Abstract
In this paper, pristine and two types of porous polydimethylsiloxane (PDMS) microparticles were fabricated using oil-in-water (O/W) single emulsion template by the needle-based microfluidic multiphase system. By manipulating the flow rate of either the dispersed or continuous phase, microparticles of various sizes were obtained. The capillary number of the continuous phase for all flow conditions applied in this study was less than 0.1, suggesting that the flow regime was dripping. The coefficients of variation (CV) of sizes under different flow conditions were less than 1.5% which indicates the particles to be highly monodispersed. The surface morphology and particle size were characterized by optical microscope and scanning electron microscope. Pristine PDMS microparticles (PDMS-P) and PDMS microparticles templated from tetrachloromethane (CCl4) and white granulated sugars (PDMS-C and PDMS-S, respectively) were prepared under the same flow conditions. Subsequently, the microparticles were adopted for treatment of a synthetic wastewater that contained organic compounds such as toluene under static and dynamic states for comparison. The effects including the releasing amount, size of particles, porosity of microparticles and initial concentration of pollutants were investigated based on the toluene concentration variation, which was quantified by a gas chromatograph-headspace sampler (GC-HS). It has been found that 50 mg of porous PDMS microparticles are capable of realising over 65% of toluene removal efficiency of 200 ppm toluene aqueous solution within 2 h. The microparticles were collected and reused 30 times with unchanged treatment capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water pollution has become a globally challenging issue in recent decades especially under an enormous pressure of the rapid population growth and expansion of industrialisation in developing countries. Organic pollutants present in wastewater such as polycyclic aromatic hydrocarbon (PAHs), benzene series (benzene, toluene, ethylbenzene and xylene, short for BTEX), volatile halogenated compounds (VHC) and other organic solvents from industries pose great harms to ground water and water sources, and even exhibit threats to human health (Tiwari et al. 2008; Kulkarni et al. 2013; Víctor-Ortega et al. 2016; Swain et al. 2018). The principal techniques for organic wastewater treatment suffer from sophisticated processes, high capital investment and operating costs (Dhote et al. 2012). For example, chemical coagulation, activated carbon absorption, and sludge precipitation are all satisfactorily efficient for removal organic pollutants from wastewater, but these methods also incur high operational costs. In addition, chemical byproducts and residuals such as corrosion control chemicals, chlorine compounds, sodium hydroxide could exacerbate contamination (Shannon et al. 2008). Moreover, considerable efforts have been spent to tackle the water pollution problems via infusion of engineering expertise and infrastructure maintenance which are nevertheless both operationally and energetically intensive (Ahmaruzzaman and Sharma 2005; Lofrano et al. 2013; Amin et al. 2014; Mohd Udaiyappan et al. 2017).
Meanwhile, sustainable development and energy shortage stimulate the emergence of novel techniques, one example of which is to synthesize functional materials that are efficient and eco-friendly, and some of the materials have been applied in pollutant removal for wastewater treatment (Scott et al. 2010; Choi et al. 2011; Calcagnile et al. 2012; Jin et al. 2015; Zhu et al. 2017). For instance, starch microparticles were fabricated using reversed phase suspension method for removing toluene in wastewater (Miao and Wang 2011). However, the investigation did not reveal the treatment efficiency of pollutant in detail such as, the amount of materials used. The polymerisation method has been developed by applying carbon tetrachloride to form porous PDMS beads to enhance the absorption ability of organic substances. However, the measurement of absorption ability suffered from limited accuracy by measuring mass variation (Li et al. 2014).
Conventional fabrication methods of microparticles include mechanical agitation, resulting in wide size distribution and uneven components. Alternatively, droplet microfluidics could lead to formation of microscale droplets with exquisite control over size, shape, structure and components (Huebner et al. 2008). The platform processes or manipulates small amounts of fluids using devices with characteristic dimensions of tens to hundreds of micrometres (Garstecki et al. 2006). As the advantages of this technology, it miniaturises the volume of reagents consumed in chemical reaction or biological assays while maintaining high sensitivity and efficiency and hence shortening the analysis time (Utada et al. 2007). Droplet microfluidics could enable the formation of multiphase emulsion templates such as single and double emulsion templates with precisely controlled compositions, structures and sizes of droplets, leading to a high monodispersity and operability of liquids (Rosca et al. 2004; Whitesides 2006; Teh et al. 2008), and a number of applications such as reagent mixing (Mengeaud et al. 2002; Ottino and Wiggins 2004), cell biology (Fu et al. 1999; Gu et al. 2011), bio-combability test (Whitesides et al. 2001; Tan et al. 2007; Prastowo et al. 2016), chemical reactions (Song et al. 2006; Mitchell et al. 2001), pharmaceutical industries (Shchukin et al. 2006), cosmetics (Karnik et al. 2008; Feczkó et al. 2010), controlled release (Hu et al. 2008), and food science and industries (Madene et al. 2006).
Among the known materials for microparticle synthesis, PDMS is a low electrical conductivity elastomeric polymer with appealing chemical and physical properties (Stroock and Whitesides 2002). Its surface could be easily modified by chemical coating or grafting according to needs; it is optically transparent in the visible/UV region so that it could be of visible priority when proceeding optical operations. This property offers feasibility of on-and off-chip detection and operations (Whitesides et al. 2001). Lee et al. 2003 found that the swelling effect of PDMS in a solvent affects the most in terms of solvent compatibility (Lee et al. 2003). They studied Hildebrand value (δH), also defined as solubility parameter, which is correlated with the compatibility of one substance dissolving in another, and it is an expression of cohesive energy density. Solvents such as diisopropylamine, tetrachloromethane, hexane, acetate, chloroform and toluene have similar solubility parameters with PDMS which thus could be regarded as highly soluble with PDMS (Koh et al. 2012). For example, toluene has a solubility parameter of 8.9 \({\text{cal}}^{1/2}{ \text{cm}}^{-3/2}\) which is close to that of PDMS 7.3 \({\text{cal}}^{1/2}{\text{cm}}^{-3/2}\)(Lee et al. 2003). The organic solvents are absorbed into PDMS bulk and held inside, resulting in volume expanding and mass increasing of PDMS. Notably, a deswelling process can lead to the recovery of its shape and mass when the absorbed solvents are released or the swelled polymers are transferred into a lower soluble solvent (Yoo et al. 1999). This may propose possible applications for recycling and reuse of PDMS materials. PDMS swelling effect by weight loss method could achieve similar experimental outcomes (Koh et al. 2012). However, most of the reports only focused on mass variance or swelling degree of bulky PDMS pieces instead of contaminant concentration variation that PDMS influenced, whilst the variation in contaminant concentration is considered as one of the most important indicators of pollutant removal. A simple method has been reported to produce AuNP/PDMS foam in situ and could realise around 42% toluene removal efficiency from a simulated wastewater with concentration of 58–100 ppm (Scott et al. 2010). The application involved addition of gold which was not cost-effective for mass production. Furthermore, the bulk foam has limited surface area-to-volume ratio compared to microparticles, it is therefore of interest to explore the possibility to enhance toluene removal efficiency using PDMS microparticles.
The motivation of the study is to unveil the correlation of water pollutant concentration with presence of microparticles and change of surface morphology in a quantitative way and thus inspire new water treatment applications using droplet microfluidics. The needle-based microfluidic device using dispensing needles and plastic linkers has been applied to form the multiphase microsystem for droplet formation. Taking advantage of this facile and cost-effective equipment, we are able to produce monodisperse oil-in-water (O/W) emulsions and use them as template to synthesize PDMS microparticles. The desirable porous structures were added to the microparticles via controlling the surface porosity of PDMS microparticles by solvent evaporation (Li et al. 2014) and sugar-templating (Zhang et al. 2013) methods. The organic substance removal capability was investigated quantitatively by gas chromatograph connected with headspace sampler (GC-HS) to quantify the absorption efficiency.
2 Experimental
2.1 Materials for microfluidic systems
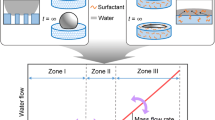
PDMS (Sylgard 184 silicone elastomer) purchased from Dow Corning (USA) was employed as disperse phase. The monomer and curing agent was mixed with mass ratio of 10:1. Tetrachloromethane (CCl4) was procured from Sigma-Aldrich (Shanghai, China) and white granulated sugar was bought from the local supermarket (Taikoo, China). Both CCl4 and sugar were served as porogens to generate porous structure on PDMS microparticles and were removed during particles formation. Polyvinyl alcohol (PVA) purchased from Sigma-Aldrich (Shanghai, China) was dissolved in DI water to prepare aqueous solution (5.0 wt%). The PVA solution was utilized as continuous phase and surfactant was added to stabilize the liquids to form PDMS microdroplets. Glue-free needle-based microfluidic device was utilized which was modified based on a method developed by Li et al. (2016). A set of the device is composed of two dispensing needles, one polypropylene (PP) cross-shaped link, two small pieces and three long pieces of silicone rubber soft tubes, one glass capillary and three syringes (refer to Fig. 1a). The needle with coloured needle holder was defined as inlet needle, whereas the other one without holder was used as outlet needle or orifice needle. They were inserted into the cross-shaped link from the opposite tips and connected with silicone rubber soft tubes. The distance between the two needles was fixed as 750 µm. The inter-needle distance could be tuned by controlling the length of soft tubes that connect the cross-link with needles. The photo of assembled microdevices was shown in Fig. S1, the colour and corresponding dimensions of needles were listed in Table S1 in the Supplementary document. In the study, 21G (Purple, i.d. = 510 µm, o.d. = 800 µm) and 20G (Pink, i.d. = 600 µm, o.d. = 900 µm) needles were employed as inlet and outlet needles, respectively, as a demonstration of microdroplet production in Fig. 1. Three 10 ml syringes were mounted onto the syringe pumps (Longer LSP02-1B) that constantly fed the liquids into the microfluidic device with controlled flow rates.
2.2 Synthesis of PDMS microdroplets and microparticles
Prior to droplet generation, the PDMS mixture was adequately stirred and then exposed to vacuum to remove air bubbles to avoid interference between air bubbles and PDMS slurry and thus ensure a stable production of microdroplets. To manufacture porous structure, 40% (w/w) CCl4 was doped with PDMS precursor, while another batch used 1% (w/w) sugar as template. As demonstrated in Fig. 1a, the dispersed liquid was injected into microdevice through the needle under a pre-set flow rate. The continuous phase, PVA aqueous solution, was pumped into the microfluidics system from the other two inlets of the cross-link in the perpendicular direction. Both flow rates could be steered from the syringe pumps. In this study, flow rate of disperse phase and continuous phase were tuned from 10 to 20 µl/min and 100 to 300 µl/min, respectively. The two immiscible fluids encountered at the exit tip of the inlet needle where a thin capillary was linked to act as the collector channel which could also facilitate observation of the formed droplets. The microdroplets were produced at the junction where the PDMS stream was pinched off by the two liquids of continuous phase. The zoomed inset in Fig. 1b of the junction area depicts that microdroplets were generated as a result of the competition between viscous shear stress and interfacial tension of the two immiscible fluids in the geometry (Li et al. 2012). The cross-shaped link provided a flow-focusing geometry while the inserted and suspended needle and the glass capillary formed a hydrodynamic focusing of co-flow geometry (Yobas 2015). The need for surface modification of wettability upon channel walls can be obviated with the facile design (Wu et al. 2017). A beaker that contained 100 ml outer phase was placed under the outlet of the glass capillary for droplet collection. Subsequently, 80 °C of thermal treatment was commenced for 90 min to allow curing of the droplets, transforming them from liquid microdroplets to solid microparticles. During this process, porogens were removed out of the droplets and left the spaces occupied as pores. The PDMS microparticles were then filtrated and washed by deionized water three times and collected for storage. In this fashion, pristine PDMS (PDMS-P, similar steps were followed except addition of porogen), porous PDMS microparticles using CCl4 (PDMS-C) and sugar (PDMS-S) were fabricated for toluene removal under flow rates of 15 and 200 µl/min for disperse phase and continuous phase, respectively. Capillary number of continuous phase (Cac) could be used to predict the flow regime, for instance, squeezing, dripping or jetting (Baroud et al. 2010).
Capillary number reflects the competition between shear force exerted by continuous phase and interfacial tension forces that resists being pinched off from stream to droplets (Fernandez-nieves et al. 2014). The values of Cac are calculated by:
where \({F_{{\text{shear}}}}\) is the shear force of continuous phase and \({F_\gamma }\) is the interfacial tension forces between the two liquid phases.
\({F_{{\text{shear}}}}\) could be expressed by a modified Stock’s drag force (Erb et al. 2011) as:
where \(\mu\) is the viscosity of the continuous phase, D is diameter of microdroplets, \({d_1}\) is diameter of the needle tube, \({v_{\text{d}}}\) and \({v_{\text{c}}}\) are average velocity of disperse and continuous phase, respectively. Meanwhile, \({v_{\text{d}}}\) and \({v_{\text{c}}}\) could be expressed in the form of flow rates as:
where \({Q_{\text{d}}}\) and \({Q_{\text{c}}}\) are flow rates of disperse and continuous phase, respectively, and \({d_2}\) is the diameter of the outlet needle.
The interfacial tension force \({F_\gamma }\) is expressed as
where \(\gamma\) is the interfacial tension between continuous phase and disperse phase. Combining Eqs. (1) to (5), \({\text{C}}{{\text{a}}_{\text{c}}}\) can be worked out as
3 Characterisation
The resulting microdroplets and microparticles were characterized by optical microscope and SEM. Observation of both microdroplets and the cured spheres were carried out under N-800F optical microscope (Ningbo Novel Optics, China). Thereafter, image capturing was completed by a computer that connected with the microscope. Image processing was done by software ImageJ, which was capable to measure the sizes of each microdroplet or microparticle. Coefficient of variation (CV) was applied to describe the particle size monodispersity based on the data of standard deviation (STD) and average diameter (AD) of particle sizes as follows:
In this study, the SEM (ZEISS Sigma 500) was used to observe surface and cross-section morphology of the microparticle. Viscosity was measured by Anton Paar Physica MCR-301 rotational rheometer (Austria). Static water contact angle (WCA) and interfacial tension were measured by Dataphysics DCAT21 contact angle meter/interface tensiometer (Germany, water droplet is 5 µl at five different positions for the same sample bed for WCA measurements) at ambient temperature. TGA measurements were carried out by TG/DTA6300, SII Nano Technology Inc, Japan.
Toluene (99.8%) bought from Aladdin (Shanghai, China) was implemented as the targeted contaminant of a simulated wastewater. 200 ppm of toluene solution was prepared by pipetting 46 µl toluene into a 200 ml volumetric flask and mixed with DI water. By the same token, other concentrations could be prepared in advance. Toluene standard samples were also purchased from Aladdin (Shanghai, China) based on which toluene concentration calibration curve was produced based on concentration gradient from 1, 10, 50, 100, 200 to 400 ppm, respectively. Oil-red was bought from Sigma-Aldrich (Shanghai, China). A shaker (GLS Aqua 12 Plus, UK) was applied to provide a shaking speed to the vials. The organic removal efficiency (η) was used to assess the removal performance of toluene by PDMS microparticles, whist Agilent7890B Gas chromatograph (GC) connected with Agilent7697 Headspace Sampler (HS) were used as analytical measurement tools. The difference in concentration of toluene before and after treatment (C0 and Ct, respectively) is an essential parameter to reflect absorption effect provided by PDMS microparticles. Hence, toluene removal efficiency at room temperature could be calculated by:
4 Results and discussion
4.1 Synthesis of microparticles
For most application cases, the dripping regime is always preferred due to its wider stable range that enables adjustment for size and structures of emulsions in a facile way. Dynamic viscosity \(\eta ~\) of 5% PVA aqueous solution was measured as 0.247 Pa s with flow rate of Qc = 200 µl/min and Qd = 15 µl/min. Interfacial tension \(\gamma\) between water and PDMS was 15.202 mN/m. Applying Eq. (6), Cac was found to be 0.06 that dripping regimes of flow conditions could thus be distinguished (Cubaud and Mason 2008; Yobas 2015). Since the droplets were formed in dripping regime, monodispersity of size and composition was expected (Utada et al. 2007). A glass slide was employed to catch the droplets for optical observation under microscope during the droplet formation. Figure 2a depicts the formed pristine PDMS microdroplets under flow rate for disperse phase Qd = 15 µl/min and the continuous phase Qc = 200 µl/min, respectively, whereas Fig. 2b shows the solidified microparticles from the same batch of droplets. At least 50 pieces of PDMS microparticles were randomly picked for characterisation, including size measurement and morphology. The measured average diameter of the microdroplets was 653 µm and that for the microparticles was 647 µm. Standard deviation and average diameters were calculated based on the measured diameter of particles. Therefore, CV of this batch was 0.67% from Eq. (7) which indicated homogeneous and uniform microparticles were generated. In addition, it was found that the particle sizes obeyed a standard distribution that most of the values converged neat the average value which was 648 µm, shown in Fig. 2c.
a The generated PDMS single emulsion microdroplets by the needle-based microfluidic device dispersed in water, photographed under a microscope. The flow rate of the disperse phase was Qd = 15 µl/min and Qc = 200 µl/min for the continuous phase. Uniform microdroplets were generated with an average diameter measured as 653 µm. The scale bar is 100 µm. b A microscope photograph of PDMS microparticles which were thermally cured from PDMS microdroplets in a. The average diameter of the solidified microparticles was 647 µm. c A standard distribution graph of sizes of PDMS microparticles fabricated under flow rates 15 and 200 µl/min for dispersed and continuous phase, respectively
To investigate the size variation with flow rates and examine their monodispersity, flow rate of one phase, continuous or disperse, was fixed while the other one will be varied as a study parameter. However, the flow rates have to be confined attributed to flow regimes. For instance, a higher flow rate of the continuous phase may cause jetting of PDMS stream which was transported further downstream and broken up at the elongated tip of the stream, leading to polydispersity of the droplets (Ward et al. 2010). On the other hand, attributed to high viscosity of PDMS, i.e., 2244 cSt, a smaller flow rate of continuous phase could result in a plug flow in squeezing regime even a failure of pinching off streams where parallel flow occurred (Li et al. 2012). The maximum and minimum flow rates of continuous phase were limited to 300 and 100 µl/min, respectively, while flow rates of disperse phase varied from 10 to 30 µl/min in the present study.
At least 50 samples were randomly collected and measured for each group of flow rate combination. Figure 3 summarises the measured particle size variation under a variety of flow rate combinations of disperse and continuous phase. The numbers of x-axis were the flow rates of the continuous phase, Qc. Three colours represent flow rates of the disperse phase Qd, which were fixed as 10, 15 and 20 µl/min, respectively. Droplet diameters were confined and reduced as the continuous phase penetrated into the microchannel with increasing flow rates from 100 to 300 µl/min for each fixed Qd (refer to the same colour). On the other hand, an increasing tendency was observed when enhancing the flow rate of the disperse phase while fixing the continuous phase. The results coupled well with the fact that higher continuous flow rate and smaller disperse phase flow rates would induce formation of the microparticles with smaller diameters (Rotem et al. 2012). The plot of Fig. 4 supports this argument which was expressed as the flow rate ratio of continuous phase and disperse phase. Malvern Mastersizer 3000 (MS3000, UK) was applied to validify the size distribution of the microparticles which were fabricated under flow rate conditions of 15–300 µl/min, as an example. At least 500 pieces were measured, and the measurement was carried out for 21 times and the results were shown in Table S2 and Fig. S2 in the Supplementary document. Additionally, microparticles with size ranging from 608 to 742 µm under variety of flow rate combinations were fabricated, while the capillary number of the continuous phase was between 0.03 and 0.08 according to Eq. (6). Coefficient of variation (CV) of particle sizes of all groups were below 1.5% under corresponding flow rate calculated by applying Eq. (7), which indicated a very high monodispersity of the microparticles were achieved (Cramer et al. 2004). Table 1 lists microparticle diameters, capillary number of continuous phase and CV affected by flow rate control, using dispersed phase flow rate of 15 µl/min as an example. From the table, it could be revealed that higher flow rate of continuous phase lead to smaller size and increment of capillary number. The information including diameters, capillary numbers and coefficient of variation of PDMS microparticles collected under diverse flow rates for all groups was summarised in Table S3 in the supplementary document. Since all groups of Ca were smaller than 0.1, the flow was controlled in a dripping regime (Cubaud and Mason 2008). Moreover, the recorded average droplet production rate was 130 pieces per minute which made it possible that the production of microparticles could be increased to 62,400 particles in around 8 h using continuous phase flow rate of 200 µl/min and dispersed phase flow rate of 15 µl/min. The throughput could be further increased via microdevice parallelisation approach which an example was demonstrated in the Supplementary document (Fig. S6; Supporting Video). This is critical as high throughput will be able to meet the ever-increasing demand of organic pollutant treatment.
The measurement and observation from figures captured by SEM corroborated well with the measured sizes from microscope photographs, as seen in Fig. 5. Figure 5a displays a SEM photograph of the pristine PDMS microparticle (defined as PDMS-P) whose diameter was 642 µm. Since no other dopant added in PDMS system, the surface appeared to be smooth and rare pores was discovered, as shown in Fig. 5b. When CCl4 was mixed into the precursor, a few pores were found on the surface of microparticle as well as its cross-sectional area, the majority of CCl4 evaporated during droplet formation process prior to the solidification stage, so that the pores left were soon refilled. Figure 5c, d depict PDMS microparticles with CCl4 as porogen (defined as PDMS-C) and both figures showed limited number of pores. For cases where sugar particles were used as porogens (PDMS-S), Fig. 5e, f demonstrate the porous surface and cross-section of the microparticles. Unevenly distributed macro-pores with ununiform pore sizes with size ranging from 500 nm to 40 µm were observed that both closed pores and large open pores were found.
The microparticles demonstrated in this section were produced at the same flow conditions that Qd = 15 µl/min and Qc = 200 µl/min. This flow rate combination was selected as a demonstration since they were in the middle of all flow rate combinations in this study, also leading to the middle value of particle size among all groups. a A SEM photograph of the pristine PDMS single emulsion microparticle (PDMS-P) whose diameter was measured as 642 µm and b its cross-sectional view. c SEM photograph of porous PDMS microparticles using CCl4 (PDMS-C) and d its cross-sectional view. e SEM photograph of porous PDMS microparticles using sugar (PDMS-S) and f its cross-sectional view. The scale bar in a, c, e is 100 µm and in b, d, f is 20 µm
4.2 Toluene removal tests in simulated wastewater by PDMS microparticles
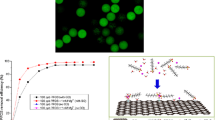
The high hydrophobicity and swelling effect of PDMS could be applied in the absorption of non-polar solvents such as oil and organic solvents. In this research, PDMS-P microparticles were employed to perform a qualitative study to validate the capability of PDMS microparticles by microfluidic methods to purify organics in water. 1 ml of toluene dyed by oil-red was mixed with 40 ml water was poured into a petri dish and 1 g of PDMS-P were dipped into the toluene–water mixture by three batches in 30 s. Absorption of pollutants happened immediately after the microparticles contacted the pollutants where the red colour gradually transferred from water into PDMS as shown in Fig. 6. This was due to the inherent high hydrophobic and superoleophilic properties of the PDMS. The 3D structure combined with the closely cross-linked Si–O bonds allowed toluene to be held in (Choi et al. 2011). The red colour in water vanished in 3 min with stirring and the particles were obtained by filtration. However, fewer studies focused on the removal of contaminants in a more quantitative manner as well as the removal over time. Since toluene has a solubility of 500–627 ppm in water (Scott et al. 2010), the following studies were based on the mitigation of ppm level of the contaminants.
Five groups of particles weighted as 50, 100, 300, 450 and 600 mg PDMS-P microparticles (645 µm in average diameter) were released into five vials with the same initial concentration of 200 ppm toluene–water systems, respectively. The vials with well-sealed threaded caps were used to avoid toluene evaporation to ambient environment. The vials were placed in static mode and sampling was made at t = 2, 4, 6, 10 and 18 h and measured by GC-HS, respectively. Another five groups of PDMS-P microparticles (645 µm in average diameter) with 50, 100, 250, 400 and 550 mg were released into five vials with the same initial conditions as above. A shaker was implemented to constantly shake the vials at a rate of 100 rpm to ensure mixing of both phases throughout the absorption experiment. Sampling was made at t = 2, 4, 6, 8 and 10 h and measured by GC-HS, respectively. As the vials were well-sealed and each sampling was finished in few seconds, the influence by evaporation of toluene has been neglected in this research. Figure 7 demonstrates the treatment results that toluene was transferred from water into PDMS microparticles over time with (expressed in inverted triangle) and without (expressed in triangle) the presence of shaker. Over 15% of toluene was uptaken within 2 h for 50, 100 and 300 mg groups, and even over 35% removal efficiency for 450 and 600 mg group, as calculated by Eq. (8). Thereafter, absorption continued but the entire absorption lasted over a long time for 10 h. This may be ascribed to the separation of toluene and water due to density difference after long time in static state that toluene rose up to the top layer where PDMS microparticles obtained more contact opportunities with toluene molecules. It was observed that the particles were attracted to the oil layer while others kept drifting and searching for the targeted pollutants from water body attributed to its water-repelling and oil-wetting properties (Choi et al. 2011). At t = 18 h, most groups achieved over 50% removal efficiency, particularly, more microparticles were capable to accomplish more treatment and thus achieve higher efficiency, as can be seen that over 75% of pollutants was removed by 600 mg of microparticles. The removal efficiencies reveal that increasing mass loading could lead to higher removal performance as larger capacities are provided. For the dynamic absorption groups, it is found that a dramatically drop of concentration over 40% of toluene was uptaken within 2 h for 50 mg and 100 mg groups, and even over 75% removal efficiency for 550 mg. Particularly, 400 mg microparticles doubled the efficiency from that of 50 mg at the second hour. However, the efficiency does not increase much with more absorbents added, such as the 500 mg of microparticles did not raise the efficiency much compared with 400 mg. After the first 2 h, the absorption slowed down significantly since most of the microparticles were almost saturated in the first 2 h. As a comparison with the static group, removal efficiency of dynamic mode after 2 h was slightly higher even with smaller mass loading (reduced by 50 mg of the last three groups) than the static group after 18 h. Thus, the shaking boosted both mixing of toluene and water and the contact between toluene and PDMS, corroborating that shaking mode could promote the absorption efficiency and significantly shortened the time consumed.
Five groups of PDMS-P microparticles with different diameters (from 450, 550, 600, 650 to 700 µm, 500 pieces for each group) were employed to treat 200 ppm toluene solution with the assistant of shaker in 2 h. The removal efficiencies were calculated after 2 h absorption, present in Fig. 8. The results implied that when saturated, at the level of individual particles, larger diameter led to higher removal efficiency due to the larger volume which could provide more capacity for the storage of toluene. On the other side, the sizes directly related to mass of particles applied as density was constant and the high monodispersity of sizes for the same type of microparticles. In regard of saving material and energy, it will be of critical importance to estimate the volume to be dealt with and offer appropriate quantity of absorbent accordingly. Thus, microfluidics affords unique advantages for tailoring materials in terms of size and composition control. As the highly monodispersed particles can allow us to estimate the number of particles or production rate is used to handle a certain amount of pollutants. Moreover, the monodispersed microparticles could be applied on the surface treatment such as chemical grafting on which the microparticles are used as substrates for generating hybrid composites, such as grafting chemicals to intensify hydrophobicity on the surface of PDMS microparticles, strengthening conductivity by grafting conductive materials or doping materials that could degrade the absorbed contaminants. In addition, the amount of materials that used for grafting or doping could be estimated in advance. The mixing of fluids could be more completed and efficient and grafting density could be ensured by microfluidics approaches.
Introducing porous structure might be conducive to improve absorption efficiency as it will enhance more contact area that could allow substantial contact between PDMS surface and targeted pollutants. 50 mg of PDMS-P, PDMS-C and PDMS-S microparticles with similar sizes of 645 µm were immerged into three groups of 200 ppm toluene solution sealed in vials. Samples were measured by GC-HS after 2 h of immersion time with shaking mode. The removal efficiency was calculated by comparing toluene concentration before (C0) and after treatment (Ct), employing Eq. (8). Table 2 summarises the results of toluene concentration at different PDMS condition and removal efficiencies of toluene by such kinds of microparticles. The results elucidate that the residue toluene concentrations after these three types of absorbent treatment were 128, 112 and 63 ppm, respectively, and the removal efficiencies of PDMS-P, PDMS-C and PDMS-S microparticles were 36%, 44% and 68.6%, respectively, as calculated by Eq. (8), indicating that the porous structure was beneficial for enhancing treatment efficiency than non-porous particles. The results supported the statements of many reports (Choi et al. 2011; Wang and Lin 2013; Zhou et al. 2013), and porous structure was conducive for microparticles to removing pollutants from water. PDMS-S possessing plentiful pores gained the highest efficiency. For the same mass loading and sizes, by endowing pores to the microparticles, the specific surface area increases so that it could provide more available contact sites for toluene molecules. Moreover, the pores by sugar leaching method resulted in small wrinkles and protrusion on surface (Fig. S3) due to the removal of sugar in the cross-linking process and thus enhanced the surface roughness. This was verified both from the SEM images and the increment of water contact angle from the data obtained for the PDMS-P and PDMS-S. The WCA was increased from 110° ± 2° to 125° ± 1.5° by the application of sugar template (refer to Fig. S4) and thus the hydrophobicity was enhanced. Meanwhile, porous structure led to lower density of PDMS microparticles that is favourable for toluene removal due to density difference in the toluene–water system. The calculated PDMS-S apparent density was 0.896 g/cm3 (with porosity 24%) which was smaller than PDMS-P (1.185 g/cm3) and water while that for PDMS-C was 1.090 g/cm3 (with porosity 8%, please refer to Supporting material for detailed calculation, Table S4). Overall, porosity is instructive both for the absorption efficiency and removal amount under limited conditions. However, if porosity was too high, partial of the interconnected structure may collapse owing to superfluous amount of porosity. In the case of PDMS-C, as exemplified from the SEM figures in Fig. 6c, d, the reduced number of pores and restricted pore sizes were attributed to the loss of CCl4 during droplet formation process so that they did not exhibit virtuous capability. There are diverse techniques to improve the absorption efficiency for instance by alteration of PDMS structures via increasing the PDMS base polymer and curing agent ratio. On the other hand, excess amount of curing agent could adversely impact the absorption, since it would result in a lower degree of swelling due to constraint on the polymer chain expansion and restraint on the mobility and thus lead to lower absorption efficiency (Yu and Han 2006).
Five groups of porous PDMS-S microparticles (average size as 645 µm) weighted as 50 mg each were dipped into five groups of simulated wastewater with initial toluene concentrations that ranged from 60 to 250 ppm, respectively. The vials were sealed in static state and sampled at 30, 60, 120, 240 and 360 min for GC-HS test. As contrast, another five groups PDMS-S microparticles (50 mg each) were tested in dynamic state with sampling points at 20, 40, 60, 90 and 120 min. The concentration variation over time was calculated based on the ratio of net absorption amount and the corresponding reaction time interval. We thus are able to evaluate the absorption rate at each time interval and suggest at which time point the experiment could be stopped due to low rates or saturation. Figure 9 demonstrated the absorption rates at each time point for microparticles in static and dynamic absorption modes. The values of each point were the averaged value based on repeated experiments for three times, with the standard deviation controlled in the range of 0.187 in static mode and 0.398 in the dynamic mode. With assistance of shaker, the porous PDMS microparticles uptook pollutants rapidly in the first 20 min and a similar amount of toluene was extracted from water into PDMS with rates around 3 ppm/min except for the 60 ppm group (1.6 ppm/min). Specifically, the poor absorption rate of 60 ppm was probably due to insufficient amount of toluene molecules in water to encounter PDMS even in the dynamic mode. Thus, this process required a longer time that toluene was constantly extracted. For 250, 215 and 185 ppm the absorption rates gradually declined from 20 to 60 min, for instance, the rates fell to 1.5 ppm/min and 1 ppm/min at 40th and 60th min, respectively, and lower than 0.5 ppm/min after 90th min. From the tendency of rate changes, it is suggested that the absorption nearly reached equilibrium, although there were plenty of remanent molecules untreated. Interestingly, for 150 ppm the rate dramatically dropped from 3.4 ppm/min (by 20 min) to 0.9 ppm/min (by 40 min). Thereafter, the rate was lower than 0.5 ppm/min for another 60 min and regarded as very slow for dynamic mode. At this point, the 50 mg of PDMS to treat 150 ppm of toluene could be considered as saturated by 60 min. For the first four groups, similar results around 120 to 130 ppm of overall concentration was reduced by 120 min which implied that the overall removal result was less related with the initial concentration. This was induced because greater quantity of toluene was attached at the surface of PDMS microparticles or attracted to its surrounding but a limited number of particles was available at the same time. Whereas the initial concentration corresponded more with the time to reach equilibrium which was reflected by absorption rates. A lower reaction rate was retained throughout the whole process for 60 ppm group, owing to insufficient amount of toluene molecules to be absorbed. A similar phenomenon could be obtained from the 60 ppm group in static mode that the rates were constantly lower than 0.5 ppm/min. The overall rates were much slower for static groups compared with dynamic ones although the total absorption amounts in the end were similar for the same initial concentration. It could be seen that the other four groups attained over 1 ppm/min rates at the first 30 min and thereafter steadily diminished to values that smaller than 0.5 ppm/min as can be seen from Fig. 9, symbols shown in open triangle. The 250 and 215 ppm groups with a rate at 0.35 ppm/min at 240 min and that reduced to 0.1 ppm/min at 360 min, whereas for 185 and 150 ppm, the rates dropped to a value smaller than 0.1 ppm/min by 240 min suggesting the absorption could be stopped. There is no doubt that 6 h is a long time and static absorption may not be an efficient approach for some industries. It is thus favourable to utilize the dynamic mode to strengthen the mixing between toluene and microparticles. Another parameter that might adversely affect the absorption was the broad pore sizes distributions regarding the irregular shapes of sugar templates as can be seen in Fig. 5 and Fig. S3. Other approaches such as emulsion templating or phase separation may provide alternative solutions to generate monodispersed pores (Grosse et al. 2008; Kim et al. 2009).
4.3 Regeneration and reuse test of microparticles
Regeneration and reuse of materials were critical in the light of energy saving and resource conservation. The swelled microparticles were filtrated after treatment and the absorbed toluene could be liberated by thermal treatment. 50 mg of porous PDMS (PDMS-S) microparticles were dispersed into 200 ppm toluene–water systems for the reuse test under dynamic state, allowing 2 h of absorption. After each treatment, the particles were filtrated and heated under 100 °C for 20 min and dried in oven for another 20 min (TGA information can be found in Fig. S5). The removal efficiency remained over 65% after 30 cycles as illustrated in Fig. 10, and the WCA after 30 times of use remained unchanged.
5 Conclusions
Pristine and two types of porous PDMS microparticles were fabricated by a needle-based microfluidic device templating from the oil-in-water (O/W) single emulsion. Desirable particle sizes could be tailored by tuning the flow rate of each phase or controlling the dimensions of the microdevice. The microparticles of different sizes have been fabricated when the capillary number of the continuous phase was controlled between 0.03 and 0.08. It was noted that all groups of produced microparticles owned size coefficient of variation (CV) less than 1.5%, suggesting highly monodispersed particles were produced. The study demonstrates the production rate of 130 microparticles per minute, leading to a total yield of 62,400 microparticles in 8 h. The fabricated microparticles have been used to treat wastewater containing toluene under static and dynamic absorption processes. A higher removal efficiency was obtained with a higher amount of the released microparticles from the same batch, and the dynamic absorption was advantageous to shorten the time for the process to reach equilibrium. The porous structure of PDMS-S not only enlarged the specific surface area to provide more absorption sites, but also enhanced the surface roughness and hydrophobicity. Keeping the releasing amount unchanged, porous microparticles endowed a higher pollutant removal efficiency that over 65% of the pollutant were removed. Absorption rates based on various absorption time intervals for different initial concentrations reflected the removal ability of the microparticles throughout the absorption process. The regeneration tests demonstrated that the microparticles were competent after 30 times of recycling with unchanged removal efficiency.
References
Ahmaruzzaman M, Sharma DK (2005) Adsorption of phenols from wastewater. J Colloid Interface Sci 287(1):14–24. https://doi.org/10.1016/j.jcis.2005.01.075
Amin MT, Alazba AA, Manzoor U (2014) A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng. https://doi.org/10.1155/2014/825910
Baroud CN, Gallaire F, Dangla R (2010) Dynamics of microfluidic droplets. Lab Chip 10(16):2032. https://doi.org/10.1039/c001191f
Calcagnile P, Fragouli D, Bayer IS, Anyfantis GC, Martiradonna L, Cozzoli PD, Athanassiou A (2012) Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 6:5413–5419. https://doi.org/10.1021/nn3012948
Choi S, Kwon T, Im H, Moon D, Baek DJ, Seol M, … Choi Y (2011) A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl Mater Interfaces. https://doi.org/10.1021/am201352w
Cramer C, Fischer P, Windhab EJ (2004) Drop formation in a co-flowing ambient fluid. Chem Eng Sci 59(15):3045–3058. https://doi.org/10.1016/j.ces.2004.04.006
Cubaud T, Mason TG (2008) Capillary threads and viscous droplets in square microchannels. Phys Fluids. https://doi.org/10.1063/1.2911716
Dhote J, Ingole S, Chavhan A (2012) Review on wastewater treatment technologies. Int J Eng Res Technol 1(5):1–10
Erb RM, Obrist D, Chen PW, Studer J, Studart AR (2011) Predicting sizes of droplets made by microfluidic flow-induced dripping. Soft Matter 7(19):8757–8761. https://doi.org/10.1039/c1sm06231j
Feczkó T, Kokol V, Voncina B (2010) Preparation and characterization of ethylcellulose-based microcapsules for sustaining release of a model fragrance. Macromol Res 18(7):636–640. https://doi.org/10.1007/s13233-010-0701-z
Fernandez-nieves A, Gordillo JM, Scott A, Gupta R, Kulkarni GU, Abate AR, Wang J (2014) Microfluidic techniques for synthesizing particles. Adv Mater 26(14):1–21. https://doi.org/10.1002/adma.201305119
Fu AY, Spence C, Scherer A, Arnold FH, Quake SR (1999) A microfabricated fluorescence-activated cell sorter. Nat Biotechnol 17(11):1109–1111. https://doi.org/10.1038/15095
Garstecki P, Fuerstman MJ, Stone H, Whitesides GM (2006) Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab Chip. https://doi.org/10.1039/b510841a
Grosse M, Lamotte M, Birot M, Deleuze H (2008) Preparation of microcellular polysiloxane monoliths. J Polym Sci Part A: Polym Chem 46:21–32. https://doi.org/10.1002/pola.22351
Gu S-Q, Zhang Y-X, Zhu Y, Du W-B, Yao B, Fang Q (2011) Multifunctional Picoliter droplet manipulation platform and its application in single cell analysis. Anal Chem 83(19):7570–7576. https://doi.org/10.1021/ac201678g
Hu SH, Tsai CH, Liao CF, Liu DM, Chen SY (2008) Controlled rupture of magnetic polyelectrolyte microcapsules for drug delivery. Langmuir 24(20):11811–11818. https://doi.org/10.1021/la801138e
Huebner A, Sharma S, Srisa-Art M, Hollfelder F, Edel JB, DeMello AJ (2008) Microdroplets: a sea of applications? Lab Chip 8(8):1244–1254. https://doi.org/10.1039/b806405a
Jin C, Han S, Li J, Sun Q (2015) Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohyd Polym 123:150–156. https://doi.org/10.1016/j.carbpol.2015.01.056
Karnik R, Gu F, Basto P, Cannizaro C, Dean L, Kyei-Manu W, Farokhzad OC (2008) Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett 8(9):2906–2912
Kim J, Taki K, Nagamine S, Ohshima M (2009) Preparation of porous poly (l-lactic acid) honeycomb monolith structure by phase separation and unidirectional freezing. Langmuir 25(19):5304–5312. https://doi.org/10.1021/la804057e
Koh KS, Chin J, Chia J, Chiang CL (2012) Quantitative studies on PDMS-PDMS interface bonding with piranha solution and its swelling effect. Micromachines 3(2):427–441. https://doi.org/10.3390/mi3020427
Kulkarni SJ, Tapre RW, Patil SV, Sawarkar MB (2013) Adsorption of phenol from wastewater in fluidized bed using coconut shell activated carbon. Procedia Eng 51(NUiCONE 2012):300–307. https://doi.org/10.1016/j.proeng.2013.01.040
Lee J, Park C, Whitesides GM (2003) Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem 75(23):6544–6554. https://doi.org/10.1021/ac0346712
Li X, Li F, Yang J, Kinoshita H (2012) Study on the mechanism of droplet formation in T-junction microchannel. Chem Eng Sci 69(1):340–351. https://doi.org/10.1016/j.ces.2011.10.048
Li N, Li T, Lei X, Fu B, Liao W, Qiu J (2014) Preparation and characterization of porous PDMS beads for oil and organic solvent sorption. Polym Eng Sci 54(12):2965–2969. https://doi.org/10.1002/pen.23860
Li T, Zhao L, Liu W, Xu J, Wang J (2016) Simple and reusable off-the-shelf microfluidic devices for the versatile generation of droplets. Lab Chip. https://doi.org/10.1039/C6LC00967K
Lofrano G, Meriç S, Emel G, Orhon D (2013) Science of the total environment chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
Madene A, Jacquot M, Scher J, Desobry S (2006) Flavour encapsulation and controlled release—a review. Int J Food Sci Technol 41(1):1–21. https://doi.org/10.1111/j.1365-2621.2005.00980.x
Mengeaud V, Josserand J, Girault HH (2002) Mixing processes in a zigzag microchannel: finite element simulations and optical study. Anal Chem 74(16):4279–4286. https://doi.org/10.1021/ac025642e
Miao Z, Wang L (2011) The performance of starch microspheres treating wastewater. ICMREE2011 Proc 2011 Int Conf Mater Renew Energy Environ 1(l):758–761. https://doi.org/10.1109/ICMREE.2011.5930918
Mitchell MC, Spikmans V, Manz A, de Mello AJ (2001) Microchip-based synthesis and total analysis systems (µSYNTAS): chemical microprocessing for generation and analysis of compound libraries. J Chem Soc Perkin Trans 1(5):514–518. https://doi.org/10.1039/b009037i
Mohd Udaiyappan AF, Hasan HA, Takriff MS, Sheikh Abdullah SR (2017) A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J Water Process Eng 20(June):8–21. https://doi.org/10.1016/j.jwpe.2017.09.006
Ottino JM, Wiggins S (2004) Introduction: mixing in microfluidics. Philos Trans A Math Phys Eng Sci 362(1818):923–935. https://doi.org/10.1098/rsta.2003.1355
Prastowo A, Feuerborn A, Cook PR, Walsh EJ (2016) Biocompatibility of fluids for multiphase drops-in-drops microfluidics. Biomed Microdev 18(6):1–9. https://doi.org/10.1007/s10544-016-0137-0
Rosca ID, Watari F, Uo M (2004) Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J Control Release 99(2):271–280. https://doi.org/10.1016/j.jconrel.2004.07.007
Rotem A, Abate AR, Utada AS, Van Steijn V, Weitz D a (2012) Drop formation in non-planar microfluidic devices. Lab Chip 12(21):4263. https://doi.org/10.1039/c2lc40546f
Scott A, Gupta R, Kulkarni GU (2010) A simple water-based synthesis of Au nanoparticle/PDMS composites for water purification and targeted drug release. Macromol Chem Phys 211(15):1640–1647. https://doi.org/10.1002/macp.201000079
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452(March):301–310. https://doi.org/10.1038/nature06599
Shchukin DG, Gorin DA, Möhwald H (2006) Ultrasonically induced opening of polyelectrolyte microcontainers. Langmuir 22(17):7400–7404. https://doi.org/10.1021/la061047m
Song H, Chen DL, Ismagilov RF (2006) Reactions in droplets in microfluidic channels. Angew Chem Int Ed 45(44):7336–7356. https://doi.org/10.1002/anie.200601554
Stroock, a. D., & Whitesides, G. M. (2002). Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis 23(20):3461–3473. https://doi.org/10.1002/1522-2683(200210)23:20%3C3461::AID-ELPS3461%3E3.0.CO;2-8
Swain AK, Sahoo A, Jena HM, Patra H (2018) Industrial wastewater treatment by aerobic inverse fluidized bed biofilm reactors (AIFBBRs): a review. J Water Process Eng 23(October 2017):61–74. https://doi.org/10.1016/j.jwpe.2018.02.017
Tan HML, Fukuda H, Akagi T, Ichiki T (2007) Surface modification of poly(dimethylsiloxane) for controlling biological cells’ adhesion using a scanning radical microjet. Thin Solid Films 515(12):5172–5178. https://doi.org/10.1016/j.tsf.2006.10.026
Teh S-Y, Lin R, Hung L-H, Lee AP (2008) Droplet microfluidics. Lab Chip 8(2):198–220. https://doi.org/10.1039/b715524g
Tiwari DK, Behari J, Sen P (2008) Application of nanoparticles in waste water treatment. Carbon Nanotubes 3(3):417–433. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Application+of+Nanoparticles+in+Waste+Water+Treatment#7
Utada aS, Chu L-Y, Fernandez-Nieves a, Link DR, Holtze C, Weitz Da (2007) Dripping, jetting, drops, and wetting: the magic of microfluidics. MRS Bull 32(09):702–708. https://doi.org/10.1557/mrs2007.145
Víctor-Ortega MD, Ochando-Pulido JM, Martínez-Férez A (2016) Phenols removal from industrial effluents through novel polymeric resins: kinetics and equilibrium studies. Sep Purif Technol 160:136–144. https://doi.org/10.1016/j.seppur.2016.01.023
Wang C, Lin S (2013) Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water, 8861–8864. https://doi.org/10.1021/am403266v
Ward T, Faivre M, Stone HA (2010) Drop production and tip-streaming phenomenon in a microfluidic flow-focusing device via an interfacial chemical reaction. Langmuir 26(12):9233–9239. https://doi.org/10.1021/la100029q
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442(7101):368–373. https://doi.org/10.1038/nature05058
Whitesides GM, Ostuni E, Jiang X, Ingber DE (2001) Soft L ithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373
Wu L, Liu X, Zhao Y, Chen Y (2017) Role of local geometry on droplet formation in axisymmetric microfluidics. Chem Eng Sci 163:56–67. https://doi.org/10.1016/j.ces.2017.01.022
Yobas L (2015) Microfluidic emulsification through a monolithic integrated glass micronozzle suspended inside a flow-focusing geometry suspended inside a flow-focusing geometry. ACS Appl Mater Interfaces. https://doi.org/10.1063/1.4919444
Yoo JS, Kim SJ, Choi JS (1999) Swelling equilibria of mixed solvent/poly(dimethylsiloxane) systems. J Chem Eng Data 44(1):16–22. https://doi.org/10.1021/je980147u
Yu K, Han Y (2006) A stable PEO-tethered PDMS surface having controllable wetting property by a swelling? Deswelling process. Soft Matter 2(8):705. https://doi.org/10.1039/b602880m
Zhang A, Chen M, Du C, Guo H, Bai H, Li L (2013) Poly(dimethylsiloxane) oil absorbent with a three-dimensionally interconnected porous structure and swellable skeleton. https://doi.org/10.1021/am4029203
Zhou X, Zhang Z, Xu X, Men X, Zhu X (2013) Facile fabrication of superhydrophobic sponge with selective absorption and collection of oil from water. Ind Eng Chem Res. https://doi.org/10.1021/ie400942t
Zhu L, Wang Y, Wang Y, You L, Shen X, Li S (2017) An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater 241:285–292. https://doi.org/10.1016/j.micromeso.2016.12.033
Acknowledgements
The authors acknowledge the financial support from the International Doctoral Innovation Centre, Ningbo Bureau of Education, Ningbo Bureau of Science and Technology, and the University of Nottingham. This research was supported by Young Scientist Program from National Natural Science Foundation of China under Grant no. NSFC51506103/E0605, and Zhejiang Provincial Natural Science Foundation of China under Grant no. LQ15E090001. The research was also supported by Inspiration Grant from Faculty of Science and Engineering, University of Nottingham Ningbo China. The authors would like to thank Ms. Qiaoqi Zhu and Ms. Tianyuan Peng for assistance on the microfluidic experiments of microparticle collection and characterization, as well as Mr. Daniel Dai from Agilent Technologies (Shanghai) Co. for helpful technical discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection “2018 International Conference of Microfluidics, Nanofluidics and Lab-on-a-Chip, Beijing, China” guest edited by Guoqing Hu, Ting Si and Zhaomiao Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 10429 KB)
Rights and permissions
About this article
Cite this article
Lian, Z., Ren, Y., He, J. et al. Microfluidic fabrication of porous polydimethylsiloxane microparticles for the treatment of toluene-contaminated water. Microfluid Nanofluid 22, 145 (2018). https://doi.org/10.1007/s10404-018-2157-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-018-2157-y