, Shen Z, Wu A (2014) Improved double emulsion technology for fabricating autofluorescent microcapsules as novel ultrasonic/fluorescent dual-modality contrast agents. Colloids Surf B Biointerfaces 116:561–567. doi:10.1016/j.colsurfb.2014.01.038
Guillot P, Colin A, Utada AS, Ajdari A (2007) Stability of a jet in confined pressure-driven biphasic flows at low Reynolds numbers. Phys Rev Lett 99:104502
Han YL, Wang W, Hu J et al (2013) Benchtop fabrication of three-dimensional reconfigurable microfluidic devices from paper–polymer composite. Lab Chip 13:4745–4749
Harada T, Discher DE (2011) Materials science: bubble wrap of cell-like aggregates. Nature 471:172–173
Hennequin Y, Pannacci N, de Torres CP, Tetradis-Meris G, Chapuliot S, Bouchaud E, Tabeling P (2009) Synthesizing microcapsules with controlled geometrical and mechanical properties with microfluidic double emulsion technology. Langmuir 25:7857–7861
Higashi S, Setoguchi T (2000) Hepatic arterial injection chemotherapy for hepatocellular carcinoma with epirubicin aqueous solution as numerous vesicles in iodinated poppy-seed oil microdroplets: clinical application of water-in-oil-in-water emulsion prepared using a membrane emulsification technique. Adv Drug Deliv Rev 45:57–64. doi:10.1016/s0169-409x(00)00100-9
Hosny KM, Banjar ZM, Hariri AH, Hassan AH (2015) Solid lipid nanoparticles loaded with iron to overcome barriers for treatment of iron deficiency anemia. Drug Des Dev Ther 9:313
Hsiung S-K, Chen C-T, Lee G-B (2006) Micro-droplet formation utilizing microfluidic flow focusing and controllable moving-wall chopping techniques. J Micromech Microeng 16:2403–2410
Hwang S, Choi C-H, Lee C-S (2012) Regioselective surface modification of pdms microfluidic device for the generation of monodisperse double emulsions. Macromol Res 20:422–428
Jaganathan M, Madhumitha D, Dhathathreyan A (2014) Protein microcapsules: preparation and applications. Adv Colloid Interface Sci 209:1–7. doi:10.1016/j.cis.2013.12.004
Jaimes-Lizcano YA, Wang Q, Rojas EC, Papadopoulos KD (2013) Evaporative destabilization of double emulsions for effective triggering of release. Colloids Surf A 423:81–88. doi:10.1016/j.colsurfa.2013.01.054
Jaworek A (2007) Electrospray droplet sources for thin film deposition. J Mater Sci 42:266–297
Jaworek A (2008) Electrostatic micro-and nanoencapsulation and electroemulsification: a brief review. J Microencapsul 25:443–468
Jayasinghe SN, Irvine S, McEwan JR (2007) Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine 2:545–553
Kaimainen M, Marze S, Järvenpää E, Anton M, Huopalahti R (2015) Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT Food Sci Technol 60:899–904. doi:10.1016/j.lwt.2014.10.016
Kazazi-Hyseni F, Landin M, Lathuile A et al. (2014) Computer modeling assisted design of monodisperse PLGA microspheres with controlled porosity affords zero order release of an encapsulated macromolecule for 3 months. Pharm Res 31:2844–2856
Kim S-H, Kim B (2014) Controlled formation of double-emulsion drops in sudden expansion channels. J Colloid Interface Sci 415:26–31
Kim S-H, Kim JW, Cho J-C, Weitz DA (2011) Double-emulsion drops with ultra-thin shells for capsule templates. Lab Chip 11:3162–3166
Koh W-G, Pishko M (2006) Fabrication of cell-containing hydrogel microstructures inside microfluidic devices that can be used as cell-based biosensors. Anal Bioanal Chem 385:1389–1397. doi:10.1007/s00216-006-0571-6
Lao K-L, Wang J-H, Lee G-B (2009) A microfluidic platform for formation of double-emulsion droplets. Microfluid Nanofluid 7:709–719. doi:10.1007/s10404-009-0430-9
Lee D, Weitz DA (2008) Double emulsion-templated nanoparticle colloidosomes with selective permeability. Adv Mater 20:3498–3503
Lee Y-H, Mei F, Bai M-Y, Zhao S, Chen D-R (2010) Release profile characteristics of biodegradable-polymer-coated drug particles fabricated by dual-capillary electrospray. J Control Release 145:58–65. doi:10.1016/j.jconrel.2010.03.014
Lensen D (2008) Polymeric microcapsules for synthetic applications. Macromol Biosci 8:991
Li X-B, Li F-C, Yang J-C, Kinoshita H, Oishi M, Oshima M (2012) Study on the mechanism of droplet formation in T-junction microchannel. Chem Eng Sci 69:340–351. doi:10.1016/j.ces.2011.10.048
Li X-B, Li F-C, Kinoshita H, Oishi M, Oshima M (2015) Dynamics of viscoelastic fluid droplet under very low interfacial tension in a serpentine T-junction microchannel. Microfluid Nanofluid 18:1007–1021
Lin Y-H, Lee C-H, Lee G-B (2008) Droplet formation utilizing controllable moving-wall structures for double-emulsion applications. J Microelectromech Syst 17:573–581
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327:46–50
Liu R, Ma G-H, Wan Y-H, Su Z-G (2005a) Influence of process parameters on the size distribution of PLA microcapsules prepared by combining membrane emulsification technique and double emulsion-solvent evaporation method. Colloids Surf B 45:144–153. doi:10.1016/j.colsurfb.2005.08.004
Liu R, Ma G, Meng F-T, Su Z-G (2005b) Preparation of uniform-sized PLA microcapsules by combining Shirasu Porous Glass membrane emulsification technique and multiple emulsion-solvent evaporation method. J Control Release 103:31–43. doi:10.1016/j.jconrel.2004.11.025
Liu L, Yang J-P, Ju X-J et al (2011) Monodisperse core-shell chitosan microcapsules for pH-responsive burst release of hydrophobic drugs. Soft Matter 7:4821–4827
Liu M, Gan L, Chen L, Zhu D, Xu Z, Hao Z, Chen L (2012) A novel liposome-encapsulated hemoglobin/silica nanoparticle as an oxygen carrier. Int J Pharm 427:354–357
Lorenceau É, Clanet C, Quéré D (2004) Capturing drops with a thin fiber. J Colloid Interface Sci 279:192–197. doi:10.1016/j.jcis.2004.06.054
Loscertales IG, Barrero A, Guerrero I, Cortijo R, Marquez M, Gañán-Calvo AM (2002) Micro/nano encapsulation via electrified coaxial liquid jets. Science 295:1695–1698. doi:10.1126/science.1067595
Marín ÁG, Loscertales IG, Márquez M, Barrero A (2007) Simple and double emulsions via coaxial jet electrosprays. Phys Rev Lett 98:014502
Martino C, Kim SH, Horsfall L, Abbaspourrad A, Rosser SJ, Cooper J, Weitz DA (2012) Protein expression, aggregation, and triggered release from polymersomes as artificial cell-like structures. Angew Chem 51:6416–6420. doi:10.1002/anie.201201443
Maruyama T, Fukui Y, Tsuchiya E, Fujii A (2012) One-step preparation of giant lipid vesicles with high encapsulation efficiency using an electrospray technique. RSC Adv 2:11672–11674
Mason N, Thies C, Cicero TJ (1976) In vivo and in vitro evaluation of a microencapsulated narcotic antagonist. J Pharm Sci 65:847–850. doi:10.1002/jps.2600650612
Moghaddam MK, Mortazavi SM, Khayamian T (2015) Preparation of calcium alginate microcapsules containing n-nonadecane by a melt coaxial electrospray method. J Electrostat 73:56–64. doi:10.1016/j.elstat.2014.10.013
Nicodemus G, Bryant S (2008) Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 14:149–165. doi:10.1089/ten.teb.2007.0332
Nisisako T, Torii T (2008) Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 8:287–293
Nossal GJV (1958) Antibody production by single cells. Br J Exp Pathol 39:544
Odenwälder PK, Irvine S, McEwan JR, Jayasinghe SN (2007) Bio-electrosprays: a novel electrified jetting methodology for the safe handling and deployment of primary living organisms. Biotechnol J 2:622–630. doi:10.1002/biot.200700031
Oh H-J, Kim S-H, Baek J-Y, Seong G-H, Lee S-H (2006) Hydrodynamic micro-encapsulation of aqueous fluids and cells via’on the fly’photopolymerization. J Micromech Microeng 16:285–291
Okochi Hideaki NM (1997) Comparative study of two preparation methods of w/o/w emulsions: stirring and membrane emulsification. Chem Pharm Bull 45:1323–1326
Okushima S, Nisisako T, Torii T, Higuchi T (2004) Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 20:9905–9908. doi:10.1021/la0480336
Palamoor M, Jablonski MM (2014) Comparative study on diffusion and evaporation emulsion methods used to load hydrophilic drugs in poly(ortho ester) nanoparticle emulsions. Powder Technol 253:53–62. doi:10.1016/j.powtec.2013.11.014
Pan J, Stephenson AL, Kazamia E, Huck WTS, Dennis JS, Smith AG, Abell C (2011) Quantitative tracking of the growth of individual algal cells in microdroplet compartments. Integr Biol 3:1043–1051
Pannacci N, Bruus H, Bartolo D et al (2008) Equilibrium and nonequilibrium states in microfluidic double emulsions. Phys Rev Lett 101:164502
Parhizkar M, Stride E, Edirisinghe M (2014) Preparation of monodisperse microbubbles using an integrated embedded capillary T-junction with electrohydrodynamic focusing. Lab Chip 14:2437–2446. doi:10.1039/C4lc00328d
Park KM, Sung H, Choi SJ, Choi YJ, Chang P-S (2014) Double-layered microparticles with enzyme-triggered release for the targeted delivery of water-soluble bioactive compounds to small intestine. Food Chem 161:53–59. doi:10.1016/j.foodchem.2014.03.125
Patel P, Irvine S, McEwan JR, Jayasinghe SN (2008) Bio-protocols for directly forming active encapsulations containing living primary cells. Soft Matter 4:1219–1229
Patel B, Gupta V, Ahsan F (2012) PEG–PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release 162:310–320. doi:10.1016/j.jconrel.2012.07.003
Pautot S, Frisken BJ, Weitz DA (2003) Production of unilamellar vesicles using an inverted emulsion. Langmuir 19:2870–2879. doi:10.1021/La026100v
Pawlik AK, Norton IT (2012) Encapsulation stability of duplex emulsions prepared with SPG cross-flow membrane, SPG rotating membrane and rotor-stator techniques—a comparison. J Membr Sci 415–416:459–468. doi:10.1016/j.memsci.2012.05.032
Perez-Moral N, Watt S, Wilde P (2014) Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocolloids 42:215–222. doi:10.1016/j.foodhyd.2014.05.023
Pimentel-González DJ, Campos-Montiel RG, Lobato-Calleros C, Pedroza-Islas R, Vernon-Carter EJ (2009) Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res Int 42:292–297. doi:10.1016/j.foodres.2008.12.002
Pott T, Bouvrais H, Meleard P (2008) Giant unilamellar vesicle formation under physiologically relevant conditions. Chem Phys Lipids 154:115–119. doi:10.1016/j.chemphyslip.2008.03.008
Prajapati J, Patel H, Agrawal YK (2012) Targeted drug delivery for central nervous system: a review. Int J Pharm Pharm Sci 3:32–38
Purnick PE, Weiss R (2009) The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol 10:410–422. doi:10.1038/nrm2698
Rabanel J-M, Hildgen P (2004) Preparation of hydrogel hollow particles for cell encapsulation by a method of polyester core degradation. J Microencapsul 21:413–431. doi:10.1080/02652040410001729223
Rakszewska A, Tel J, Chokkalingam V, Huck WTS (2014) One drop at a time: toward droplet microfluidics as a versatile tool for single-cell analysis. NPG Asia Mater 6:e133
Rife JC, Bell MI, Horwitz JS, Kabler MN, Auyeung RCY, Kim WJ (2000) Miniature valveless ultrasonic pumps and mixers. Sens Actuators A 86:135–140. doi:10.1016/S0924-4247(00)00433-7
Rodríguez-García R, Mell M, López-Montero I, Netzel J, Hellweg T, Monroy F (2011) Polymersomes: smart vesicles of tunable rigidity and permeability. Soft Matter 7:1532. doi:10.1039/c0sm00823k
Rosca ID, Watari F, Uo M (2004) Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release 99:271–280. doi:10.1016/j.jconrel.2004.07.007
Rotem A, Abate AR, Utada AS, Van Steijn V, Weitz DA (2012) Drop formation in non-planar microfluidic devices. Lab Chip 12:4263–4268
Schroën K, Bliznyuk O, Muijlwijk K, Sahin S, Berton-Carabin CC (2015) Microfluidic emulsification devices: from micrometer insights to large-scale food emulsion production. Curr Opin Food Sci 3:33–40
Seiffert S, Thiele J, Abate AR, Weitz DA (2010) Smart microgel capsules from macromolecular precursors. J Am Chem Soc 132:6606–6609. doi:10.1021/ja102156h
Seo M, Paquet C, Nie Z, Xu S, Kumacheva E (2007) Microfluidic consecutive flow-focusing droplet generators. Soft Matter 3:986–992
Shah RK, Shum HC, Rowat AC et al (2008) Designer emulsions using microfluidics. Mater Today 11:18–27. doi:10.1016/S1369-7021(08)70053-1
Shao T, Feng X, Jin Y, Cheng Y (2013) Controlled production of double emulsions in dual-coaxial capillaries device for millimeter-scale hollow polymer spheres. Chem Eng Sci 104:55–63
Shum HC, Kim J-W, Weitz DA (2008) Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J Am Chem Soc 130:9543–9549
Sim SPC, Kang TG, Yobas L, Holtze C, Weitz DA (2010) The shape of a step structure as a design aspect to control droplet generation in microfluidics. J Micromech Microeng 20:035010
Staff RH, Landfester K, Crespy D (2013) Recent advances in the emulsion solvent evaporation technique for the preparation of nanoparticles and nanocapsules. In: Percec V (ed) Hierarchical macromolecular structures: 60 years after the staudinger Nobel Prize II. Springer, pp 329–344
Stano P, Luisi PL (2010) Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem Commun 46:3639–3653. doi:10.1039/b913997d
Sugiura S, Nakajima M, Yamamoto K, Iwamoto S, Oda T, Satake M, Seki M (2004) Preparation characteristics of water-in-oil-in-water multiple emulsions using microchannel emulsification. J Colloid Interface Sci 270:221–228
Sun BJ, Shum HC, Holtze C, Weitz DA (2010) Microfluidic melt emulsification for encapsulation and release of actives. ACS Appl Mater Interfaces 2:3411–3416
Sun Y, Zheng Y, Ran H et al (2012) Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablation. Biomaterials 33:5854–5864
Suvorov VG, Litvinov EA (2000) Dynamic Taylor cone formation on liquid metal surface: numerical modelling. J Phys D Appl Phys 33:1245
Takeuchi S, Garstecki P, Weibel DB, Whitesides GM (2005) An axisymmetric flow-focusing microfluidic device. Adv Mater 17:1067–1072
Tal-Figiel B (2007) The formation of stable w/o, o/w, w/o/w cosmetic emulsions in an ultrasonic field. Chem Eng Res Des 85:730–734
Teh SY, Lin R, Hung LH, Lee AP (2008) Droplet microfluidics. Lab Chip 8:198–220. doi:10.1039/b715524g
Terwagne D, Gilet T, Vandewalle N, Dorbolo S (2010) From a bouncing compound drop to a double emulsion. Langmuir 26:11680–11685. doi:10.1021/la101096q
Thompson KL, Mable CJ, Lane JA, Derry MJ, Fielding LA, Armes SP (2015) Preparation of pickering double emulsions using block copolymer worms. Langmuir 31:4137–4144. doi:10.1021/acs.langmuir.5b00741
Utada AS, Lorenceau E, Link DR, Kaplan PD, Stone HA, Weitz DA (2005) Monodisperse double emulsions generated from a microcapillary device. Science 308:537–541. doi:10.1126/science.1109164
Utada AS, Fernandez-Nieves A, Gordillo JM, Weitz DA (2008) Absolute instability of a liquid jet in a coflowing stream. Phys Rev Lett 100:014502
Vandergraaf S, Schroen C, Boom R (2005) Preparation of double emulsions by membrane emulsification? A review. J Membr Sci 251:7–15. doi:10.1016/j.memsci.2004.12.013
Vladisavljević GT, Williams RA (2005) Recent developments in manufacturing emulsions and particulate products using membranes. Adv Colloid Interface Sci 113:1–20
Vladisavljević GT, Shimizu M, Nakashima T (2004) Preparation of monodisperse multiple emulsions at high production rates by multi-stage premix membrane emulsification. Journal of Membrane Science 244:97–106. doi:10.1016/j.memsci.2004.07.008
Wagdare NA, Marcelis A, Ho OB, Boom RM, van Rijn CJM (2010) High throughput vegetable oil-in-water emulsification with a high porosity micro-engineered membrane. J Membr Sci 347:1–7
Wang C, Ge Q, Ting D et al (2004) Molecularly engineered poly (ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat Mater 3:190–196
Wang W, Zhang MJ, Chu LY (2014) Microfluidic approach for encapsulation via double emulsions. Curr Opin Pharmacol 18C:35–41. doi:10.1016/j.coph.2014.08.003
Wei Q, Wei W, Tian R, Wang L-y SuZ-G, Ma G-H (2008) Preparation of uniform-sized PELA microspheres with high encapsulation efficiency of antigen by premix membrane emulsification. J Colloid Interface Sci 323:267–273
Wei Y, Wang Y, Wang L, Hao D, Ma G (2011) Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification. Colloids Surf B 87:399–408
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373
Wu B, Gong H-Q (2012) Formation of fully closed microcapsules as microsensors by microfluidic double emulsion. Microfluid Nanofluid 14:637–644. doi:10.1007/s10404-012-1083-7
Wu J, Kong T, Yeung KWK, Shum HC, Cheung KMC, Wang L, To MKT (2013) Fabrication and characterization of monodisperse PLGA–alginate core–shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater 9:7410–7419. doi:10.1016/j.actbio.2013.03.022
Wu J, Fan Q, Xia Y, Ma G (2015) Uniform-sized particles in biomedical field prepared by membrane emulsification technique. Chem Eng Sci 125:85–97
Yang Y, Bajaj N, Xu P, Ohn K, Tsifansky MD, Yeo Y (2009) Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 30:1947–1953. doi:10.1016/j.biomaterials.2008.12.044
Young E, Alper H (2010) Synthetic biology: tools to design, build, and optimize cellular processes. J Biomed Biotechnol 2010:130781. doi:10.1155/2010/130781
Yow HN, Routh AF (2006) Formation of liquid core-polymer shell microcapsules. Soft Matter 2:940–949
Yuan QC, Williams RA (2014) Precision emulsification for droplet and capsule production. Adv Powder Technol 25:122–135. doi:10.1016/j.apt.2013.10.006
Zamani M, Prabhakaran MP, San Thian E, Ramakrishna S (2014) Protein encapsulated core–shell structured particles prepared by coaxial electrospraying: investigation on material and processing variables. Int J Pharm 473:134–143
Zhang J, Coulston RJ, Jones ST, Geng J, Scherman OA, Abell C (2012) One-step fabrication of supramolecular microcapsules from microfluidic droplets. Science 335:690–694
Zhang Y, Ho YP, Chiu YL et al (2013) A programmable microenvironment for cellular studies via microfluidics-generated double emulsions. Biomaterials 34:4564–4572. doi:10.1016/j.biomaterials.2013.03.002
Zhao C-X, Middelberg APJ (2011) Two-phase microfluidic flows. Chem Eng Sci 66:1394–1411. doi:10.1016/j.ces.2010.08.038
Zhao X, Wu J, Gong F-L, Cui J-M, Janson J-C, Ma G-H, Su Z-G (2014) Preparation of uniform and large sized agarose microspheres by an improved membrane emulsification technique. Powder Technol 253:444–452. doi:10.1016/j.powtec.2013.12.012
Zinchenko A, Devenish SR, Kintses B, Colin PY, Fischlechner M, Hollfelder F (2014) One in a million: flow cytometric sorting of single cell-lysate assays in monodisperse picolitre double emulsion droplets for directed evolution. Anal Chem 86:2526–2533. doi:10.1021/ac403585p
Zou G-K, Song Y-L, Zhou W et al (2012) Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radiol 114:284–289
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (11372243, 51476128), the International Science and Technology Cooperation Program of China (2013DFG02930) and the National Key Scientific Apparatus Development of Special Item (2013YQ190467).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chong, D., Liu, X., Ma, H. et al. Advances in fabricating double-emulsion droplets and their biomedical applications. Microfluid Nanofluid 19, 1071–1090 (2015). https://doi.org/10.1007/s10404-015-1635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1635-8