Abstract
Purpose
In high-intensity focused ultrasound (HIFU) comprising high-intensity burst ultrasound (triggering pulse) and medium-intensity continuous wave ultrasound (heating wave), optimizing the effects of the triggering pulse conditions on the coagulated volume may help to reduce treatment times.
Methods
HIFU combined with a triggering pulse was applied to chicken deep pectoral muscles. The acoustic power of the heating wave was set to 36, 54, or 72 W. Four different triggering pulse conditions were used: heating wave only; or pulse widths and pulse repetition frequencies of 30 μs and 1 kHz, 300 μs, and 100 Hz, or 3 ms and 10 Hz.
Results
Compared to the heating wave only condition, the coagulated volume was significantly larger under all conditions that included a triggering pulse. Significant differences were also observed in the ellipticity of the coagulated region between some conditions.
Conclusion
HIFU combined with a triggering pulse may play an important role in reducing treatment times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-intensity focused ultrasound (HIFU) is a medical procedure that involves focusing ultrasonic energy radiated from outside the body on a focal point. This action induces strong vibrational energy that generates coagulative necrosis in tumors, mostly through thermal action. The procedure only involves sending ultrasonic energy into the body from outside, with no incisions made in the skin, and so is bloodless and minimally invasive. This modality is being used to treat brain diseases and tumors in the prostate, breast, liver, and pancreas, among others [1–3].
While this method shows very strong potential, two major problems are encountered with current HIFU procedures. First, the coagulated volume from each dose of irradiation is very small and forms a long, thin ellipsoid shape. The actual shape depends on the device used and the specific irradiation conditions, but is 3 × 3×12 mm3 for existing liver treatment instruments [1], so irradiation of the entire tumor region is very time-consuming. According to previous studies [1–3], an average of 2.5 h is required to treat a uterine fibroid with a mean size of 77.3 cm3, during which time the patient is not allowed to move. The second problem is that the ultrasonic energy used is much stronger than that of diagnostic ultrasound devices, and is reflected and absorbed at boundaries in the body such as skin, fascia, and bone, which may potentially result in severe burn injuries if focal points form outside the target [4]. Patients are currently required to ameliorate heating of the skin by contact with cold water, but this does not represent a complete solution. These two problems can be solved by developing methods to enable enlargement of the coagulated volume per dose of irradiation and to reduce the intensity of ultrasonic energy irradiated from outside the body.
Various methods are being investigated to enlarge the coagulated volume. One such method is the use of cavitation bubbles generated by ultrasonic waves [5]. As these cavitation bubbles implode under pressure, a sonochemical reaction occurs that has antitumor effects and amplifies the thermal activity of the ultrasonic waves [4, 6]. Conventional HIFU procedures use irradiation methods that do not generate cavitation bubbles, which are difficult to control and can cause skin burns [4]. However, some newer studies have been focusing on sonodynamic therapy, which efficiently uses the high-intensity energy generated by the implosion of cavitation bubbles [5, 7]. One such study [8] used high-intensity burst ultrasound (a triggering pulse) to induce cavitation bubbles, followed by medium-intensity continuous wave ultrasound (a heating wave) to collapse the cavitation bubbles and amplify the thermal action. As a result, the output of heating waves can be reduced, potentially reducing both the risk of burns outside the target and treatment time. While some reports have described the effectiveness of using a triggering pulse [8, 9], more investigation is needed to determine optimal irradiation conditions and effects on enlarging the coagulated volume.
In the present study, HIFU combined with a triggering pulse was applied to chicken deep pectoral muscles to investigate the conditions required to enlarge the coagulated volume, induce implosion of cavitation bubbles, and avoid coagulation of the tissue surface when using a triggering pulse.
Materials and methods
Experimental setup and methods
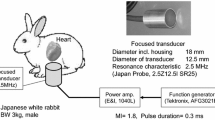
An overview of the experimental setup is shown in Fig. 1a. Chicken deep pectoral muscle was used as the target for HIFU irradiation. The muscle was immersed in 37 °C degassed water for 1 h and then sealed in a 0.04-mm-thick plastic bag. A positioning stage was used to fix the HIFU transducer to the focal position in the water bath. A degasser (ERC-3502W; ERC, Saitama, Japan) was used to create degassed water. The dissolved oxygen volume was kept at ≤3.0 mg/L by constant degassing circulation. A heater (Power Safe Pro 300; Marukan, Osaka, Japan) was used to maintain a degassed water temperature of 37.0–37.9 °C.
We used an annular HIFU transducer with a lead zirconate titanate (PZT) ceramic element (resonant frequency, 1.08 MHz; focal length, 75 mm; outer diameter, 75 mm; inner diameter, 37 mm). A diagnostic ultrasound probe (EUP-C532; Hitachi Aloka Medical, Tokyo, Japan) was placed inside the inner diameter of the HIFU transducer, and changes in B-mode images from an ultrasound diagnosis system (EUB-7500; Hitachi Aloka Medical) were measured during irradiation. The HIFU transducer was energized and driven by a radiofrequency amplifier (A300; E&I, Rochester, NY, USA). The input signal to the amplifier was generated by a function generator (WF1974; NF, Kanagawa, Japan) controlled by software we originally developed.
We used 12 different combinations of irradiation conditions based on preliminary experiments (Fig. 2). The heating wave total acoustic power (TAP) was 36, 54, or 72 W, and the intensity of focal region I spta was 1.0, 1.5, and 2.0 kW/cm2, respectively. The triggering pulse duration and pulse repetition frequency (PRF) were set as shown in Table 1. Triggering pulse TAP was set to 358 W for Conditions b–d. The heating wave TAP was evaluated by the buoyancy method [10], and triggering pulse TAP was estimated by data extrapolation (Fig. 3). The duration of irradiation was 10 s for all conditions. In each chicken deep pectoral muscle, irradiation was focused on four points 10–12 mm below the surface (Fig. 4). This was repeated on 60 muscles. The software that we developed randomly selected irradiation conditions, and the operator did not know which condition had been selected when performing irradiation. A limit was imposed on the software so that, by the end of the experiment, the sample size of each irradiation condition became the same number. To eliminate interactions, we took a break of ≥30 s between irradiation sessions, and set each target about 13–15 mm apart using the positioning stage.
The drive voltage waveform during HIFU combined with a triggering pulse. The triggering pulse induces formation of cavitation bubbles in the tissue, and the heating wave induces coagulation in the target. Pulse width and pulse repetition frequency were set to: a heating wave only, with no triggering pulse; b 30 μs, 1 kHz; c 300 μs, 100 Hz; or d 3 ms, 10 Hz
Evaluation methods
The coagulated volume was evaluated by ellipsoid approximation (Fig. 1b) [8]. Specifically, the depth and width of the coagulated region in a cut section were measured using electronic calipers (PC-15JN; Mitutoyo, Kanagawa, Japan), and the coagulated volume (V) was calculated using Formula 1 below. Formula 2 below was used to define ellipticity (f) to evaluate the shape of the coagulated volume. The closer the ellipticity is to 0, the more spherical the volume is.
Triggering implosion of cavitation bubbles was evaluated ultrasonographically. When observing a target region with the ultrasound diagnosis system during HIFU irradiation, irregular noise was sometimes visible across the entire image (Fig. 5). In a preliminary study, we noted that coagulated regions tended to be larger when such noise was observed. We hypothesized that this noise represented cavitation noise generated by intense implosion of cavitation bubbles, and added this noise to the list of items to be evaluated in the experiment. We observed the duration of cavitation noise and defined it as present when noise was observed for 1/4 of the total irradiation time (i.e., 2.5 s). We then calculated the ratio of trials out of 20 (n = 20) in which cavitation noise was present to determine the cavitation noise incidence rate.
For evaluation of tissue surface coagulation (assuming burns), we determined coagulation to be present if it could be seen with the naked eye. We also calculated the ratio out of 20 trials in which such coagulation was observed to determine the surface coagulation incidence rate.
Statistical analysis
Statistical software (JMP9.0; SAS Institute Japan, Tokyo, Japan) was used to compare results among different conditions. Volume and ellipticity were evaluated by one-way analysis of variance (ANOVA). Pairs that showed significant differences were then tested using the Tukey–Kramer honestly significant difference (HSD) test. Cavitation bubble implosion rate and surface coagulation were tested with Pearson’s Chi squared test, and residual analysis was performed on pairs that differed significantly. The level of significance was set to p < 0.05.
Results
Coagulated volume
Figure 6a shows the mean coagulated volume and 95 % confidence interval for each condition. Under all heating wave TAP conditions (36, 54, and 72 W), trials with a triggering pulse (Conditions b–d) all resulted in significantly larger coagulated volumes than trials with no triggering pulse (Condition a). As one-way ANOVA revealed significant differences in coagulated volume between triggering conditions for each heating wave power of 36, 54, and 72 W (p < 0.0001 for each), Tukey–Kramer HSD tests were performed to compare groups. Significant differences between groups are also shown in Fig. 6a.
Coagulated region ellipticity
Figure 6b shows mean ellipticity and the 95 % confidence interval for each condition. Ellipticity was significantly lower under Conditions c and d compared to Condition a when heating wave TAP was 36 and 54 W. At 72 W, ellipticity was significantly lower under Condition d than under Condition a. As one-way ANOVA revealed significant differences in ellipticity between trigger conditions for each heating wave power of 36, 54, and 72 W (36 W, p = 0.0219; 54 W, p = 0.0003; 72 W, p = 0.0060), Tukey–Kramer HSD tests were performed to compare groups. Significant differences between groups are also shown in Fig. 6b.
Cavitation noise incidence rate
Cavitation noise incidence rates for each condition are shown in Fig. 6c. According to Pearson’s Chi squared tests, cavitation noise differed between trigger conditions at 36, 54, and 72 W (p < 0.0001 for each), so residual analysis was performed. Significant differences between groups are shown in Fig. 6c.
Coagulation at tissue surface
The tissue surface coagulation incidence rates for each condition are shown in Fig. 6d. According to Pearson’s Chi squared tests, no differences in surface coagulation incidence rates were evident between trigger conditions at 36 W (p = 0.5618). Differences were seen, however, at 54 W and 72 W (p = 0.0003, p = 0.0007, respectively).
Relationship between cavitation noise generation and coagulated volume
Table 2 shows mean coagulated volume and 95 % confidence interval for each heating wave power divided according to the presence of cavitation noise. According to t tests, coagulated volume differed significantly at all powers depending on whether cavitation noise was present.
Discussion
In the present study, we performed HIFU irradiation on chicken deep pectoral muscles; evaluated changes in coagulated region volume and shape, cavitation noise generation, and coagulation in the tissue surface when combined with a triggering pulse; and examined triggering pulse irradiation conditions. Under all conditions that included a triggering pulse, coagulated volume was significantly larger. In addition, ellipticity was significantly smaller under some conditions, with the coagulation shape approaching that of a sphere. A significant rise in cavitation noise incidence rate was also observed. Surface coagulation, as one side effect, was shown to be a result of significantly increasing the triggering pulse. Coagulated volume was significantly larger when cavitation noise was present as compared to when it was absent. Although the time-averaged TAP under Conditions b–d was about 12–27 % larger than cases without a triggering pulse, the coagulated volume was at least 1.5 times larger. This suggests that combined use with a triggering pulse induces the implosion of cavitation bubbles, accelerating enlargement of the coagulated volume.
Only triggering pulse conditions differed between Conditions b, c, and d, while total acoustic energy irradiated (the product of acoustic power and irradiation time) was the same across conditions. Under Condition b, short triggering pulses were applied frequently. Under Condition d, comparatively longer triggering pulses were applied less frequently, and Condition c was intermediate between these two conditions. According to results, coagulated volume increased in the order of b < c < d. Meanwhile, a greater tendency for tissue surface coagulation (burns) was seen when heating wave acoustic power was larger, indicating a suitable range for heating wave power. The influence that this condition exerts on the result is large, though the mechanism is not clear. The frequency of the cavitation noise incidence rate varies according to a trigger condition at 36 W, whereas the noise occurs stably in the case of 72 W. When the trigger pulse duration is long, cavitation bubbles expand greatly, and it may be stochastically in the environment where the collapse of cavitation bubbles more readily occurs. In addition, there may be a possibility that a long triggering pulse in itself becomes the energy supplier to cavitation bubbles. Further investigation is necessary to shed light on this mechanism.
Based on these results, for the categories of parameters examined in this experiment, optimal irradiation conditions for HIFU combined with a triggering pulse to increase coagulated volume and generate a spherical region of coagulation, induce implosion of cavitation bubbles, and prevent coagulation at the surface were either 36 W, 3 ms, and 10 Hz or 54 W, 300 μs, and 100 Hz. Use of these conditions increased the coagulated volume by about two- to fourfold compared to conventional HIFU methods that used only a heating wave, potentially reducing irradiation time by 25–50 %. According to previous studies [1, 3], about 2.5 h is required to treat a uterine fibroid with a mean size of 77.3 cm3. By applying a triggering pulse with the conditions revealed in the present study, this treatment time might be reduced. Moreover, as the coagulation shape becomes more spherical, margin setting and other aspects of treatment planning may be carried out more efficiently, helping to reduce the proportion of the target that remains untreated.
As the present study was only a basic experiment using chicken deep pectoral muscles, we were only able to evaluate acute thermocoagulation of muscular tissue from ultrasonic energy. Other effects must be considered in actual living tissues, such as the heat sink effect from blood, so the results of this study cannot necessarily be applied as is. However, there have been reports of chemical antitumor effects [11, 12] that work by generation of singlet oxygen and heat shock proteins from implosion of cavitation bubbles [13]. We can thus expect that addition of these effects in living tissue may potentially increase the efficiency of treatment.
Conclusion
In the present study, we examined a new method of HIFU treatment that added use of a triggering pulse in an attempt to improve the problem of long treatment times with current HIFU treatment. We conducted a basic experiment on chicken deep pectoral muscles and discovered the optimal conditions for inducing implosion of cavitation bubbles with a triggering pulse, increasing the coagulated volume and changing the shape, and preventing coagulation at the tissue surface. These findings will help advance the development of HIFU treatment methods with fewer side effects and shorter treatment times.
References
Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumors. Nat Rev Cancer. 2005;5:321–7.
ter Haar GR. High intensity focused ultrasound for the treatment of tumors. Echocardiography. 2001;18:317–22.
Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol. 2012;81:3624–30.
Frenkel V, Kimmel E, Iger Y. Ultrasound-induced cavitation damage to external epithelia of fish skin. Ultrasound Med Biol. 1999;25:1295–303.
Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–63.
Kim YS, Rhim H, Choi MJ, et al. High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol. 2008;9:291–302.
Frenkel V, Li KC. Potential role of pulsed-high intensity focused ultrasound in gene therapy. Future Oncol. 2006;2:111–9.
Takagi R, Yoshizawa S, Umemura S. Enhancement of localized heating by ultrasonically induced cavitation in high intensity focused ultrasound treatment. Jpn J Appl Phys. 2010;49:07HF21.
Inaba Y, Yoshizawa S, Umemura S. Coagulation of Large Regions by Creating Multiple Cavitation Clouds for High Intensity Focused Ultrasound Treatment. Jpn J Appl Phys. 2010;49:07HF22.
Shaw A. A buoyancy method for the measurement of total ultrasound power generated by HIFU transducers. Ultrasound Med Biol. 2008;34:1327–42.
Feril LB Jr, Kondo T, Cui ZG, et al. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett. 2005;221:145–52.
Hundt W, Yuh EL, Steinbach S, et al. Comparison of continuous vs. pulsed focused ultrasound in treated muscle tissue as evaluated by magnetic resonance imaging, histological analysis, and microarray analysis. Eur Radiol. 2008;18:993–1004.
Hundt W, Yuh EL, Steinbach S, et al. Mechanic effect of pulsed focused ultrasound in tumor and muscle tissue evaluated by MRI, histology, and microarray analysis. Eur J Radiol. 2010;76:279–87.
Acknowledgments
We would like to thank Shin Yoshizawa and Shin-ichiro Umemura for assistance with the use of the HIFU transducer. This research was partially supported by the Ministry of Economy, Trade and Industry, Japan (METI, Standardization Promotion Project). A part of this research was supported by a grant from the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program),” initiated by the Council for Science and Technology Policy (CSTP).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Abe, N., Nakamoto, H., Suzuki, T. et al. Ex vivo evaluation of high-intensity focused ultrasound with ultrasonic-induced cavitation bubbles. J Med Ultrasonics 41, 3–9 (2014). https://doi.org/10.1007/s10396-013-0469-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-013-0469-9