Abstract
Nickel oxide (NiO) nanoparticles are essential to developing a wide range of important industrial products, examples of which include electrodes, catalysts, and sensors, leading to diverse applications from electrochemical detection to energy storage and environmental remediation. NiO nanoparticles exhibit higher reaction selectivity under solar-driven conditions. Thus, they are good candidates for photocatalysts, which can generate strong oxidizing and reducing agents for photodegradation of organic pollutants and other target molecules under normal temperature and pressure conditions, giving rise to versatile applications for energy and environmental remediation. The conventional strategies of NiO nanoparticle synthesis can be broadly categorized into three themes: solid-phase method, liquid-phase method, and vapor-phase method. Recently, microfluidic reactors hold great promise for nanomaterial synthesis due to the thermal homogeneity across the reactor and rapid heat transfer ensured by the large ratio of surface area to volume. The exquisite control over the size, structure and composition of the droplet by microfluidic emulsification technology outperforms the traditional microemulsion method. Herein, we present an overview of the latest advances in fabrication of NiO nanoparticles using different approaches including both conventional methods and microfluidic methods, and focus on the fundamentals of each formation process with the main advantages and disadvantages discussed. This review also provides comparative overview of influence of synthesis conditions on size and morphologies of NiO nanoparticles. We also summarized the development of NiO-based photocatalysts in environmental applications. The perspectives for future research are also discussed. It can be envisioned that success in microfluidic method will continue to inspire novel approaches to drive the rapid evolution of the NiO synthesis technologies in future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles are ubiquitous in our daily life and are of great interest in several areas, forming the basis for an astounding array of applications of technological and scientific importance. Nanoparticles can be broadly categorized as those with one of its characteristic lengths in the dimension range between 1 and 100 nm. They can be used as nano-building blocks of more sophisticated nanocomposites. The particles with size reduction to nanometer-scale will possess the properties deviated from their bulk ones significantly because of the large specific surface area and unique quantum phenomenon (Greenham et al. 1997; Yang et al. 2019; Wang et al. 2021; Santhi et al. 2004; Stickler et al. 2021), thus possessing remarkably superior advantages in chemical, photology, thermology, and magnetism areas. With the rapid development of nanotechnology, nanoparticles have been widely used in medicine (Kung et al. 2020), biology (Restaino and White 2019; Furtado et al. 2018), electrical engineering (Jayathilaka et al. 2019), sensing (El-Shamy 2021), energy (Ma et al. 2020) and environmental applications (Peng 2002). Therefore, nanoparticles have attracted great interest in recent years and remain a hot research topic in the near future.
The metal-oxide nanoparticle is one of the most important sub-classes of nanoparticles. The past decade has witnessed an explosion in the development of metal-oxide nanoparticles with controlled compositions, sizes, shapes, and structures for industrial applications. For example, titanium dioxide (TiO2) nanoparticles can remove a range of organic species via photodegradation under UV irradiation, thus it has been widely utilized in environmental pollution mitigation for water purification and air pollution treatment. One can find many excellent review papers on TiO2 (Palmas et al. 2021; Hasan and Rana 2021; Jaji et al. 2020). NiO is also a burgeoning metallic oxide because it is naturally abundant and environmentally friendly with high thermal and chemical stability (Ghosh et al. 2016). It can be applied as a combustion catalyst (Liu et al. 2017), anode interfacial layer in solar cells (Irwin et al. 2008), anode material in batteries (Li et al. 2021), gas sensors (Yang et al. 2021), and magnetic materials (Kumar and Das 2021). NiO has face center cubic crystal structure and ferromagnetic properties with a Neel temperature of 525 K (Rinaldi-montes et al. 2016a; b). It is a p-type semiconductor with a wide bandgap of 3.6–4.0 eV and possesses peculiar magnetic and electric behavior depending on the particle size (Khatri and Rana 2020; Pooyandeh et al. 2021; Mohseni Meybodi et al. 2012). The unique chemical and physical characteristics render NiO particularly suitable for photocatalytic applications.
Different synthesis methods of NiO electrode material for supercapacitor have been reviewed (Kate et al. 2018), the methods for NiO nanostructure synthesis and characterization of material properties have been reviewed (Bonomo 2018), and the overview has been provided for the synthesis and applications of nickel nanoparticles in size range of 1–100 nm based on solvothermal, physical, and chemical approaches (Jaji et al. 2020).
Microfluidics has emerged as a promising tool in nanomaterial formation, and it is also of importance to assess the application of microfluidics for NiO nanoparticle synthesis, which has not yet been sufficiently reviewed. This motivates us to undertake the review study in a more systematical fashion. The content of the review paper is organized as the following: in the first section, the synthetic approaches for NiO nanoparticles are systematically surveyed, including solid-phase method, liquid-phase method such as direct precipitation method, homogeneous precipitation method, sol–gel method, hydrothermal method, microemulsion method, organic complex precursor method, polymer-network gel method and biosynthesis method, as well as vapor-phase method. In the subsequent section, the principles and applications of microfluidics in fabricating NiO nanoparticles are reviewed. The representative applications using NiO nanoparticles for environmental remediation via photocatalytic approaches are also reviewed and discussed, considering the ever-increasing demands of NiO in renewable and sustainable applications. The summary of NiO synthesis methods and future development perspectives is finally presented.
2 Conventional synthesis methods
The synthesis methods of nanoparticles can be divided into two major types: top-down and bottom-up. The former refers to crushing the bulky material into small-size substances by mechanical techniques, while the latter refers to building the nanoparticles via a chemical process. NiO nanoparticle formation process mainly relies on the bottom-up method through chemical reactions, obeying the classical nucleation theory, which involves three stages: nucleation, growth, and aging (Köhler et al. 2013). Adjusting the temperature of heat treatment, changing the ratio of water or pre-dehydration before the calcination can efficiently avoid nanoparticle aggregation during the formation process (Zhang 2014). The NiO nanoparticle synthesis methods can be classified into the following three categories according to the variations in the reaction media and environment. All the approaches have been intensively investigated to synthesize NiO nanomaterials with tailored composition, size, shape, and crystalline structure.
2.1 Solid-phase method
The solid-phase method is a traditional and long-standing way of fabricating nanomaterials; it has advantages of low cost, solvent-free, high selectivity, and can be applied in industrial production at a relatively mild reaction environment. However, it has drawbacks such as limited accuracy, relatively low efficiency, and difficulty in controlling the particle characteristic properties (Zhang and Qiu 2009). Solid powders are normally used as precursors or reactants. Once homogeneously mixed, the input energy is required to initiate the reaction. Therefore, the high reaction temperature is usually indispensable for the solid-phase method. The microwave reaction system can be applied in solid-phase method, microwave can uniformly radiate to each part of reactants, it can cause the rotation, vibration, and swing of particles, therefore, increasing the efficiency of particles collision, and shortening the reaction time. As shown in Scheme 1, the solid reactants are grinded into powders, then mixed through vibration or rotation, and the heat treatment via calcination is applied to facilitate the pyrolytic reaction before the nanoparticles can be synthesized.
Xia et al. (2015) used nickel nitrate and oxalic acid as reactants with heat treatment at the temperature of 400 °C for 4 h. Wang et al. (2005) used Ni(OAc)2·4H2O and Tween 80 as the precursor, grinded them into the powder, and then applied the heat treatment by the calcination at 400 °C for 2 h and drying the samples for 4 h under 80 °C. NiO nanoparticles with a well mesoporous structure were synthesized, as shown in Fig. 1. Hosny (2011) produced 8-nm nanoparticles through a semi-solid-phase reaction with a 700 °C decomposition process.
The TEM of NiO nanoparticle samples synthesized by solid-phase method, reproduced with permission (Wang et al. 2005) from Elsevier
2.2 Liquid-phase method
The liquid-phase method can be classified into several subtypes, such as direct precipitation method, homogeneous precipitation method, sol–gel method, hydrothermal method, microemulsion method, organic complex precursor method, biosynthesis method and polymer-network gel method, according to the different liquid media that have been used. The liquid-phase method mostly uses metallic sault solution to separate the metal element into ions. The heat treatment, hydrolysis, or other processes can be applied to obtain the material precipitation or crystal, and dehydration will turn the precipitation into the target powders (Zhang and Qiu 2009). The NiO nanoparticles fabricated by the liquid-phase method normally feature with relatively uniform distribution of particle size, thus high monodispersity; thus, this method has been most widely used nowadays, owing to the controllable reaction conditions (Karatutlu et al. 2018). The representative samples fabricated by each type of liquid-phase method are shown in Fig. 2.
A Scanning electron micrographs (SEM) images of powders synthesized by direct precipitation method. Reproduced with permission (Bahadur et al. 2008) from Elsevier. B TEM image of NiO nanoparticles synthesized by homogeneous precipitation. Reproduced with permission (Deng and Chen 2004) from Elsevier. C SEM images of sol–gel product. Reproduced by permission (Thota and Kumar 2007) from Elsevier. D SEM (a, b, c) and TEM (d, e, f) images of particles synthesized by the hydrothermal method. Reproduced with permission (Cao et al. 2020) from Elsevier. E TEM image of NiO nanoparticles synthesized by the microemulsion method. Reproduced with permission (Han et al. 2004) from Elsevier. F SEM image of NiO nanoparticles at high magnification (Tao and Wei 2004). Reproduced with permission (Tao and Wei 2004) from Elsevier. G SEM images of NiO nanoflowers synthesized by polymer gel method. Reproduced with permission (Munkaila et al. 2021) from Elsevier
2.2.1 Direct precipitation method
The direct precipitation method has been widely adopted in fabricating ultrafine particles. This method uses a chemical reaction to precipitate intact wedges. Then, precipitation will turn into nanopowders through purification, grinding, and heat treatment process (Karatutlu et al. 2018). Bahadur et al. (2008) used sodium hydroxide (NaOH) and nickel nitrate (Ni(NO3)2) as the precursor, reacting and leading to formation of a wet cake of nanocrystalline NiO, which was subsequently dried and grinded into powders. The grinding process gives rise to big variations in the particle dimension, and distribution of density, thus lacking physical integrality.
2.2.2 Homogeneous precipitation method
The homogeneous precipitation method adopts a principle similar to that of the direct precipitation method; it also used the chemical reaction to produce solid precipitation. However, it keeps the precipitation in solution at a balance condition and makes the precipitate at a uniform speed (Pan et al. 2021). It is attained through controlling the concentration of the precipitant. Therefore, this method is stable, balanced, and able to fabricate high-quality particles. Deng and Chen (2004) fabricated the NiO nanopowders with the purity of 99.73%, cube structure, and averaged size of 9 nm through homogeneous precipitation. Despite the high-quality nanopowders they have synthesized, they used NiCl2·6H2O solution and NH3· H2O as pre-reactants. The ammoniate products will pollute the environment, so this method needs an extra purification process.
2.2.3 Sol–gel method
This method uses high chemical activity substances as a precursor, through hydrolyze, alcoholization, condensation, and other chemical reactions to get stable sol with relative uniform distribution of the nanoparticles (Kumar and Han 2019; Zorkipli et al. 2016). Then, heat treatment was utilized to obtain metal nanoparticles. The final particles can reach atom level and possess a very small grain diameter. Thota and Kumar (2007) adopted nickel acetate tetrahydrate and oxalic acid as reactants, ethyl alcohol as a solute, undergoing 400 °C calcination. The black powders of NiO nanoparticles were fabricated by Pooyandeh et al. (2021) using the sol–gel methods with nickel nitrate hexahydrate and nickel chloride hexahydrate as the precursor, and magnetic stirring was applied in the fabricate process, after quiescence for a short time, the solution was filtrated to form the NiO nanoparticles.
2.2.4 Hydrothermal method
The hydrothermal method refers to the nanoparticle fabrication process in which a solution was sealed in a high-pressure vessel with the chemical reaction occurring at high pressure and temperature condition, reactants will undergo dissolving, recrystallization, and heat treatment to form the final products (Sree et al. 2020). Scheme 2 shows the typical synthesis mechanisms and procedures of hydrothermal method, the chemical reactions take place in a solution to trigger the formation of the nanoparticles, followed by the heat treatment to remove wastes and obtain the purified products. It has the advantages such as intact development of grain, relative uniform distribution of particle diameter, less aggregation of particles, obviation of calcination at high temperature in the final process, and in particular, it is a cost-effective and facile synthesis process. However, it is more time-consuming than most other methods. Adschiri et al. applied the continuous hydrothermal method for synthesis of 10 various metal-oxide nanomaterials including NiO nanoparticles, using a microreactor with inner diameter of microchannel to be 9.5 mm. They synthesized NiO nanomaterial with an average size of 200 nm in 2 min (Adschiri et al. 1992). Cao et al. (2020) successfully fabricated three hierarchical NiO microspheres through the hydrothermal method. The molar ratio of 1,2-Propanediol and water was kept at 1:1 in the solution. Nickel nitrate (Ni(NO3)2·6H2O), urea (CO(NH2)2, and citric acid were chosen as reactants and the ratio was kept at 2:1:1. NiO microspheres were self-assembled and grew in a uniform condition. Nickel nitrate and NH2CONH2 are also widely used reactants in the hydrothermal method. An accurate calculation is needed to determine the ratio between each reactant.
Scheme of hydrothermal decomposition method for producing the NiO nanoparticles. Reproduced with permission (Lv et al. 2015) from Elsevier
2.2.5 Microemulsion method
This method works through two insoluble solvents, for example, oil/water system, to form an emulsion in the presence of a surfactant, or cosurfactants, which can decrease the water/oil surface tension to 1–10 mN m−1, facilitating the formation of emulsions (Malik et al. 2012). Meanwhile, surfactants and cosurfactants will create a transient interfacial tension to prevent the droplets from coalescence (Ita 2020). The formation of microemulsions normally needs centrifugation or magnetic stirring to accelerate the dispersion process. For example, Han et al. (2004) used cyclohexane as oil phase, Triton X100 as surfactants, and hexyl alcohol as cosurfactants, which were mixed at a ratio of 5:3:2 with the aid of magnetic force to form emulsions effectively.
Fabricating nanoparticles through microemulsion method has multiple advantages, such as relatively simple experiment setup, low energy cost, and facile operation. Moreover, the range of particle diameter distribution is relatively narrow, and the particle diameter can be efficiently controlled; second, choosing appropriately different surfactants and cosurfactants can lead to the formation of nanoparticles with a special property (Malik et al. 2012), and the particles will have less aggregation. They are stable due to the presence of surfactants and cosurfactants.
2.2.6 Organic coordination compound precursor method
This method used coordination compounds which are the substances with central metal atoms surrounded by nonmetal atoms (Crichton 2012), and are easy to be removed by pyrolytic reactions as a dispersing agent, then mixed with a metal ion to activate the coordination reaction. During the reaction, the dispersing agent will separate particles and prevent them from agglomeration. The reaction will lead to the fabrication of a highly dispersed precursor, which undergoes heat treatment to produce the target particles. Tao and Wei (2004) selected polymer as a dispersing agent, nickel acetate as reactants, which were mixed to form the precursor at a raised environment temperature of 373 K. NiO nanoparticles were fabricated when the temperature was further raised to 673 K with homogeneous size distribution of around 30 nm as shown in Fig. 2.
2.2.7 Polymer-network gel method
The polymer-network gel method can successfully fabricate the relatively pure phase of NiO particles with a uniform distribution of particle diameter and facile control over the shape of the crystalline grain. The formation of a macromolecular chain will make metal sault ion evenly distributed in the sol–gel. Drying and calcining the sol–gel will lead to the fabrication of the nanoparticles. Liu et al. (2003) adopted acrylamide free radical polymerization and N,N′-methylene diacylamine bifunctional group using nickel nitrate aqueous solution as raw material, water-soluble propylene Amide monomer and N,N′-methylene diacylamine act as network agents, ammonium sulfate as initiator, finally, obtaining NiO particles with size ranging in 15–20 nm. Munkaila et al. (2021) synthesized NiO nanoflower using amphiphilic block copolymer as reactants in one-pot synthesis method. The NiO nanoflowers with mesoporous structure were successfully fabricated, as shown in Fig. 2.
2.2.8 Biosynthesis method
The biosynthesis techniques are very effective in synthesizing metal-oxide nanoparticles. It has drawn much attention as it is both environmentally and economically friendly, it does not require complex operation, and it is innoxious to cell and other biosystems. It uses the small natural molecules in biosystems in combination with the conventional synthesis methods (mainly liquid-phase method) to fabricate nanoparticles and has been proved to have better quality and productivity compared to the conventional methods (Sudhasree et al. 2014; Najjar et al. 2021; Imran Din and Rani 2016; Arumugam et al. 2021). Scheme 3 shows the basic principle of the biosynthesis method. The extract substance of plant can be incorporated in the synthesis process of the nanoparticles. Its pH value, concentration, reaction time, and temperature will directly influence the quality of final products. Sabour et al. (2020) applied the biosynthesis method, using Rheum Turkestanicum plant extractions, and successfully fabricated CeO2 nanoparticles with average diameter of 30 nm. Similarly, Najjar et al. (2020) used gelatin as stabilizing agent, and sol–gel method to successfully synthesize SnO2 nanoparticles with an average diameter of 27 nm.
The diagram of biosynthesis principles for forming the metal-oxide nanoparticles. Reproduced with permission of (Shah et al. 2015) from MDPI
Sabouri et al. used tragacanth as stabilizing agents via the co-precipitation method to synthesize nanosheets with comparable high quality and low toxicity to cells. Its absorption efficiency of anionic dyes and cationic dyes reaches 82% and 60%, respectively (Sabouri et al. 2020a, b). The same group also combined the bio-method with the sol–gel method. They successfully synthesized NiO nanoparticles with an average diameter of 59 nm. They used Arabic gum as stabilizing agent, nickel nitrate (Ni (NO3)2·6H2O) as reactants, forming an aerogel at 80 °C, enabling the formation of NiO nanoparticles via the biology activities (Sabouri et al. 2021). Therefore, biosynthesis method paves a promising way to synthesize NiO nanoparticles in future.
2.3 Vapor-phase method
The vapor-phase method uses high pressure and temperature vapor to carry the precursor and use its energy to finish the reaction. The most used vapor-phase method is spray pyrolysis, which applies the high temperature and pressure at the nozzle to stimulate the pyrolytic reaction, and the precipitation reaction occurs simultaneously (Wuled Lenggoro et al. 2003). As the metal salt solution sprays out from the nozzle, it will instantly turn into vapor and separate into nanoparticles. Due to the rapid solution evaporation and release of vapor, it is hard to control the particle characteristics such as crystalline shape, particle diameter, and phase purity using this method. However, as it possesses the advantages of a large production rate and facile operation, it has wide industrial production potential (Karatutlu et al. 2018). Scheme 4 shows the different stages of the process. Mixture of air and fuel will provide an environment with high temperature and high pressure, and the precursors are sprayed into droplets prior to the taking place of the pyrolytic reaction and evaporation process. Precursor droplets will start to nucleate and grow to form nanoparticles.
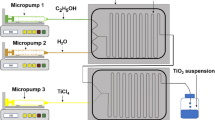
Wuled Lenggoro et al. (2003) used low-pressure spray pyrolysis and successfully synthesized the NiO nanoparticles with an average size of 20 nm. The conversion process and results under different condition are shown in Fig. 3. The solution contains precursor driven by pump going through the channels and is spurted at the nozzle, and the solubility of precursor decides the final type of particles. High-solubility precursor produces nanoparticles, and low-solubility precursor becomes sub-microparticles. Oh et al. (2007) synthesized hollow spherical nanoparticles with uniform distribution of particle diameter of around 20 nm, and well-dispersed nanocrystalline structures. Moravec et al. (2011) fabricated NiO nanoparticles with average diameter about 50 nm by pyrolysis and reduction reaction through 1.5 cm inner diameter nozzle. They proved the production rate include particle size and particle size distribution increase with the increasing of saturation temperature.
Conversion process from droplet to particles of low-pressure spray pyrolysis. Reproduced with permission (Wuled Lenggoro et al. 2003) from Elsevier
2.4 The advantages and limitations of conventional methods
Each method has its own advantages and challenges, as summarized in Table 1. The liquid-phase method is the most widely used method in industrial production and lab-scale synthesis. The size of nanoparticles is more uniform and controllable than the solid-phase and vapor-phase methods. The solid-phase method widely uses pyrolysis or microwave, and it is convenient to operate; it has low requirements on the reaction environment and low cost, and the produced particles have a relatively uniform distribution of diameter. However, its quality is relative hard to control and always accompanied by agglomeration. The vapor-phase method has great potential in industrial manufacture, and it has great efficiency with relatively facile operation. However, it has relatively high requirements on the reaction environment and complexity of the devices.
3 Overview of microfluidic systems and applications
Microemulsion has become one of the most effective approaches for nanoparticle formation. However, the traditional methods for microemulsion formation suffer from poor control over the size distribution. An alternative way is to use microfluidics system, in which the fluid flow is constrained in geometry at the microscale, the fluid flow characteristics will undergo significant changes due to the fascinating competition among forces. Parameters describing microfluidic systems can be described by the balance of inertial force, viscous drag forces, buoyant forces, and interfacial tension forces; the balance of each of these forces gives rise to key dimensionless numbers: the Reynolds number (Re), Weber number (We), Bond number (Bo), capillary number (Ca) and flow rate ratio. The viscous force and surface tension will become dominant in the typical flow regimes at low Reynolds number (Bragheri et al. 2016). Microfluidic chips have been applied in biomedicine (Wu et al. 2016a, b), organic synthesis (Zhao et al. 2019), microreactor (Schrimpf et al. 2019), biomimicking (Xu et al. 2020), and chemical analysis (Wu et al. 2016a, b). The microfluidic chip can integrate multiple fluid operations and functions within a portable device at a highly exquisite level (Chen et al. 2021; Stroock 2008; Abedini-Nassab et al. 2021). More demanding applications have been increasing exponentially, and the application areas expand from microreactors for catalysis and chemical synthesis to point-of-care diagnostics, from drug delivery to cell/molecule compartmentalization and diagnostic testing.
In a microdevice, the flows through a microchannel and can be manipulated in a droplet-wise dispersed way (Lian et al. 2020a, b; Lim et al.2017; Ren and Leung 2016; Ren et al. 2015) or a continuous way (Leung and Ren 2014; Ren et al. 2013; Ren et al. 2013; Leung and Ren 2013). The droplet microfluidics has been widely used in producing microcarriers (Choi et al. 2016a, b), microcapsules (Shirk et al. 2013), and cell-laden microgels (Choi et al. 2016a, b), because fast reaction times in such small compartments are induced by the high surface area to volume ratios, efficient heat and mass transfer, and short diffusion distances. This mechanism can form droplets with uniform size distribution in a very narrow range. Droplet microfluidic device is composed of microchannels and microchambers (Ren et al. 2013). A wide range of materials such as metal (Singh et al. 2010), silicon (Singh et al. 2010), glass (Campbell et al. 2020), polymers (Boodaghi and Shamloo 2020), and ceramics (Malecha et al. 2019) have been used to fabricate microfluidic devices. Each material has its own advantages, demerits, and application areas. For example, metal has the advantages such as low cost, ease machining, and high stiffness. However, it is relatively hard to monitor the status of inner fluid and reaction because it is not transparent (James et al. 2020). Using silicon-based materials for microfluidic chips enables multiple advantages as it is stable, easy to design, and it has special semiconductor characteristics. However, its transparency and price remain big problems (Singh et al. 2010). Glass has perfect transparency and chemical stability along with other advantages’ however, its high fabrication expense hinders its application in making microdevices (Niculescu et al. 2021a; b). Ceramics has unique chemical properties and high-temperature stability; however, due to the restriction of porosity, processing techniques, and brittleness, it is relatively hard to use (Singh et al. 2010). Polydimethylsiloxane (PDMS) is one of the most well-known polymer materials used in microfluidics because it is cheap, easy to design, mechanically flexible, and chemically stable, making it a very popular material in lab on a chip areas, however, it still has the problem of molecular diffusion and porosity (Nielsen et al. 2020). The technique used in the fabrication of microfluidic devices is, therefore, material dependent. Glass and silicon are mainly used by chemical methods, for example, wet and dry etching and electrochemical discharge machining (Hwang et al. 2019). Other materials like metals, are mainly used in mechanical processes, for example, micromachining (Faustino et al. 2016), micro-milling (Faustino et al. 2016), ultrasonic machining (Hwang et al. 2019), blasting (Jáuregui et al. 2010), injection (Gale et al. 2018), and polymers are normally used by soft lithography.
Microfluidics technology has been drawing a lot of attention since the early 1990s (Manz et al. 1990), and it has been applied to create a stable pre-reaction environment for chemical reactions. In addition, microfluidics can also efficiently separate different substances in a solution by the characteristic properties such as the density difference, examples including plasma separation, circular tumor cell capture, and detection (Wu et al. 2016a, b). Microfluidic technology has evolved rapidly in the past decade and demonstrated to be a promising solution with the capability to conduct multiple biological or chemical reactions in parallel, thus accelerating measurement outcomes of the conventional laboratory tests (Niculescu et al. 2021a; b), such as fast detection of circulating tumor cells (Xu et al. 2020) and environmental detection and remediation (Yew et al. 2019; Lian et al. 2020a, b). In addition, microfluidics has versatile control over the flow conditions, giving rise to the ability to synthesize micro/nanostructured materials with high monodispersity in size and shape distribution (Lian et al. 2018). Microfluidics has been widely used in biology and biomedical engineering for studies related to genes, cells, and proteins. The combination of microfluidics with biomolecular and tissue engineering provides a new direction for simulating the human internal environments (Tian et al. 2019) and biomimicking in vivo environments (Xu et al. 2020).
4 Fabrication of nanoparticles using microfluidic technology
The nanomaterial formation process can be achieved using a microfluidic platform with accurate control and monitor over the nucleation and growth of nanoparticles (Jensen 2017). Zhang et al. (2017) provided an overview on mixing, flow dynamics, and mass and heat transfer in microreactors along with three strategies for scaling-up microreactors: parallel numbering-up, consecutive numbering-up, and scale-out. They also proposed a possible methodology to design microchemical systems. Wang et al. (2014) had developed a new strategy for scaling-up of a microsieve dispersion reactor. Deposition methods can be applied to create a thin coating on the particles surface and form a layered structure (Jocic 2016), it has been widely used in food (Wang et al. 2018), medicine (Dai et al. 2021), textiles (Yip and Luk 2016), materials (Zhang et al. 2014) and environmental protection. Zhao et al. (2020) successfully used a microfluidic interfacial synthetic method to fabricate covalent organic polymer microcapsules, and it has been widely used in wastewater treatment and environmental protection.
Microfluidics can produce particles with high purity, complex structures and a highly evenly distribution of particle sizes. Lian et al. (2020a, b) successfully applied microfluidics in fabricating TiO2 nanoparticles through surfactant wrapping sol–gel method, with TiO2 nanoparticles evenly distributed on the outer surface of the multiple wall carbon nanotubes (MWCNTs), and it has the ability to absorb Rhodamine B for wastewater treatment. Du et al. (2011) fabricated SiO2 nanoparticles using membrane microreactor, they suggested a gas–liquid precipitation reaction system which can guarantee the quality meanwhile saving the cost. The same group improved the synthesized process of BaSO4 by adding microbubbles created by the microreactor (Du et al. 2013). CaCO3 nanoparticles were fabricated by Wang et al. (2007) via integration of a microstructured reactor and the microfluidics to enhance mass transfer in the mixing process. It is feasible to apply microfluidic technology in the synthesis process of NiO nanoparticles. Most nanomaterial growth process involves three steps: nucleation, growth, and aging. As shown in Scheme 5, the microfluidic system enables accurate control over the flow conditions of different phases which are pumped into the microchannels, and the synthesis of nanomaterials can be achieved in the microreactors, with yielded products adopting different forms such as sphere-shaped, non-sphere-shaped particles and capsules, and fibers. The microfluidic platform provides a powerful mixing ability that can significantly stimulate the nucleation process. Besides, the stable fluid flow environment is suitable for the growth of large size particles and the formation of complex-shaped particles (Chen et al. 2021).
Another attribute making microfluidics competitive for synthesizing NiO nanoparticles is that as the synthesis reaction of nanoparticles is mainly chemical reaction dominant, less time-consuming procedures will be favorable, and this can benefit from rapid mixing of reactants which can be achieved using microfluidic platforms. Liu et al. (2014) used microfluidic reactors connected in series for fabricating AlPO4-5 which is a very important crystalline microporous aluminophosphate material, the single-phase AlPO4-5 was produced in 1 min. In comparison, it will take the conventional methods, such as the hydrothermal methods, a few days to fabrication the same products. Furthermore, the same group used a millimeter-sized continuous flow reactor to synthesize ZSM-5 (Liu et al. 2016). The reaction time has been successfully shortened into seconds.
The rapid development of microfluidic reactor makes it possible to fabricate complex structured nanoparticles with an accurate control over the process. Liang et al. (2020) developed a continuous mode microfluidic reactor, which can effectively enhance heat transfer and mixing. They successfully synthesized Ni0.6Mn0.2Co0.2CO3 with uniform particle size distribution, good thermal stability, and homogeneous transition metal distribution. As the NiO nanoparticle fabrication process normally involves high pressure, either the number of channels could be increased, the channel cross-section could be enlarged, or the flow rate in the channels could be increased to cope with a high mass flow rate (Kockmann et al. 2006). Ceramics microreactors can be used when high temperature is required for the formation of NiO nanoparticles.
Kawasaki et al. (2010a, b) used a T-shaped micromixer and successfully applied a continuous supercritical hydrothermal method to synthesize NiO nanoparticles with size of 20–50 nm. Meanwhile, when they decreased the tube diameter from 2.3 to 0.3 mm, the size of NiO nanoparticles changed from 54.3 to 20.1 nm. Sue et al. used a central collision type micromixer for synthesis of Fe2O3 and NiO nanoparticles. They investigated the effects of the key factors such as residence time and temperature in the synthesis process. They used Fe(NO)3 and Ni(NO)2 as reactants in the continuous hydrothermal method and successfully fabricated nickel ferrite with a diameter less than 5 nm (Sue et al. 2011a, b). They also used a 0.3 mm T-shape micromixer to successfully fabricate NiO nanoparticles with an average diameter of 16 nm. They used preheated water flow in the T-shape tube to provide the energy for the hydrothermal reaction shown in Scheme 6 (Sue et al. 2011a, b). Kiwamu et al. (2009) invented a new type of micromixer for synthesis of nanoparticles. As shown in Scheme 6B, they adopted a T-shaped micromixer with the tube inner diameter to be 0.18 mm. The completed flow system consists of the pumps, reactor and other devices. The micromixer was positioned at upstream of the reactor, the powerful mixing ability of the microfluidic mixer provided the highly uniform mixture solution. With hydrothermal reaction, they used this new mixer to achieve the fabrication of NiO nanoparticles with size of 18.2 nm and 23.5 nm, respectively. Kawasaki et al. (2010a, b) developed a new swirl micromixer for synthesis of NiO nanoparticles. Compared to a conventional T-shaped mixer, the average diameter of particles has been reduced from 47 to 20 nm with monodispersed size distribution. Moreover, computational fluid dynamic simulation results suggested that the swirl mixer can enable rapid and homogeneous mixing of fluids, therefore, producing high-quality nanoparticles. Zhao et al. (2015) constructed various NiO nanoarrays through Y-shaped needles, and they used a pump to drive the solution flow through the needles; then, the reactions occurred in microtubes to form nanosheets with a thickness of about 40 nm; they used the nanosheets to modify microchannels to enhance its ability to absorb protein. Xia et al. used the aerosol decomposition method in microreactor with inner diameter of 13 mm to successfully fabricate NiO nanoparticles with an average size of 10 nm. Furthermore, they investigated the influence of the addition of salts (Xia et al. 2002). Lu et al. (2019) reported the synthesis of NiO nanoparticles with size of 4–6 nm encapsulated in the carbonization of eggshell membrane using a green and facile approach for hydrogen evolution reaction electrocatalysts. The eggshell was applied as the microreactor. They also synthesized NiO/C nanocomposites with higher catalytic activity and smaller Tafel slope. The method can dispose eggshell waste and synthesize NiO nanoparticles simultaneously. Yoko et al. (2020) applied a T-shaped micromixer system to investigate the correlation between the mass transfer rate and Reynolds number and the kinetics under reaction control conditions, and evaluated the reaction kinetics of synthesis for the nickel nitrate to nickel oxide reaction that took place in a wide range of temperatures and pressures around the critical point of water. The NiO nanomaterials with diameter ranging in 10–40 nm had been fabricated. Xu (2017) investigated the most appropriate mixer design for a novel two-stage reactor by computational fluid dynamics modeling, constructed a two-stage continuous hydrothermal flow synthesis microreactor for synthesis of different metal oxides including NiO nanoplates with thickness of 3.4–54.3 nm. Michalska et al. (2021) deposited high-quality NiO film with thickness of 30 nm using a Tesla-valve micromixer. The key synthesis conditions for NiO nanomaterial formation using microfluidic reactors are summarized in Table 2 which demonstrates that the morphology and size of NiO nanomaterials can be well tuned via control over the flow rate and the dimensions of the microreactor systems.
In summary, microfluidics has the intrinsic superior ability in enhancing mixing, heat, and mass transfer and provides a stable chemical reaction environment, giving rise to these advantages as follows:
-
1.
Microfluidics enables high mixing efficiency even in laminar flow at low Reynolds number. It can accelerate the formation of precursor and shorten the reaction time significantly. Meanwhile, a microfluidic mixer, either in a passive or active way, can rapidly lead to homogeneous mixing of reactants, hence enhancing the quality of the final products.
-
2.
Stable flow environment can facilitate the reaction with very stable conditions. A microfluid reactor with a uniform fluid flow can provide a very stable chemical environment, which is suitable for NiO nanoparticle synthesis.
-
3.
Improved heat transfer ability can decrease the energy loss and make the reaction take place rapidly; most synthesis methods of NiO nanoparticles need heat treatment, microfluidic reactor accelerates the process of heat transfer and facilitates the nucleation and growth of nanoparticles.
The detailed comparison between conventional synthesis methods and microfluidic synthesis methods has been shown in Table 3. Compared with the conventional methods, the microfluidic technology provides a number of merits for nanoparticle synthesis, such as highly monodispersed particle size distribution, and more accurate control over the particle size and the particle loading efficiency by tuning the flow and reaction conditions, as well as the microreactor dimensions (Shepherd et al. 2021).
Owing to the exquisite control over the particle diameter, shape, and other properties in a tunable way, microfluidic systems have been demonstrated to be useful platforms for synthesizing nanoparticles of organic polymers, oxides, semiconductors, and metals as well as hybrid structures combining multiple materials and functionalities. Despite the rapid development of microfluidic technologies for nanomaterial synthesis, the novel approach still faces some challenges. First, it is important to identify and understand the mechanisms of channel clogging, such as constriction by deposition and accumulation on the walls or particle agglomeration, which remains challenging during the material synthesis process via the microfluidic approach. The microdevices will have to be cleaned thoroughly or even completely replaced by a new one. The surface modifications and flow modulation can potentially address these issues. For example, the contact between reactants and microreactor wall can be minimized using droplet flow or applying special coating on the reactor wall can efficiently isolate control the particle agglomeration (Zardi et al. 2021). Mitigation of the clogging in microchannels can also be achieved using pulsatile driven flow instead of static flow and this will significantly delay the formation of clogging (Dincau et al. 2022). Second, the throughput of microfluidic approach remains far from meeting the practical industrial demands. This challenge can be addressed by scale-up approach via increasing the number of devices running in a parallel mode. Third, the design of microfluidic chips requires substantial efforts to explore the optimal parameters, such as the number of microchambers and channel size (length, width, height). In addition, the fabrication process of microfluidic chips greatly relies on expensive laboratories and extremely sophisticated instruments (Niculescu et al. 2021a, b). Therefore, the development of microfluidic technology for nanomaterial synthesis requires more in-depth investigations and multidisciplinary integration of material science and fluid mechanics in particular. An effective way to address the fabrication challenge is to apply the commercially available off-shelf devices to build the micromixers and micropumps, this will obviate the need of photolithography method and clean room; therefore, it will significantly decrease the fabrication cost.
5 Photocatalytic applications of NiO nanomaterials
Photocatalysis is capable of photodegradation of the organic contamination, and the light absorption involved process can accelerate a photo-reaction by a catalyst, which has no change in itself and is not consumed during the chemical reactions. Photocatalysis is an eco-friendly technique to address energy and environmental challenges (Koe et al. 2020). Semiconductor metal oxides such as NiO (Sun et al. 2018), TiO2 (Wang et al. 2020), MoS2 (Chandrabose et al. 2021), ZnO (Abdullah et al. 2020), and Al2O3 (Li et al. 2016) can be activated by exposure to UV–visible light appropriate to its bandgap energy to catalyze a redox reaction at its surface. As shown in Scheme 7, the electrons of a semiconductor are excited from the valence band (VB) to the conduction band (CB) by photoluminescence. The photoelectrons and holes subsequently experience spatial separation and transfer to their acceptors. The oxidation and reduction reactions will require more positive hole potential and more negative CB potential to favor the reactions (Liu et al. 2020; Byrne et al. 2015). Recently, NiO have been used as photocatalyst to degrade organic dyes, such as methyl orange (MO) and methylene blue (MB) (Ma et al. 2021). The electron and hole formation will contribute to oxidize/reduce organic pollutants, while the holes of NiO surface will adsorb and trap water molecules, with the causation of hydroxyl radicals oxidation. At the same time, the oxygen molecules produce anionic superoxide radicals, which will degrade MB and MO to CO2, and H2O (Sabouri et al. 2020a, b).
Among the transition metal oxides, TiO2 is a very good photocatalyst and has been used in many photocatalytic systems, due to the high photoconversion efficiency, high stability, and high specific surface area (Shrestha et al. 2010). However, the efficiency of TiO2 is limited by wideband energy which is closed to 3 eV, and fast recombination of photo-generated electrons and holes, indicating only about 5% of the solar energy spectrum can be used (Mohapatra et al. 2007; Jasim et al. 2020). NiO is a p-type semiconductor with low cost, high optical transmittance, high specific surface area, and can be shaped into complex structures (Chinnappan et al. 2018). It can form a p–n junction material with an n-type semiconductor. The electric field arising from the p–n junction via the combination of p-type and n-type semiconductors can significantly restrain the recombination of photo-generated electrons and holes. NiO nanoparticles can significantly enhance the photocatalytic hydrogen evolution from aqueous methanol because NiO nanoparticles exhibit high activity attributed to ease of trapping photo-generated electrons (Wu et al. 2014; Faisal et al. 2018); therefore, the combination of NiO and TiO2 can significantly increase the efficiency of photocatalyst. This approach can also be extended to other n-type semiconductors, such as SnO2 (Suvith et al. 2020) and ZnO (Abdullah et al. 2020). Hu and Teng (2010) loaded NiO on n-type NaTaO3 to trigger the water-splitting reaction under UV light exposure, the final production rate of H2 reached 9000 μmol g−1 h−1. Sun et al. (2021) used p–n heterojunction between NiO and Cadmium Sulfide (CdS) to induce hydrogen spillover effect, remarkably improving the H2 evolution performance with production rate to be 243.9 mmol/g/h. In addition, NiO as cocatalyst not only can cooperate with a metallic oxide but also with another catalyst to induce a synergetic effect. Liu et al. (2018) loaded NiO on the surface of g-C3N4, the created C–O–Ni bond significantly improved hydrogen evolution and stability, the inner electric field created by heterojunctions can drive the migration of the photo-generated electrons through the C–O–Ni linkage from g-C3N4 to NiO, which facilitated the charge separation. Khatri and Rana (2020) adopted Fe doping on the NiO nanoparticles through the chemical co-precipitation method to form photocatalysis dyes. It largely increased the photocatalysis efficiency and can be considered as promising photocatalysts for organic pollutant treatment. Lin et al. (2018) applied two-dimensional amorphous NiO nanostructure instead of nanocrystal NiO particles in solar H2 evolution, the NiO nanoparticles can work alone as a photocatalyst and functions stably and efficiently in the chemical reactions. Table 4 shows the representative applications using NiO as the photocatalysts or co-photocatalysts, and it demonstrates that the addition of NiO can significantly improve the photocatalytic efficiency. NiO nanoparticles with high photocatalytic activities can be used as self-cleaning transparent nanocoating for solar cells to prevent the deposition of dust and air pollutants on the solar cells over time. It can be envisioned that NiO nanoparticles can find more diverse applications, especially in environmental fields.
6 Conclusions and outlook
NiO nanoparticles have attracted increasing attention because of unique chemical and physical properties. We provide a comprehensive review of the state-of-art synthesis methods of NiO nanoparticles, combining both the conventional methods and the latest advances in applying microfluidics as a promising alternative for the highly efficient synthesis of NiO nanoparticles.
The liquid-phase method is the most widely used method in industrial production and lab-scale synthesis. The size of nanoparticles is more uniform and controllable than the solid-phase and vapor-phase methods. With the development of microfluidic technology, the traditional synthesis method can be improved to achieve a higher production rate, expanded range of applications, and higher product quality. Moreover, the integration of multiple functions on the chip-based microfluidic systems shows a promise for the holistic realization of nanoparticles with demanded optical, electronic, and catalytic properties by controlling the channel geometries and flow conditions. Nevertheless, the novel approach still faces some challenges. First, it is important to identify and understand the mechanisms of channel clogging, and mitigate the problem by surface modifications and flow modulation. Second, the throughput of microfluidic approach remains far from meeting the practical industrial demands. This challenge can be addressed by scale-up approach via increasing the number of devices running in a parallel mode. Third, the fabrication process of microfluidic chips greatly relies on expensive laboratories and extremely sophisticated instruments, and this can be addressed by applying the commercially available off-shelf devices to build the micromixers and micropumps in the microreactor systems. Accuracy and repeatability are also very crucial, and it is expected that automated apparatus should be used as much as possible without much intervention from human operators to ensure the quality consistency of NiO nanoparticles during the production process from one batch to another batch. More efforts are, therefore, required in the future to develop microfluidics based technologies for NiO nanoparticle synthesis in a more cost-effective and energy-efficient way, as well as exploring their photocatalytic capabilities for environmental remediation to more expanded extent.
References
Abdullah H, Gultom NS, Kuo D-H (2020) Depletion-Zone size control of p-type NiO/n-type Zn(O, S) nanodiodes on high-surface-area SiO2 nanoparticles as a strategy to significantly enhance hydrogen evolution rate. Appl Catal b: Environ 261:118223
Abedini-Nassab R, Pouryosef Miandoab M, Şaşmaz M (2021) Microfluidic synthesis, control, and sensing of magnetic nanoparticles: a review. Micromachines 12:768
Adschiri T, Kanazawa K, Arai K (1992) Rapid and continuous hydrothermal synthesis of boehmite particles in subcritical and supercritical water. J Am Ceram Soc 75:2615–2618
Arumugam J, Thambidurai S, Suresh S, Selvapandiyan M, Kandasamy M, Pugazhenthiran N, Karthick Kumar S, Muneeswaran T, Quero F (2021) Green synthesis of zinc oxide nanoparticles using Ficus carica leaf extract and their bactericidal and photocatalytic performance evaluation. Chem Phys Lett 783:139040
Bahadur J, Sen D, Mazumder S, Ramanathan S (2008) Effect of heat treatment on pore structure in nano-crystalline NiO: a small angle neutron scattering study. J Solid State Chem 181:1227–1235
Barakat A, Al-Noaimi M, Suleiman M, Aldwayyan AS, Hammouti B, Hadda TB, Haddad SF, Boshaala A, Warad I (2013) One step synthesis of NiO nanoparticles via solid-state thermal decomposition at low-temperature of novel aqua(2,9-dimethyl-1,10-phenanthroline)NiCl2 complex. Int J Mol Sci 14:23941–23954
Bonomo M (2018) Synthesis and characterization of NiO nanostructures: a review. J Nanopart Res 20:222
Boodaghi M, Shamloo A (2020) A comparison of different geometrical elements to model fluid wicking in paper-based microfluidic devices. AIChE J 66:e16756
Bragheri F, Martinez Vazquez R, Osellame R (2016) Chapter 12.3 - Microfluidics. In: Baldacchini T (ed) Three-dimensional microfabrication using two-photon polymerization. William Andrew Publishing, Oxford
Byrne JA, Dunlop PSM, Hamilton JWJ, Fernández-Ibáñez P, Polo-López I, Sharma PK, Vennard ASM (2015) A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 20:5574–5615
Campbell SB, Wu Q, Yazbeck J, Liu C, Okhovatian S, Radisic M (2020) Beyond polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.0c00640
Cao S, Peng L, Han T, Liu B, Zhu D, Zhao C, Xu J, Tang Y, Wang J, He S (2020) Hydrothermal synthesis of nanoparticles-assembled NiO microspheres and their sensing properties. Physica e: Low-Dimensional Syst Nanostructures 118:113655
Chandrabose G, Dey A, Gaur SS, Pitchaimuthu S, Jagadeesan H, Braithwaite NSJ, Selvaraj V, Kumar V, Krishnamurthy S (2021) Removal and degradation of mixed dye pollutants by integrated adsorption-photocatalysis technique using 2-D MoS2/TiO2 nanocomposite. Chemosphere 279:130467
Chen T, Yin S, Wu J (2021) Nanomaterials meet microfluidics: improved analytical methods and high-throughput synthetic approaches. TrAC, Trends Anal Chem 142:116309
Chinnappan A, Dongxiao J, Jayathilaka WADM, Baskar C, Qin X, Ramakrishna S (2018) Facile synthesis of electrospun C@NiO/Ni nanofibers as an electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy 43:15217–15224
Choi C-H, Lee H, Abbaspourrad A, Kim JH, Fan J, Caggioni M, Wesner C, Zhu T, Weitz DA (2016a) Triple emulsion drops with an ultrathin water layer: high encapsulation efficiency and enhanced cargo retention in microcapsules. Adv Mater 28:3340–3344
Choi C-H, Wang H, Lee H, Kim JH, Zhang L, Mao A, Mooney DJ, Weitz DA (2016b) One-step generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell. Lab Chip 16:1549–1555
Crichton RR (2012) Chapter 2—basic coordination chemistry for biologists. In: Crichton RR (ed) Biological inorganic chemistry, 2nd edn. Elsevier, Oxford
Dai X, Ruan J, Guo Y, Sun Z, Liu J, Bao X, Zhang H, Li Q, Ye C, Wang X, Zhao C-X, Zhou F, Sheng J, Chen D, Zhao P (2021) Enhanced radiotherapy efficacy and induced anti-tumor immunity in HCC by improving hypoxia microenvironment using oxygen microcapsules. Chem Eng J 422:130109
Deng X, Chen Z (2004) Preparation of nano-NiO by ammonia precipitation and reaction in solution and competitive balance. Mater Lett 58:276–280
Dincau BM, Tang C, Dressaire E, Sauret A (2022) Clog mitigation in a microfluidic array via pulsatile flows. Soft Matter. https://doi.org/10.1039/D2SM00013J
Du L, Tan J, Wang K, Lu Y, Luo G (2011) Controllable preparation of SiO2 nanoparticles using a microfiltration membrane dispersion microreactor. Ind Eng Chem Res 50:8536–8541
Du L, Wang YJ, Lu YC, Luo GS (2013) Process intensification of BaSO4 nanoparticle preparation with agitation of microbubbles. Powder Technol 247:60–68
El-Shamy AG (2021) New nano-composite based on carbon dots (CDots) decorated magnesium oxide (MgO) nano-particles (CDots@MgO) sensor for high H2S gas sensitivity performance. Sens Actuators, B Chem 329:129154
Faisal M, Harraz FA, Ismail AA, El-Toni AM, Al-Sayari SA, Al-Hajry A, Al-Assiri MS (2018) Novel mesoporous NiO/TiO2 nanocomposites with enhanced photocatalytic activity under visible light illumination. Ceram Int 44:7047–7056
Faustino V, Catarino SO, Lima R, Minas G (2016) Biomedical microfluidic devices by using low-cost fabrication techniques: a review. J Biomech 49:2280–2292
Furtado D, Björnmalm M, Ayton S, Bush AI, Kempe K, Caruso F (2018) Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater 30:1801362
Gale BK, Jafek AR, Lambert CJ, Goenner BL, Moghimifam H, Nze UC, Kamarapu SK (2018) A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 3:60
Ghosh P, Senthilarasu S, Nixon T, Krishnamurthy S (2016) Influence of nanostructures in perovskite solar cells. In: Reference module in materials science and materials engineering. Elsevier
Greenham NC, Peng X, Alivisatos AP (1997) Charge separation and transport in conjugated polymer/cadmium selenide nanocrystal composites studied by photoluminescence quenching and photoconductivity. Synth Met 84:545–546
Han DY, Yang HY, Shen CB, Zhou X, Wang FH (2004) Synthesis and size control of NiO nanoparticles by water-in-oil microemulsion. Powder Technol 147:113–116
Hasan I, Rana, A (2021) A review on in SITU green synthesis of titanium dioxide nanoparticles and their photocatalytic activities. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2021.04.287
Hosny NM (2011) Synthesis, characterization and optical band gap of NiO nanoparticles derived from anthranilic acid precursors via a thermal decomposition route. Polyhedron 30:470–476
Hu C-C, Teng H (2010) Structural features of p-type semiconducting NiO as a co-catalyst for photocatalytic water splitting. J Catal 272:1–8
Hwang J, Cho YH, Park MS, Kim BH (2019) Microchannel fabrication on glass materials for microfluidic devices. Int J Precis Eng Manuf 20:479–495
Imran Din M, Rani A (2016) Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: a green adeptness. Int J Anal Chem 2016:3512145
Irwin MD, Buchholz DB, Hains AW, Chang RPH, Marks TJ (2008) p-Type semiconducting nickel oxide as an efficiency-enhancing anode interfacial layer in polymer bulk-heterojunction solar cells. Proc Natl Acad Sci 105:2783–2787
Ita K (2020) Chapter 6—Microemulsions. In: Ita K (ed) Transdermal drug delivery. Academic Press, Cambridge
Jaji N-D, Lee HL, Hussin MH, Akil HM, Zakaria MR, Othman MBH (2020) Advanced nickel nanoparticles technology: from synthesis to applications. Nanotechnol Rev 9:1456–1480
James M, Revia RA, Stephen Z, Zhang M (2020) Microfluidic synthesis of iron oxide nanoparticles. Nanomaterials 10:2113
Jasim MM, Azeez Dakhil OA, Abdullah HI (2020) Synthesis of NiO/TNTs p-n junction for highly photocatalysis activity under sunlight irradiation. Solid State Sci 107:106342
Jáuregui AL, Siller HR, Rodríguez CA, Elías-Zúñiga A (2010) Evaluation of micromechanical manufacturing processes for microfluidic devices. Int J Adv Manuf Tech 48:963–972
Jayathilaka WADM, Qi K, Qin Y, Chinnappan A, Serrano-García W, Baskar C, Wang H, He J, Cui S, Thomas SW, Ramakrishna S (2019) Significance of nanomaterials in wearables: a review on wearable actuators and sensors. Adv Mater 31:1805921
Jensen KF (2017) Flow chemistry—microreaction technology comes of age. AIChE J 63:858–869
Jocic D (2016) 14—Smart coatings for comfort in clothing. In: Hu J (ed) Active coatings for smart textiles. Woodhead Publishing, Sawston
Karatutlu A, Barhoum A, Sapelkin A (2018) Chapter 1—Liquid-phase synthesis of nanoparticles and nanostructured materials. In: Barhoum A, Salam A, Makhlouf H (eds) Emerging applications of nanoparticles and architecture nanostructures. Elsevier, Amsterdam
Kate RS, Khalate SA, Deokate RJ (2018) Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: a review. J Alloy Compd 734:89–111
Kawasaki S-I, Sue K, Ookawara R, Wakashima Y, Suzuki A (2010a) Development of novel micro swirl mixer for producing fine metal oxide nanoparticles by continuous supercritical hydrothermal method. J Oleo Sci 59:557–562
Kawasaki S-I, Sue K, Ookawara R, Wakashima Y, Suzuki A, Hakuta Y, Arai K (2010b) Engineering study of continuous supercritical hydrothermal method using a T-shaped mixer: experimental synthesis of NiO nanoparticles and CFD simulation. J Supercrit Fluids 54:96–102
Khatri A, Rana PS (2020) Visible light assisted photocatalysis of Methylene Blue and Rose Bengal dyes by iron doped NiO nanoparticles prepared via chemical co-precipitation. Physica B 579:411905
Kiwamu S, Akira S, Yukiya H, Hiromichi H, Kunio A, Yoshihiro T, Satoshi Y, Takeshi F (2009) Hydrothermal-reduction synthesis of Ni nanoparticles by superrapid heating using a micromixer. Chem Lett 38:1018–1019
Kockmann N, Kiefer T, Engler M, Woias P (2006) Convective mixing and chemical reactions in microchannels with high flow rates. Sens Actuators, B Chem 117:495–508
Koe WS, Lee JW, Chong WC, Pang YL, Sim LC (2020) An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ Sci Pollut Res Int 27:2522–2565
Köhler JM, Li S, Knauer A (2013) Why is micro segmented flow particularly promising for the synthesis of nanomaterials? Chem Eng Technol 36:887–899
Kumar S, Das J (2021) Synthesis, structural and magnetic properties of NiO nanospheres and rGO-NiO nanocomposites and observing magnetocaloric effect in rGO-NiO nanocomposites. Mater Sci Eng, B 265:115007
Kumar A, Han SS (2019) Chapter 13—Bioactive glass–based composites in bone tissue engineering: synthesis, processing, and cellular responses. In: Holban A-M, Grumezescu AM (eds) Materials for biomedical engineering. Elsevier, Amsterdam
Kung C-T, Gao H, Lee C-Y, Wang Y-N, Dong W, Ko C-H, Wang G, Fu L-M (2020) Microfluidic synthesis control technology and its application in drug delivery, bioimaging, biosensing, environmental analysis and cell analysis. Chem Eng J 399:125748
Leung WW-F, Ren Y (2013) Crossflow and mixing in obstructed and width-constricted rotating radial microchannel. Int J Heat Mass Transf 64:457–467
Leung WW-F, Ren Y (2014) Scale-up on mixing in rotating microchannel under subcritical and supercritical operating modes. Int J Heat Mass Transf 77:157–172
Li B, Yuan H, Yang P, Yi B, Zhang Y (2016) Fabrication of the composite nanofibers of NiO/γ-Al2O3 for potential application in photocatalysis. Ceram Int 42:17405–17409
Li L, Xiao R, Tao X, Wu Y, Jiang L, Zhang Z, Qing Y (2021) Free-standing electrodes via coupling nanostructured Ni–NiO with hierarchical wood carbon for high-performance supercapacitors and Ni–Zn batteries. J Power Sources 491:229618
Lian Z, Ren Y, He J, Chen GZ, Koh KS (2018) Microfluidic fabrication of porous polydimethylsiloxane microparticles for the treatment of toluene-contaminated water. Microfluid Nanofluid 22:145
Lian Z, Chan Y, Luo Y, Yang X, Koh KS, Wang J, Chen GZ, Ren Y, He J (2020a) Microfluidic formation of highly monodispersed multiple cored droplets using needle-based system in parallel mode. Electrophoresis 41:891–901
Lian Z, Wei C, Gao B, Yang X, Chan Y, Wang J, Chen GZ, Koh KS, Shi Y, Yan Y, Ren Y, He J, Liu F (2020b) Synergetic treatment of dye contaminated wastewater using microparticles functionalized with carbon nanotubes/titanium dioxide nanocomposites. RSC Adv 10:9210–9225
Liang H, Yuan S, Shi L, Zhao Y, Wang Z, Zhu J (2020) Highly-ordered microstructure and well performance of LiNi0.6Mn0.2Co0.2O2 cathode material via the continuous microfluidic synthesis. Chem Eng J 394:124846
Lim CN, Koh KS, Ren Y, Chin JK, Shi Y, Yan Y (2017) Analysis of liquid-liquid droplets fission and encapsulation in single/two layer microfluidic devices fabricated by xurographic method. Micromachines 8:49
Lin Z, Du C, Yan B, Wang C, Yang G (2018) Two-dimensional amorphous NiO as a plasmonic photocatalyst for solar H2 evolution. Nat Commun 9:4036
Liu SF, Wu CY, Han XZ (2003) Preparation of nanoscale NiO powders by polymer-network gel process. Chin J Inorg Chem 19(6):624–626
Liu Z, Wakihara T, Nishioka D, Oshima K, Takewaki T, Okubo T (2014) Ultrafast continuous-flow synthesis of crystalline microporous aluminophosphate AlPO4-5. Chem Mater 26:2327–2331
Liu Z, Okabe K, Anand C, Yonezawa Y, Zhu J, Yamada H, Endo A, Yanaba Y, Yoshikawa T, Ohara K, Okubo T, Wakihara T (2016) Continuous flow synthesis of ZSM-5 zeolite on the order of seconds. Proc Natl Acad Sci 113:14267
Liu F, Sang Y, Ma H, Li Z, Gao Z (2017) Nickel oxide as an effective catalyst for catalytic combustion of methane. J Nat Gas Sci Eng 41:1–6
Liu J, Jia Q, Long J, Wang X, Gao Z, Gu Q (2018) Amorphous NiO as co-catalyst for enhanced visible-light-driven hydrogen generation over g-C3N4 photocatalyst. Appl Catal B 222:35–43
Liu B, Wu H, Parkin IP (2020) New insights into the fundamental principle of semiconductor photocatalysis. ACS Omega 5:14847–14856
Lu S, Hummel M, Gu Z, Gu Y, Cen Z, Wei L, Zhou Y, Zhang C, Yang C (2019) Trash to treasure: a novel chemical route to synthesis of NiO/C for hydrogen production. Int J Hydrogen Energy 44:16144–16153
Lv P, Zhao H, Zeng Z, Gao C, Tianhou Z (2015) Self-assembled three-dimensional hierarchical NiO nano/microspheres as high-performance anode material for lithium ion batteries. Appl Surf Sci 329:301–305
Ma X, Sheikholeslami M, Jafaryar M, Shafee A, Nguyen-Thoi T, Li Z (2020) Solidification inside a clean energy storage unit utilizing phase change material with copper oxide nanoparticles. J Clean Prod 245:118888
Ma X, Liu X, Zhang X, Piao C, Liu Z, Fang D, Wang J (2021) Construction of dual Z-scheme NiO/NiFe2O4/Fe2O3 photocatalyst via incomplete solid state chemical combustion reactions for organic pollutant degradation with simultaneous hydrogen production. Int J Hydrogen Energy 46:31659–31673
Malecha K, Jasińska L, Grytsko A, Drzozga K, Słobodzian P, Cabaj J (2019) Monolithic microwave-microfluidic sensors made with low temperature Co-fired ceramic (LTCC) technology. Sensors 19:577
Malik MA, Wani MY, Hashim MA (2012) Microemulsion method: a novel route to synthesize organic and inorganic nanomaterials: 1st nano update. Arab J Chem 5:397–417
Manz A, Graber N, Widmer HM (1990) Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens Actuators, B Chem 1:244–248
Michalska M, Surmiak MA, Maasoumi F, Senevirathna DC, Chantler P, Li H, Li B, Zhang T, Lin X, Deng H, Chandrasekaran N, Peiris TAN, Rietwyk KJ, Chesman ASR, Alan T, Vak D, Bach U, Jasieniak JJ (2021) Microfluidic processing of ligand-engineered NiO nanoparticles for low-temperature hole-transporting layers in perovskite solar cells. Solar RRL 5:2100342
Mohapatra SK, Misra M, Mahajan VK, Raja KS (2007) Design of a highly efficient photoelectrolytic cell for hydrogen generation by water splitting: application of TiO2-xCx nanotubes as a photoanode and Pt/TiO2 nanotubes as a cathode. J Phys Chem C 111:8677–8685
Mohseni Meybodi S, Hosseini SA, Rezaee M, Sadrnezhaad SK, Mohammadyani D (2012) Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method. Ultrason Sonochem 19:841–845
Moravec P, Smolík J, Keskinen H, Mäkelä J, Bakardjieva S, Levdansky V (2011) NiOx nanoparticle synthesis by chemical vapor deposition from nickel acetylacetonate. Mater Sci Appl 2:258–264
Munkaila S, Bentley J, Schimmel K, Ahamad T, Alshehri SM, Bastakoti BP (2021) Polymer directed synthesis of NiO nanoflowers to remove pollutant from wastewater. J Mol Liq 324:114676
Najjar M, Hosseini HA, Masoudi A, Hashemzadeh A, Darroudi M (2020) Preparation of tin oxide (IV) nanoparticles by a green chemistry method and investigation of its role in the removal of organic dyes in water purification. Res Chem Intermed 46:2155–2168
Najjar M, Hosseini HA, Masoudi A, Sabouri Z, Mostafapour A, Khatami M, Darroudi M (2021) Green chemical approach for the synthesis of SnO2 nanoparticles and its application in photocatalytic degradation of Eriochrome Black T dye. Optik 242:167152
Niculescu A-G, Chircov C, Bîrcă AC, Grumezescu AM (2021a) Nanomaterials synthesis through microfluidic methods: an updated overview. Nanomaterials (basel, Switzerland) 11:864
Niculescu A-G, Chircov C, Bîrcă AC, Grumezescu AM (2021b) Fabrication and applications of microfluidic devices: a review. Int J Mol Sci 22:2011
Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT (2020) Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem 92:150–168
Oh SW, Bang HJ, Bae YC, Sun Y-K (2007) Effect of calcination temperature on morphology, crystallinity and electrochemical properties of nano-crystalline metal oxides (Co3O4, CuO, and NiO) prepared via ultrasonic spray pyrolysis. J Power Sources 173:502–509
Palmas S, Mais L, Mascia M, Vacca A (2021) Trend in using TiO2 nanotubes as photoelectrodes in PEC processes for wastewater treatment. Curr Opin Electrochem 28:100699
Pan T, Deng H, Kang S, Zhang Y, Lian W, Zhang C, He H (2021) Facile homogeneous precipitation method to prepare MnO2 with high performance in catalytic oxidation of ethyl acetate. Chem Eng J 417:129246
Peng X (2002) Green chemical approaches toward high-quality semiconductor nanocrystals. Chemistry A European Journal 8:334–339
Pooyandeh S, Shahidi S, Khajehnezhad A, Mongkholrattanasit R (2021) In situ deposition of NiO nano particles on cotton fabric using sol–gel method- photocatalytic activation properties. J Market Res 12:1–14
Ren Y, Leung WWF (2016) Numerical investigation of cell encapsulation for multiplexing diagnostic assays using novel centrifugal microfluidic emulsification and separation platform. Micromachines 7:17
Ren K, Zhou J, Wu H (2013) Materials for microfluidic chip fabrication. Acc Chem Res 46:2396–2406
Ren Y, Liu Z, Shum HC (2015) Breakup dynamics and dripping-to-jetting transition in a Newtonian/shear-thinning multiphase microsystem. Lab Chip 15:121–134
Restaino SM, White IM (2019) A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal Chim Acta 1060:17–29
Rinaldi-Montes N, Gorria P, Martínez-Blanco D, Fuertes AB, Puente-Orench I, Olivi L, Blanco JA (2016a) Size effects on the Néel temperature of antiferromagnetic NiO nanoparticles. AIP Adv 6:056104
Rinaldi-Montes N, Gorria P, Martínez-Blanco D, Fuertes AB, Puente Orench I, Olivi L, Blanco J (2016b) Size effects on the Néel temperature of antiferromagnetic NiO nanoparticles. AIP Adv 6:056104
Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M (2020a) Tragacanth-mediate synthesis of NiO nanosheets for cytotoxicity and photocatalytic degradation of organic dyes. Bioprocess Biosyst Eng 43:1209–1218
Sabouri Z, Sabouri M, Amiri MS, Khatami M, Darroudi M (2020b) Plant-based synthesis of cerium oxide nanoparticles using Rheum turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater Tech. https://doi.org/10.1080/10667857.2020.1863573
Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M (2021) Green-based bio-synthesis of nickel oxide nanoparticles in Arabic gum and examination of their cytotoxicity, photocatalytic and antibacterial effects. Green Chem Lett Rev 14:404–414
Santhi A, Umadevi M, Ramakrishnan V, Radhakrishnan P, Nampoori VPN (2004) Effect of silver nano particles on the fluorescence quantum yield of Rhodamine 6G determined using dual beam thermal lens method. Spectrochim Acta Part A Mol Biomol Spectrosc 60:1077–1083
Schrimpf M, Esteban J, Rösler T, Vorholt AJ, Leitner W (2019) Intensified reactors for gas-liquid-liquid multiphase catalysis: from chemistry to engineering. Chem Eng J 372:917–939
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8:7278–7308
Shepherd SJ, Issadore D, Mitchell MJ (2021) Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 274:120826
Shirk K, Steiner C, Kim JW, Marquez M, Martinez CJ (2013) Assembly of colloidal silica crystals inside double emulsion drops. Langmuir 29:11849–11857
Shrestha NK, Yang M, Nah Y-C, Paramasivam I, Schmuki P (2010) Self-organized TiO2 nanotubes: Visible light activation by Ni oxide nanoparticle decoration. Electrochem Commun 12:254–257
Singh A, Malek CK, Kulkarni SK (2010) Development in microreactor technology for nanoparticle synthesis. Int J Nanosci 09:93–112
Sree GS, Botsa SM, Reddy BJM, Ranjitha KVB (2020) Enhanced UV–Visible triggered photocatalytic degradation of Brilliant green by reduced graphene oxide based NiO and CuO ternary nanocomposite and their antimicrobial activity. Arab J Chem 13:5137–5150
Stickler BA, Hornberger K, Kim MS (2021) Quantum rotations of nanoparticles. Nat Rev Phys 3:589–597
Stroock AD (2008) Chapter 17 - MICROFLUIDICS. In: Ligler FS, Taitt CR (eds) Optical biosensors, 2nd edn. Elsevier, Amsterdam
Sudhasree S, Shakila Banu A, Brindha P, Kurian GA (2014) Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol Environ Chem 96:743–754
Sue K, Aoki M, Sato T, Nishio-Hamane D, Kawasaki S-I, Hakuta Y, Takebayashi Y, Yoda S, Furuya T, Sato T, Hiaki T (2011a) Continuous hydrothermal synthesis of nickel ferrite nanoparticles using a central collision-type micromixer: effects of temperature, residence time, metal salt molality, and NaOH addition on conversion, particle size, and crystal phase. Ind Eng Chem Res 50:9625–9631
Sue K, Kawasaki S-I, Suzuki M, Hakuta Y, Hayashi H, Arai K, Takebayashi Y, Yoda S, Furuya T (2011b) Continuous hydrothermal synthesis of Fe2O3, NiO, and CuO nanoparticles by superrapid heating using a T-type micro mixer at 673K and 30MPa. Chem Eng J 166:947–953
Sun X, Zhang Y, Li P, Guo D, Zi H, Guo J, Li Y (2018) Heterostructure nano-NiO/BiOCl composites with advanced adsorption and photocatalytic performance for organic dyes. J Alloy Compd 736:22–28
Sun G, Xiao B, Shi J-W, Mao S, He C, Ma D, Cheng Y (2021) Hydrogen spillover effect induced by ascorbic acid in CdS/NiO core-shell p-n heterojunction for significantly enhanced photocatalytic H2 evolution. J Colloid Interface Sci 596:215–224
Suvith VS, Devu VS, Philip D (2020) Facile synthesis of SnO2/NiO nano-composites: structural, magnetic and catalytic properties. Ceram Int 46:786–794
Tao D, Wei F (2004) New procedure towards size-homogeneous and well-dispersed nickel oxide nanoparticles of 30 nm. Mater Lett 58:3226–3228
Thota S, Kumar J (2007) Sol–gel synthesis and anomalous magnetic behaviour of NiO nanoparticles. J Phys Chem Solids 68:1951–1964
Tian C, Tu Q, Liu W, Wang J (2019) Recent advances in microfluidic technologies for organ-on-a-chip. TrAC, Trends Anal Chem 117:146–156
Wang Y, Zhu J, Yang X, Lu L, Wang X (2005) Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochim Acta 437:106–109
Wang K, Wang YJ, Chen GG, Luo GS, Wang JD (2007) Enhancement of mixing and mass transfer performance with a microstructure minireactor for controllable preparation of CaCO3 nanoparticles. Ind Eng Chem Res 46:6092–6098
Wang K, Lu Y, Luo G (2014) Strategy for scaling-up of a microsieve dispersion reactor. Chem Eng Technol 37:2116–2122
Wang Y, Jia J, Shao J, Shu X, Ren X, Wu B, Yan Z (2018) Preservative effects of allicin microcapsules on daily foods. LWT 98:225–230
Wang D, Li Q, Miao W, Liu Y, Du N, Mao S (2020) One-pot synthesis of ultrafine NiO loaded and Ti3+ in-situ doped TiO2 induced by cyclodextrin for efficient visible-light photodegradation of hydrophobic pollutants. Chem Eng J 402:126211
Wang X, Han T, Sun Y, Geng H, Li B, Dai H (2021) Effects of nano metal oxide particles on activated sludge system: Stress and performance recovery mechanism. Environ Pollut 285:117408
Wu Z, Wang Y, Sun L, Mao Y, Wang M, Lin C (2014) An ultrasound-assisted deposition of NiO nanoparticles on TiO2 nanotube arrays for enhanced photocatalytic activity. Journal of Materials Chemistry A 2:8223–8229
Wu J, He Z, Chen Q, Lin J-M (2016a) Biochemical analysis on microfluidic chips. TrAC, Trends Anal Chem 80:213–231
Wu J, Wang X, Lin Y, Zheng Y, Lin J-M (2016b) Peroxynitrous-acid-induced chemiluminescence detection of nitrite based on Microfluidic chip. Talanta 154:73–79
Wuled Lenggoro I, Itoh Y, Iida N, Okuyama K (2003) Control of size and morphology in NiO particles prepared by a low-pressure spray pyrolysis. Mater Res Bull 38:1819–1827
Xia YS, Liu JT, Lu QL, Bao DC (2015) Preparation and characterization of high surface area mesoporous nickel oxide. Chem Res Appl 27(02):127–132
Xia B, Lenggoro IW, Okuyama K (2002) Nanoparticle separation in salted droplet microreactors. Chem Mater 14:2623–2627
Xu X, Jiang Z, Wang J, Ren Y, Wu A (2020) Microfluidic applications on circulating tumor cell isolation and biomimicking of cancer metastasis. Electrophoresis 41:933–951
Yang G, Terzis A, Zarikos I, Hassanizadeh SM, Weigand B, Helmig R (2019) Internal flow patterns of a droplet pinned to the hydrophobic surfaces of a confined microchannel using micro-PIV and VOF simulations. Chem Eng J 370:444–454
Yang J, Han W, Ma J, Wang C, Shimanoe K, Zhang S, Sun Y, Cheng P, Wang Y, Zhang H, Lu G (2021) Sn doping effect on NiO hollow nanofibers based gas sensors about the humidity dependence for triethylamine detection. Sens Actuators, B Chem 340:129971
Yew M, Ren Y, Koh KS, Sun C, Snape C (2019) A review of state-of-the-art microfluidic technologies for environmental applications: detection and remediation. Glob Chall 3:1800060
Yip J, Luk MYA (2016) 3 - Microencapsulation technologies for antimicrobial textiles. In: Sun G (ed) Antimicrobial textiles. Woodhead Publishing, Sawston
Yoko A, Tanaka Y, Seong G, Hojo D, Tomai T, Adschiri T (2020) Mixing and solvent effects on kinetics of supercritical hydrothermal synthesis: reaction of nickel nitrate to nickel oxide. The Journal of Physical Chemistry C 124:4772–4780
Zhang W (2014) Nanoparticle aggregation: principles and modeling. Adv Exp Med Biol 811:19–43
Zhang W, Liao LP, Zhao Y (2014) 12 - Incorporating microcapsules in smart coatings for corrosion protection of steel. In: Makhlouf ASH (ed) Handbook of smart coatings for materials protection. Woodhead Publishing, Sawston
Zhang J, Wang K, Teixeira AR, Jensen KF, Luo G (2017) Design and scaling up of microchemical systems: a review. Annu Rev Chem Biomol Eng 8:285–305
Zhao D, Wang G, He Z, Wang H, Zhang Q, Li Y (2015) Controllable construction of micro/nanostructured NiO arrays in confined microchannels via microfluidic chemical fabrication for highly efficient and specific absorption of abundant proteins. Journal of Materials Chemistry B 3:4272–4281
Zhao Y, Liao Z, Xiang Z (2019) Microfluidics for synthesis and morphology control of hierarchical porous covalent organic polymer monolith. Chem Eng Sci 195:801–809
Zhao Y, Zhang M, Wen X, Xiang Z (2020) Microfluidic interface boosted synthesis of covalent organic polymer capsule. Green Chem Eng 1:63–69
Zorkipli NNM, Kaus NHM, Mohamad AA (2016) Synthesis of NiO Nanoparticles through Sol-gel Method. Proc Chem 19:626–631
Xu Y (2017) Continuous hydrothermal flow synthesis of functional oxide nanomaterials used in energy conversion devices. Department of Energy Conversion and Storage, Technical University of Denmark 236p
Zardi P, Carofiglio T, Maggini M (2022) Mild microfluidic approaches to Oxide nanoparticles synthesis. Chem Eur J 28:e202103132
Zhang Y, Qiu YR (2009) Preparation and application of NiO nanoparticles. J Chem Ind Eng 30(02):51–54
Acknowledgements
This work was financially supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LY19E060001 and LQ19F050003, Ningbo Science and Technology Bureau under Service Industry Science & Technology Programme with project code 2019F1030. The Zhejiang Provincial Department of Science and Technology is acknowledged for this research under its Provincial Key Laboratory Programme (2020E10018). The authors acknowledge the financial support from Nottingham Ningbo China Beacons of Excellence Research and Innovation Institute. Jiayou Mou also acknowledges the financial support by the scholarship from New Material Institute, University of Nottingham Ningbo China.
Funding
This work was funded by Natural Science Foundation of Zhejiang Province (Grant nos: LY19E060001, LQ19F050003); Ningbo Municipal Bureau of Science and Technology (Grant no: 2019F1030).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mou, J., Ren, Y., Wang, J. et al. Nickel oxide nanoparticle synthesis and photocatalytic applications: evolution from conventional methods to novel microfluidic approaches. Microfluid Nanofluid 26, 25 (2022). https://doi.org/10.1007/s10404-022-02534-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-022-02534-2