Abstract
Hydatid disease, caused by Echinococcus granulosus, is a zoonotic infection encountered worldwide. Though involvement of the liver and lungs is quite common, pelvic involvement is rarely reported, with the incidence being 0.2–2.2 %. Ovarian and broad ligament hydatids are rare entities and are usually seen after rupture of a hepatic hydatid cyst. These cysts are usually asymptomatic, and a high index of clinical suspicion coupled with unequivocal imaging findings is required to make an accurate and timely diagnosis. We present a case of multifocal hydatid disease in a female child involving the lungs and liver and provide an account of the quintessential radiological findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydatid disease is a parasitic zoonosis caused by the larval stage of Echinococcus granulosus and, uncommonly, Echinococcus multilocularis. Canines are the definite hosts of Echinococcus, and human beings and cattle are intermediate hosts. Epidemiologically, it is found everywhere in the world, and is especially common in Asian and African countries. The most common organ to be involved in hydatid disease is the liver (60–70 %), followed by the lungs (10–25 %), while ovarian and parametrial involvement is extremely uncommon [1]. Multiorgan involvement is exceedingly rare and occurs in less than 2 % of the cases. Diagnosis is usually established by serological tests and radiological imaging. The optimal treatment of such cases includes albendazole followed by surgical excision.
Case report
An 8-year-old female child from a rural background presented to the pediatric outpatient department with complaints of difficulty in breathing and recurrent abdominal pain. The patient had no history of any other chronic illness. On examination, vital signs of the patient were stable. General examination revealed pallor. On respiratory examination, there was reduced air entry bilaterally, and fine crepitations were auscultated in bilateral lung bases. Abdominal examination revealed tender hepatomegaly. The rest of the examination was within normal limits.
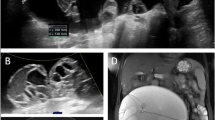
Preliminary routine investigations revealed raised eosinophil count (17 %). Chest radiograph of the patient revealed large, well-defined, spherical radio-opaque shadows, with no air–fluid levels or apparent calcification, in bilateral mid lung zones (Fig. 1). The medial border of the right-sided lesion was indistinct from the right cardiac border. There was no evidence of effusion. The patient was then subjected to abdominal ultrasound examination, which revealed hepatomegaly, with multiple multilocular cystic lesions with multiple internal septations, and no calcifications, distributed in both lobes of the liver (Figs. 2, 3). No free fluid was detected. A provisional diagnosis of multifocal hydatid disease was made.
a Transabdominal ultrasonography with a 3.75-MHz curvilinear probe showing well-defined multilocular cystic lesions in the right hepatic lobe giving rise to a honeycomb appearance and b the same lesion with a linear 7.5-MHz probe showing the “double wall” of these cystic lesions. Note the outer wall (straight white arrow) representing the pericyst and the inner layer (curved white arrow) representing the ectocyst. No definite evidence of calcifications is seen
Thereafter, the patient underwent computed tomography (CT) scanning of the thorax and the abdomen to confirm the diagnosis, ascertain the extent of the lesion, and rule out other possibilities. Contrast-enhanced CT scan of the thorax revealed well-defined, unilocular, spherical, fluid attenuation, cystic lesions with minimally enhancing walls in the right middle lobe and left lower lobe (Fig. 4). There was no evidence of calcifications within the lesions. No definite mediastinal lymphadenopathy was noted, and no apparent communication with bronchi was seen. CT scan of the abdomen revealed multiple multilocular cystic lesions, with thick internal septations producing a honeycomb or cartwheel appearance. These lesions were present in both hepatic lobes (Fig. 5).
ELISA for echinococcal immunoglobulin E was 49.7 Ku/L. The clinical picture combined with the radiopathological appearance strongly favored the diagnosis of multifocal hydatidosis. The patient was started on albendazole, and after 2 weeks she was taken up for surgery.
A midline incision was made and the surgical field involving the peritoneal cavity was packed with sponges dipped in 0.5 % silver nitrate. The largest of the hepatic cysts was then aspirated through a suction device and daughter cysts were evacuated. The residual pericystic cavity was then filled with normal saline and closed by purse-string sutures. Three more similar cysts were excised in this fashion. The cyst wall was sent for histopathological examination, which confirmed the diagnosis of hydatid cyst. No postoperative complications were seen. No definite surgical procedure was planned for pulmonary hydatid cysts, which showed marked regression in size following medical therapy.
Discussion
Hydatid disease is worldwide in distribution and is especially common in countries where animal husbandry is largely practiced. The most common organs to be involved include the liver and lungs. Many authors report pulmonary involvement to be the commonest in the pediatric population [2, 3]. It is hypothesized that increased compliance of lung tissue as compared to the liver favors expansion and growth of these cysts. Pelvic hydatid cysts are rarely primary and are most commonly seen secondary to inoculation from rupture of a hepatic hydatid [4–6].
Clinically, these cysts are usually asymptomatic when small. With growth in size, symptoms secondary to a mass effect of these cysts arise. Large hepatic hydatid cysts may cause abdominal pain and obstructive jaundice. Cough and chest pain are the most common features of pulmonary hydatids. Symptoms may also arise due to complications like rupture of cyst, secondary infection, and formation of cystobiliary fistulas, which may include anaphylaxis, urticaria, or cholangitis.
Pathologically, hydatid cysts consist of three layers. The outermost layer is fibrous and is called pericyst. It is formed due to inflammatory changes secondary to activation of the immune system of the host. Pericyst is absent in hydatid cysts located in brain and lungs. The middle layer is acellular and is called the laminar membrane or the ectocyst, and the inner layer is the germinal layer, which is the vital layer and harbors the parasites, also known as endocyst [7, 8]. The fluid within the cyst is alkaline and is highly antigenic. Peritoneal spillage of this fluid can result in severe anaphylactic reaction.
Imaging plays a pivotal role in the diagnosis of these lesions. The most common imaging modalities include ultrasonography, CT scan, and magnetic resonance imaging (MRI). Plain chest radiograph is a valuable tool in the initial diagnosis of pulmonary hydatid. Several radiographic signs of pulmonary hydatid cysts have been described in the literature, including notch sign, water-lily sign, meniscus sign, annular solar eclipse, and the migratory sign.
Ultrasonography is an essential tool in the diagnosis of hydatid cysts. Several sonographic signs have been described in relation to hydatid cysts. On ultrasonography, the cyst wall is seen as two echogenic lines separated by linear hypoechogenicity [9], the so-called “double wall” sign. The motion of fine echogenic debris with change in the patient’s posture can be well appreciated, and is also referred to as the “snow storm” sign [9]. Detachment of the endocyst from the pericyst can result from trauma, degeneration or host tissue reaction. In such cases, visible “floating membranes” can be appreciated within the cyst. In some cases, a focal rent in the wall can also be noticed. When the detachment is complete, the sonographic “water-lily sign” can be seen, which is so named because of the resemblance of pulmonary hydatids to waterlily. With disease progression, the physical properties of the hydatid fluid also undergo transition from watery to more viscid fluid, resulting in the folds of the germinal layer becoming immobile and being seen as serpentine structures entrapped within an echogenic matrix. This appearance is called the “congealed water-lily” sign. Daughter cysts are visualized as multiple cysts separated from each other by well-defined walls and may give rise to a multilocular or honeycomb appearance of these lesions. In conditions where the cyst cavity is completely obliterated by the matrix, the cyst may appear as a solid mass [10]. Calcification, both within the wall as well as within the matrix, can also be well depicted by ultrasound. Ultrasound stands out as the most sensitive imaging modality in detection of septa, membranes, and hydatid sand [9].
CT scan clearly depicts most of the findings that are seen on sonography. On contrast-enhanced CT scan, these cysts show a high attenuation wall. Calcifications, detached membranes, and daughter cysts can be easily seen on CT scan. CT scan is also the investigation of choice in detection of cyst infection. On MRI, these cysts contain a hypointense peripheral rim on T2W sequences, which is represented by pericyst, which is rich in collagen [11]. The daughter cysts are hypointense on T1W sequences and hyperintense on T2W sequences in comparison with cyst fluid. Ovarian and broad ligament hydatids are extremely rare, and in a review of 532 cases by Bickers et al. [12], only two cases of broad ligament hydatids were identified. Most of the ovarian hydatid cysts are seen in elderly and middle-aged females. The treatment of hydatid disease is chiefly surgical, and albendazole is given preoperatively to reduce their size. The overall prognosis of these cysts is good with reported recurrence of 7–14 % [13]. Recurrence is chiefly attributed to rupture of the cyst. In cases of ovarian hydatids, cystectomy is the treatment of choice, while complete cyst excision with preservation of maximum possible pulmonary tissue is advocated for lung hydatids.
References
Tsaroucha AK, Polychronidis AC, Lyrantzopoulos N, et al. Hydatid disease of the abdomen and other locations. World J Surg. 2005;29:1161–5.
King CH. Cestodes (tapeworms). In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious disease. 6th ed. New York: Churchill Livingstone; 2005. p. 3290–2.
Karaoglanoglu N, Kurkcuoglu IC, Gorguner M, et al. Giant hydatid lung cysts. Eur J Cardiothorac Surg. 2001;19(6):914–7.
Gueddana F, Chemmen Lebbene L, Lebi I, et al. Intrauterine hydatidosis: a case report. J Obstet Gynecol Reprod Biol (Paris). 1990;19:725–7.
Komongui DG, Diouf A, Dao B, et al. Hydatid cyst of the uterus (apropos of a case seen at the gynecologic and obstetrical clinic of the C.H.U. of Dakar). Dakar Med. 1990;35:162–7.
Tarcoveanu E, Dimofte G, Bradea C, et al. Multiple peritoneal hydatid disease after rupture of a multivesicular hepatic hydatid cyst: case report. J Gastrointestin Liver Dis. 2006;15:301–5.
Pumarola A, Rodriguez-Torres A, García-Rodriguez JA, et al. Microbiología y parasitologíamédica. 2nd ed. Spain: Salvat; 1990.
King CH. Cestodes (tapeworms). In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. p. 2544–53.
Moguillanski SJ, Gimenez CR, Villavicencio RL. Radiología de la hidatidosis abdominal. In: Stoopen ME, Kimura K, Ros PR, editors. Radiología e imagendiagnóstica y terapeútica: abdomen, vol. 2. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 47–72.
Gharbi HA, Hassine W, Brauner MW, et al. Ultrasound examination of the hydatid liver. Radiology. 1981;139:459–63.
Davolio SA, Canossi GC, Nicoli FA, et al. Hydatid disease: MR imaging study. Radiology. 1990;175:701–6.
Bickers WM. Hydatid disease of the female pelvis. Am J Obstet Gynaecol. 1970;107:477–83.
Xu MQ. Hydatid disease of the lung. Am J Surg. 1985;150(5):568–73.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jha, A., Ullah, E., Gupta, P. et al. Sonography of multifocal hydatidosis involving lung and liver in a female child. J Med Ultrasonics 40, 471–474 (2013). https://doi.org/10.1007/s10396-013-0444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-013-0444-5