Abstract
Purpose
Acoustic radiation force impulse (ARFI) is a modality for elasticity imaging of various organs using shear waves. In some situations, the heart is a candidate for elasticity evaluation with ARFI. Additionally, an ultrasound contrast agent (UCA) provides information on the blood flow conditions of the cardiac muscle. This study aimed to evaluate ARFI’s effect on the heart concomitantly with UCA administration (i.e., perfluorobutane).
Methods
Ultrasound with ARFI was applied to the hearts of male Japanese white rabbits (n = 3) using a single-element focused transducer with or without UCA administration. They were exposed to ultrasound for 0.3 ms with a mechanical index (MI) of 1.8. UCA was administered in two ways: a single (bolus) injection or drip infusion. Electrocardiograms were recorded to identify arrhythmias during ultrasound exposure.
Results
Extrasystolic waves were observed following ultrasound exposure with drip infusion of UCA. Life-threatening arrhythmia was not observed. The frequency of the extra waves ranged from 4.2 to 59.6 %. With bolus infusion, extra waves were not observed.
Conclusions
Arrhythmogenicity was observed during ultrasound (MI 1.8) with ARFI and concomitant administration of UCA in rabbits. Although the bolus administration of UCA was similar to its clinical use, which may not cause extra cardiac excitation, cardiac ultrasound examinations with ARFI should be carefully performed, particularly with concomitant use of UCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic radiation force impulse (ARFI) [1–3] is a technology that uses shear waves to evaluate the elasticity of various organs, including the heart muscle [4]. When biological tissues are exposed to the focused ultrasound with higher intensity, a shear wave is generated at the focal area. As ARFI continues to progress, the safety of this technique is under investigation [6, 7]. In addition, even a mechanical index (MI) of ARFI <1.9 with concomitant administration of an ultrasound contrast agent (UCA) may reinforce the intensity of the ultrasound by scattering microbubbles and/or causing cavitation [5]. We previously reported on the arrhythmogenetic effect of ultrasound with ARFI and an MI of 4.0 [6]. Subsequently, we proposed an examination of more limited situations to identify the effects of ARFI during ultrasonic elasticity imaging of the heart with concomitant administration of UCA to confirm the safety of this new technology.
Materials and methods
Subjects
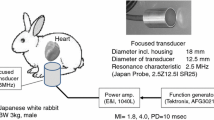
Three male Japanese white rabbits were placed in the supine position under general anesthesia. After shaving the rabbits’ chests, an axial cardiac image (left ventricle) was depicted through the intercostal space using an ultrasonic diagnostic system (UF-400AX; Fukuda Denshi, Tokyo, Japan) equipped with a 7.5-MHz probe (FVT-LS386-9A). The ARFI transducer was also placed on the chest wall using ultrasound gel. The needle electrodes were inserted subcutaneously on the bilateral upper chest to obtain electrocardiograms (ECGs), which were recorded using the PowerLab™ LabChart™ Teaching System (LabChart™; AD Instruments Pty, Castle Hill, Australia).
Acoustic radiation force impulse and sonication system
Ultrasound exposure was derived from a focused transducer (Japan Probe, Kanagawa, Japan) that was custom made for this study (Fig. 1) [6, 7]. The focus was 20 mm from the transducer surface. The signal generated by the function generator (AFG3021B, Tektronix, Beaverton, OR, USA) was amplified using an RF Power Amplifier (1040L, Electronics & Innovation, Rochester, NY, USA) to produce an ARFI. The wave form data were collected as previously reported [6]. The ARFI ultrasound exposure started at 40 ms before the peak of the T wave on the rabbits’ ECGs. This period contains the vulnerable interval that easily produces arrhythmia, including life-threatening ones, by external stimulation [8]. The ultrasound exposure settings included a pulse duration of 0.3 ms and an MI of 1.8 (Fig. 1). The ultrasound exposure was synchronized with the rabbits’ heart beats at one pulse per six heart beats. For each condition of UCA administration (i.e., none, a single injection, or drip infusion), 50 exposures were performed. Analysis of the timing of the exact ARFI exposure on ECG was also performed. The exact exposure timing was classified by the difference from the peak of the T wave: >30 ms before the T wave’s peak was pre-vulnerable, 0–30 ms before the T wave’s peak was vulnerable, and after the T wave’s peak was post-vulnerable.
Ultrasound contrast agent
A gas-containing microbubble contrast agent, perfluorobutane (Sonazoid™; Daiichi-Sankyo, Tokyo, Japan), was used. Two methods of injection were tested: (1) a single (bolus) injection of 0.8 µL of microbubbles followed by ultrasound exposure 10 min after injection and (2) ARFI application 3 min after a drip infusion (1.6 µL of microbubbles per 50 mL of saline) was started. The infusion speed was 50 mL of saline per 30 min.
Statistical analyses
All the statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [9]. R is a free software environment for statistical computing and graphics. More precisely, it is a modified version of the R commander designed, which adds statistical functions that are frequently used in biostatistics. The frequency of extrasystolic waves was compared using Fisher’s exact test. A probability (P) value of <0.05 was considered to be statistically significant.
Results
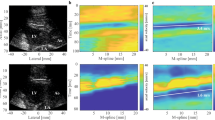
By using ultrasound focused on the heart with ARFI (MI: 1.8), extrasystolic waves (QRS complex) were observed only after continuous administration of UCA (Fig. 2). The frequency of the extra waves ranged from 4.2 to 59.6 %. Almost all the extra waves (97.2 %) had a narrow QRS complex. No extra waves were found without UCA or with bolus administration of UCA, and this was statistically significant (Table 1; P < 0.05). Except for one wide QRS extra wave (Fig. 2b), an extra QRS complex was observed with an 80–125 ms delay in ARFI exposure. After the extrasystolic wave, the regular QRS complex appeared with the doubled RR interval (Fig. 2). With regard to the timing of the exact ARFI exposure with continuous infusion of UCA on ECG, there were no differences in the extra wave frequency according to the exposure timing (relatively pre-vulnerable, vulnerable, and post-vulnerable) (Table 2). During these experimental sessions, life-threatening arrhythmia was not observed.
The upper trace shows the electrocardiogram (ECG) of a rabbit. The lower trace shows the mechanical sensing signal generated by our exposure control device that indicates an R wave followed by the acoustic radiation force impulse (ARFI) exposure. An extrasystolic wave (arrowhead) on ECG is evoked by ultrasound with ARFI and an ultrasound contrast agent (UCA). The timing of ARFI’s exposure (black arrow) is also shown (MI 1.8). a An extrasystolic wave is observed and the RR interval doubled because the regular QRS complex is delayed. The x-axis scale represents 100 ms. b A wide QRS complex and a narrow complex are observed on the same trace. The x-axis scale represents 250 ms
Discussion
Even with an MI of 1.8, using ARFI on a rabbit heart evoked extrasystolic waves with concomitant administration of UCA. Previous studies on rats reported that arrhythmia was evoked by a combination of diagnostic ultrasound and UCA [10, 11]; however, our findings showed that increased microbubbles in the cardiac muscle and/or the blood stream in the cardiac chamber may impact the extrasystolic wave production in rabbits.
We found no extra waves during the bolus infusion of UCA. A reason for this may be that UCA does not deposit much in the cardiac muscle. It was previously reported that perfluorobutane is minimally deposited in the cardiac muscle of rats [12]. To evoke extrasystolic waves in our study, more microbubbles would be needed in the cardiac muscle and/or cardiac chamber (blood stream) during continuous UCA infusion. Thus, the threshold of the microbubble concentration needed to evoke extrasystolic waves should be elucidated.
In our previous report [6], extra waves were not found using ARFI ultrasound (MI: 1.8) with UCA administration, even as a continuous infusion. The methodology for the experiment in the present study was exactly the same as that in the previous study; therefore, the reason for this difference may be a trivial deviation in the transducer on the chest wall, causing individual differences between the rabbits and/or differences in the exact focus site in the cardiac chamber (e.g., the ventricle, atrium, or cardiac muscle). A limitation of the current study was that pre-exposure positioning and exposure were performed using different a probe/transducer. Thus, the ARFI exposure site was not observed in real time during exposure. If cardiac imaging and ARFI exposure are obtained using one transducer, this limitation and any uncertain factors will be reduced.
The destruction of microbubbles caused by ARFI ultrasound with a higher pressure amplitude may stimulate cardiac myocytes and the impulse conduction system of the heart. In the cardiac ventricle, Purkinje fibers are found just beneath the endocardium in all mammals, including rabbits [13]. With the stimulation starting in the cardiac chamber, the fibers would be activated and produce extrasystolic waves. Since almost all of the wave forms of the extrasystolic waves had a narrow QRS pattern with or without a P wave, ARFI stimulation via the UCA response may affect the upper stream of the cardiac impulse conduction system (the atrium and/or atrio-ventricular node) instead of the ventricle myocytes. This was supported by the observed delay in the extrasystolic wave from ARFI exposure, which was similar to the PR interval. To resolve the mechanism of extrasystolic wave generation, further studies are needed to explore the pathways in detail (e.g., try some different exposure sites of the heart).
Although this study was restricted to animals, the arrhythmogenicity of ARFI with concurrent UCA administration may exist in all species, including humans, and the risk of more serious arrhythmia may occur during routine examinations. Moreover, ultrasound with ARFI and UCA can be used concomitantly for evaluating cardiac stiffness and perfusion in a clinical setting [14]. A normal heart’s response to ARFI ultrasound cannot be applied to a diseased heart. Thus, we suggest that ARFI should not be applied to the heart after UCA administration until more studies demonstrate its safety in humans.
Conclusions
In our animal study, extrasystolic waves were observed under conditions that resembled the clinical use of ARFI with concomitant UCA. To produce extra waves, a higher MI and higher density of UCA may be needed; therefore, we recommend that ultrasound practitioners should know this effect, because this phenomenon may occur in some human settings. Cardiac stiffness should be evaluated carefully using this ultrasound modality and no UCA.
References
Nightingale K. Acoustic radiation force impulse (ARFI) imaging: a review. Curr Med Imaging Rev. 2011;7:328–39.
Nightingale K, Bentley R, Gregg ET. Observation of tissue response to acoustic radiation force: opportunities for imaging. Ultrason Imaging. 2002;14:522–9.
Nightingale K, Soo MS, Nightingale R, et al. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–35.
Hsu SJ, Bouchard RR, Dumont DM, et al. In vivo assessment of myocardial stiffness with acoustic radiation force impulse imaging. Ultrasound Med Biol. 2007;33:1706–19.
Miller DL. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and internal cavitation. Prog Biophys Mol Biol. 2007;93:314–30.
Ishiguro Y, Sasanuma H, Nitta N, et al. The arrhythmogenetic effect of ultrasonic exposure with acoustic radiation force (ARF) impulse on the rabbit heart with ultrasound contrast agent; perfluorobutane. J Med Ultrasonics. 2015;42:47–50.
Nitta N, Ishiguro Y, Sasanuma H, et al. Experimental system for in situ measurement of temperature rise in animal tissue under exposure to acoustic radiation force impulse. J Med Ultrasonics. 2015;42:39–46.
Kirchhof PF, Fabritz CL, Zabel M, et al. The vulnerable period for low and high energy T-wave shocks: role of dispersion of repolarisation and effect of d-sotalol. Cardiovasc Res. 1996;31:953–62.
Kanada Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Zachary JF, Hartleben SA, Frizzell LA, et al. Arrhythmias in rat hearts exposed to pulsed ultrasound after intravenous injection of a contrast agent. J Ultrasound Med. 2002;21:1347–56.
Miller DL, Li P, Dou C, et al. Influence of contrast agent dose and ultrasound exposure on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Radiology. 2005;237:137–43.
Toft KG, Hustvedt SO, Hals PA, et al. Disposition of perfluorobutane in rats after intravenous injection of sonazoid. Ultrasound in Med. & Biol. 2006;32:107–14.
Tranum-Jensen J, Wilde AA, Vermeulen JT, et al. Morphology of electrophysiologically identified junctions between Purkinje fibers and ventricular muscle in rabbit and pig hearts. Circ Res. 1991;69:429–37.
Rakhit DJ, Becher H, Monaghan M, et al. The clinical applications of myocardial contrast echocardiography. Eur J Echocardiogr. 2007;8:524–9.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research B (23300194) and C (26350544) from the Japan Society for the Promotion of Science and a Research and Development Committee Program of the Japan Society of Ultrasonics in Medicine. This work was also supported in part by a project of the Ultrasound Equipment and Safety Committee of the Japanese Society of Ultrasonics in Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical consideration
All institutional and national guidelines for the care and use of laboratory animals were followed.
About this article
Cite this article
Ishiguro, Y., Nitta, N., Taniguchi, N. et al. Ultrasound exposure (mechanical index 1.8) with acoustic radiation force impulse evokes extrasystolic waves in rabbit heart under concomitant administration of an ultrasound contrast agent. J Med Ultrasonics 43, 3–7 (2016). https://doi.org/10.1007/s10396-015-0654-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-015-0654-0