Abstract
Defining the linkages between landscape change, disease ecology and human health is essential to explain and predict the emergence of Plasmodium knowlesi malaria, a zoonotic parasite residing in Southeast Asian macaques, and transmitted by species of Anopheles mosquitos. Changing patterns of land use throughout Southeast Asia, particularly deforestation, are suggested to be the primary drivers behind the recent spread of this zoonotic parasite in humans. Local ecological changes at the landscape scale appear to be increasing the risk of disease in humans by altering the dynamics of transmission between the parasite and its primary hosts. This paper will focus on the emergence of P. knowlesi in humans in Malaysian Borneo and the ecological linkage mechanisms suggested to be playing an important role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Southeast Asia, the zoonotic Plasmodium knowlesi has emerged to become the fifth malarial parasite infecting humans, presenting a serious public health threat in the region (Moyes et al. 2014; Rajahram et al. 2016). At least nine Southeast Asian countries have confirmed human cases (Shearer et al. 2016). The highest incidence rates are found in Malaysia where 69% of all current malaria cases (n = 2627) are P. knowlesi, found mostly as a mono-infection, and concentrated in the states of Sabah and Sarawak (World Health Organization 2017). Reported knowlesi cases increased in Malaysia from 376 in 2008 to 1604 by 2016 (8 of these imported from Indonesia, Papua New Guinea and Thailand) (World Health Organization 2017).
Routinely, microscopy misidentifies P. knowlesi as P. malariae (in 97.2% of cases), or to a lesser degree as P. falciparum or P. vivax (William et al. 2014, Singh and Daneshvar 2013). Notwithstanding this, data from Sabah of PCR-confirmed cases were 18 times higher (n = 1067) in 2013 than notifications in 2004 (n = 59) (William et al. 2013, 2014). This represented 62% of all malaria notifications in Sabah in 2013 (William et al. 2013, 2014). The situation in Sarawak is similar, with a significant trend of increasing incidence detected from 1992 to 2014 (P < 0.001), rising notably in 2008 (Ooi et al. 2017). Projections suggest that the incidence rate from 2010 will triple by 2040 (Ooi et al. 2017). William et al. (2014) describe these trends as an accurate reflection of the parasites’ emergence in humans in Malaysian Borneo.
The P. knowlesi parasite was brought to Southeast Asia with its hosts prior to human settlement in the region (Lee et al. 2011; 2009). Current research points to deforestation and anthropogenic land-use change causing increased contact between humans, mosquito vectors and the macaque hosts of P. knowlesi in Malaysian Borneo (Manin et al. 2016). Primates, along with their roles as seed dispersers, are being lost from the tropical forests and marginalized into habitats that overlap increasingly with human habitation (Estrada et al. 2017). Deforestation decimates mammalian biodiversity resulting in a corresponding loss of the dilution effect (Civitello et al. 2015; Wilcove et al. 2013; Yue et al. 2015). This may concentrate the knowlesi parasite in the macaque hosts making them more infectious to humans, in a process comparable to that for hantavirus in rodents when small-mammal diversity was reduced (Suzán et al. 2009; Mills 2006). Furthermore, the altered biotic and abiotic conditions arising from land-use change, with the creation of forest fringes, may be creating favourable environments for the mosquito vectors of the parasite (Tan et al. 2008; Brant et al. 2016; Wong et al. 2015b; Yakob et al. 2018).
These anthropogenic changes to the landscape in Malaysian Borneo are influencing the dynamics of parasite transmission between mosquito vectors, macaques and humans (Brock et al. 2016). Recent modelling suggests that the vectors display differing transmission responses under varying scenarios of macaque–human host availability (Yakob et al. 2018). Further analysis suggests that P. knowlesi is adapting to changes in the distribution and vectorial capacity of its vectors in Malaysian Borneo (Benavente et al. 2017). The exact mechanism by which the current land-use changes are affecting host and vector abundance, distribution and behaviour, resulting in an increased risk of P. knowlesi malaria in humans, is yet to be determined.
Anthropogenic Environmental Changes
Deforestation
Tropical forests are home to at least two-thirds of the world’s biodiversity even though they cover less than 10% of the land surface of the Earth (Bradshaw et al. 2009; Raven 1988). Within 1.4% of the tropical forest land area (including islands such as Borneo) are found 44% and 35% of the world’s plant and animal species, respectively—‘hot spots’ of biodiversity (Myers et al. 2000). These forests contain countless endemic species which hold valuable genetic information and are being lost to future generations (Tanner and Kirk 2008). Recent satellite analysis shows that net deforestation is continuing across the tropical forest belt (Hansen et al. 2013). The primary economic drivers responsible are large-scale commercial agriculture and land-use intensification (Geist and Lambin 2002; DeFries et al. 2010; Rudel 2017; Leblois et al. 2017; Barbier 2004; Malhi et al. 2014). Selective logging is a further pressure with over 400 million hectares of tropical forest around the world currently under designation as logging concessions (Martin et al. 2015).
Southeast Asia could be considered the tropical region of greatest environmental concern (Sodhi et al. 2012; Hughes 2017), having been referred to as ‘an impending disaster’ with predictions suggesting three quarters of its original forests along with 42% of its biodiversity could be gone by 2100 (Sodhi et al. 2004). Rates of deforestation in Southeast Asia are high and accelerating, with Malaysia having the highest level of deforestation in relation to land area (Hansen et al. 2013).
The island of Borneo has long been known as a major centre of biodiversity and endemism, a location rich in evolutionary history (Woodruff 2010). Between 1990 and 2009, almost 80% of the land surface of Malaysian Borneo had been impacted by forest logging or clearing, leaving only 8% in Sabah and 3% in Sarawak covered by intact forests within protected areas (Bryan et al. 2013). A total of 70% of the subregions’ lowland forests (773,000 km2) and 65% of its peat swamp forests (96,000 km2) had been lost by 2010 (Wilcove et al. 2013). Sarawak (as well as the eastern lowlands of Sumatra) lost around half of its peatland swamp forest alone between 2000 and 2010 (Miettinen et al. 2011). These forests are being converted to industrial plantations of oil palm (Elaeis guineensis) and pulpwood (Gaveau et al. 2016; Bryan et al. 2013). Oil palm has been the principal driver of deforestation in Malaysian Borneo over the past four decades clearing 4.2 Mha for the establishment of estates (Gaveau et al. 2016).
Forest Fragments and Fringes
Deforestation not only converts virgin forest to anthropogenic homogenous land uses, but also fragments remaining forest cover into small reserves that sit as disconnected forest patches within this mosaic of homogeneity (Taubert et al. 2018; Cushman et al. 2017; Tanner and Kirk 2008). There can be a marked difference in how ecological groups and individual species respond to forest fragmentation with the physical and biotic changes that arise at the abrupt margins of the forest edge (Laurance et al. 2018; Barros and Honório 2015; Despommier et al. 2006; Loh et al. 2016). Not only is biodiversity affected through restrictions to habitat range, but alterations to ecosystem functions such as forest hydrology, carbon storage and biochemical cycles also occur (Laurance et al. 2018).
In Borneo, the largest forest fragment contains 18% of remaining forest cover (Taubert et al. 2018) with many smaller forest fragments interspersed with oil palm plantations and logging concessions over the island (Scriven et al. 2017; Brühl et al. 2003). Alarmingly however, even a medium-sized forest fragment (42.9 km2) sampled in Sabah reflected a sharp decline in species richness and abundance for leaf litter ant communities. Using these useful ecosystem disturbance indicators, Bruhl et al. (2003) showed that the number of ant species declined by 47.5% (n = 48) in the aforementioned forest fragment when compared with contiguous undisturbed forest (n = 101). This finding is disturbing because most forest fragments in Sabah are smaller than this. One fragment of only 0.46 km2 was noted to be dominated by invasive ant species including the highly destructive Anoplolepis gracilipes (Brühl et al. 2003; Brühl and Eltz 2010). Similar findings have been recorded from Amazonian research which compared ant species richness between two forest fragments, both only 1 km2, compared to continuous forest areas. Both fragments had reduced species richness and 65.8% (n = 27) of species had greater nest densities in the continuous forest (Carvalho and Vasconcelos 1999).
The size of forest fragments clearly has a direct influence on biodiversity and the species which can survive in situ, with ‘bigger is better’ being the general rule (Keinath et al. 2017). However, a key finding from the Biological Dynamics of Forest Fragments Project (BDFFP) in the Amazon is that even fragments less than 0.1 km2 have ecological value, and that the wider surrounding landscape and even regional and global ecological conditions have a vital role to play in influencing local biodiversity (Laurance et al. 2018). This result was also deduced from a meta-analysis of over 1000 species of vertebrates and invertebrates within habitat patches that varied in isolation and area by 8 and 12 orders of magnitude, respectively. The surrounding matrix of land use may have an even more important influence on the occupancy of many species than actual fragment size (Prugh et al. 2008). For example, Scriven et al. (2017) found that less than 50% of butterfly species from a Borneo rainforest were able to cross the boundary of an oil palm plantation to reach further forest habitat because their larval host plants were not found within the oil palm.
Reduced Habitat Complexity of Oil Palm Estates
In relation to animal biodiversity, there is a marked difference between forest conversion to oil palm and selective logging in that selectively logged forests have shown the ability to retain a degree of their faunal community which can recover with time, whereas oil palm plantations are monocultures, found to be relatively devoid of vertebrate species (Fitzherbert et al. 2008; Wilcove and Koh 2010; Tuck et al. 2016; Bell 2015). Fitzherbert et al. (2008) compared thirteen studies on animal biodiversity between oil palm and undisturbed forest and showed that on average only 15% of the taxa from primary forests were found in oil palm plantations with vertebrate taxa consistently less than half that of the pristine forest. In Sabah, high mammal species diversity found in an undisturbed forest habitat was reduced to fourteen species at the forest edge and then to only one to two species at a distance of 2 km into a nearby oil palm plantation (Yue et al. 2015).
Invertebrates also show overall reduced species richness compared with primary and secondary forests, with a significant alteration to the species assemblages present (Turner and Foster 2009; Gray et al. 2015, 2017; Chung et al. 2000; Luke et al. 2017b; Mercer et al. 2014; Savilaakso et al. 2014). In one study, arthropod biomass was reduced by 87.5% in epiphytes, by 72.4% in the leaf litter and by 37.9% in the canopy when the primary forest was compared to an oil palm estate (Turner and Foster 2009). Further examples of taxa that were reported as depauperate in oil palm plantations compared with undisturbed forest include macro-fungi (Shuhada et al. 2017), bats (Fukuda et al. 2009), birds (Aratrakorn et al. 2006), lizards (Glor et al. 2001), small mammals (Cusack 2011) and frogs (Faruk et al. 2013).
Herbivores become more abundant and predators less diverse and abundant in oil palm estates, when compared to logged habitats (Chung et al. 2000) with the more generalist and opportunistic species predominating in the oil palm (Wang et al. 2016; Patz et al. 2004; Loh et al. 2016). For example, species of the invasive Rattus genus which readily feed on oil palm seeds are commonly found in abundance in oil palm landscapes (Cusack 2011; Tanner and Kirk 2008), as is the bearded pig—Sus barbatus (Love et al. 2017). In relation to ground-dwelling ant species in Sabah, highest abundances were recorded for non-forest species, with the most common species Anoplolepis gracilipes, present at 70% of bait sites in oil palm (Brühl and Eltz 2010).
In Borneo, streams in oil palm plantations were found to have lower riparian quality compared with logged forests, resulting in warmer water temperature, shallower depths and more sand (Luke et al. 2017a). Changes to stream ecosystems in this manner reduce the suitable habitat for benthic macroinvertebrates and alter community composition and diversity (Burdon et al. 2013). Studies have recorded an absence of dragonfly larvae (Odonata) (Luke et al. 2017b) as well as absences of Coleoptera (beetles) and Hemiptera (true bugs) from streams within oil palm plantations (Mercer et al. 2014). Retaining riparian buffer zones in oil palm streams in Sabah helped to mitigate the impact on the macroinvertebrate community (Chellaiah and Yule 2018).
A recent study from Colombia set out to determine whether there was a critical threshold of oil palm land cover which triggered a significant decline in mammalian species richness. The authors found that between 45% and 75% of oil palm cover in the landscape correlated with a strong indication of community compositional change for most mid–large-sized mammals (Pardo et al. 2018). Oil palm cover of 75% or more resulted in a decline of nearly all the terrestrial mammals in this area.
Ways to improve biodiversity and ecological functioning in oil palm estates have become a crucial focus for research in order to make them more sustainable and environmentally acceptable (Ashraf et al. 2018; Azhar et al. 2015, 2017). Studies from Peninsular Malaysia show that polyculture cropping in oil palm estates where other income-producing plants are included increases habitat heterogeneity and biodiversity for bird species richness and abundance (Yahya et al. 2017). Also, alley cropping systems which alternate the oil palm with a secondary food crop have been found to increase arthropod biodiversity as well as the number of predators and decomposers, enhancing the overall biodiversity and ecological functioning of the plantation (Ashraf et al. 2018). Other research in eastern Sabah has studied agroforestry combinations in oil palm utilizing Tectona grandis (teak) and Aquilaria sp. (agarwood) with positive results for carbon stock and biodiversity enhancement compared to the monoculture (Suardi et al. 2016; James et al. 2016).
Azhar et al. (2015) compared small-scale and large-scale management of oil palm plantations in Peninsular Malaysia and found that small-scale farmers produced much higher habitat heterogeneity measures than the large-scale producers, improving benefits for biodiversity. From an economic perspective, however, large-scale, intensively managed oil palm estates have the most capacity for biodiversity improvement (bird species richness and abundance) through tree enrichment at a relatively low cost (Teuscher et al. 2015).
The importance of connecting forest fragments and increasing habitat heterogeneity in and around oil palm estates can scarcely be overstated (Koh 2008). Biodiversity is more positively affected in oil palm estates by having old-growth forest patches retained in proximity, than by enhancing the local vegetation structure within the estate (Koh 2008). To this end, the establishment of wildlife corridors between disturbed habitats such as oil palm is seen as increasingly important (Brodie et al. 2015a), as is the need to maintain the coverage of oil palm within surrounding land uses to below the threshold limit (45–75%) determined for supporting mammalian biodiversity (Pardo et al. 2018).
Forest Restoration
The widespread and pervasive damage from human activities to the planet’s ecosystems, with the resulting loss of essential ecosystem services (ES) and biodiversity, has brought about an urgent focus on restoration efforts (Hobbs and Norton 1996; Hobbs and Cramer 2008; Budiharta et al. 2016). Globally, estimates suggest that 2 billion hectares of forest could be brought under restoration management (Crouzeilles et al. 2016), which is defined as the ‘process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed’ (SER 2004).
Forests such as the tropical peat swamps of Southeast Asia store an enormous amount of carbon which is released to the atmosphere upon degradation, and only an estimated 6% of these ecosystems remained in a pristine condition in 2015 (Graham et al. 2017). Therefore, the goals of tropical forest restoration are manyfold and may include carbon sequestration, biodiversity conservation, preservation and improvements to water supplies, and support for human habitats and livelihoods (Holl 2017).
In Borneo, forest restoration is urgently needed in forest fragments and in underproductive oil palm plantations to increase available habitat and forest connectivity for the wildlife (Hearn et al. 2018; Yeong et al. 2016). Fragmented forest patches often undergo repeated logging for Dipterocarpaceae trees and as such can become highly degraded (Yeong et al. 2016). Forest restoration in Sabah follows an enrichment planting technique where seedlings of dipterocarps (and small numbers of other species) are planted along lines (or gaps) cleared out of the vegetation leftover from the previous forest management practice (Hector et al. 2011). This technique is used to accelerate the recovery of dipterocarps where natural regeneration would be insufficient in secondary forests as this family are late successional species (Romell et al. 2008). These seedlings are then managed over the following few years to encourage growth by clearing away invasive vines and competing pioneer species such as Macaranga spp. (Hector et al. 2011).
Although deforestation clearly affects ecosystem services and biodiversity negatively, restoration does not hold the promise of a linear and predictable return to pristine conditions (Hobbs et al. 2006). In many cases, ecological restoration produces ‘novel’ ecosystems that are irreversibly different from original conditions (Hobbs et al. 2006, 2009). Research undertaken within restored forests in Sabah on avian and leaf litter detritivore biodiversity demonstrated that species richness was diminished in both cases, when compared to the unlogged forest (Cosset and Edwards 2017; Edwards et al. 2012). In their recent study, Cosset and Edwards (2017) noticed that the bird species proliferating in the restored forests were closely related, exhibiting similar functional traits, resulting in an overall reduction in functional richness when compared with the unlogged forest.
Similar findings have been recorded from the forests in Sarawak, Borneo. Natural regeneration in forests of northern Sarawak produced lower species diversity for trees, fungi and army ants even after 20–60 years of growth (Takano et al. 2014). Also in Sarawak, edge-dwelling butterfly species richness was still three times lower in the restored fallows than in the primary forest after the same time frame of 20–60 years. Importantly, after the first 20 years, the rate of recovery slowed and depended upon connection to the primary forest (Itioka et al. 2015). Specialist species are less able to survive habitat loss and less able to recolonize restored forest than more generalist species (Loh et al. 2016).
Biodiversity and Loss of the Dilution Effect
It may be conjectured that the most biodiverse habitats, harbouring maximal species richness (and therefore animal disease), would pose the greatest disease threat to humans from zoonotic spillover when contact occurs (Mills 2006; Ostfeld and Keesing 2017). For this to be the case, pathogen diversity would have to be a function of host diversity and human intrusion into highly biodiverse environments would result in exposure to a more diverse pathogen pool (Murray and Daszak 2013). This is termed the ‘amplification effect’ which simply states that increased species diversity increases the disease risk for humans (Keesing et al. 2006). The logic behind this argument—that high host diversity translates into high pathogen diversity—is relatively well established in the scientific literature (Ostfeld and Keesing 2017; Morand et al. 2014).
However, for the ‘amplification effect’ to occur, a high pathogen diversity must also translate into a relatively high zoonotic pathogen diversity, where the potential for spillover to humans is likely. This situation is not currently supported by the literature, with overall host–pathogen diversity not directly translating into a high overall diversity of zoonotic species (Ostfeld and Keesing 2017). Biodiversity may be a source of pathogens, but the loss of biodiversity appears to be more highly correlated with an increase in zoonotic emerging infectious disease (Morand et al. 2014). In work on the ecology of Lyme disease in the USA, it was discovered that infection risk varied inversely with vertebrate host diversity (Ostfeld and Keesing 2000a, b).
Loss of biodiversity is hypothesized to result in an increase in emerging zoonotic diseases as a result of losing vertebrate species that either predate upon, compete with or simply dilute the overall host diversity pool (Civitello et al. 2015; McCallum 2015; Ostfeld 2009; Ostfeld and Keesing 2000b; Keesing et al. 2006; Levi et al. 2016). Species-rich communities allow pathogens to ‘waste time’ by infecting hosts which have a low competency for disease transmission (Johnson and Thieltges 2010). In lower-diversity habitats, there is a tendency for more transmission events to occur between a single species (the preferred host species) which results in more efficient transmission of the pathogen and therefore a higher prevalence and greater risk for humans and wildlife (Mills 2006; Johnson et al. 2013; Cunningham et al. 2017).
Levi et al. (2016) studied changes in vertebrate community composition and the effect upon tick nymphs infected with Lyme bacterium. Their results suggest that diverse host communities appear to provide two types of dilution hosts: ones that occur with enough abundancy that they absorb blood meals away from the most competent hosts and alternatively ones that can reduce the abundance of the most competent hosts through competition and predation. In relation to malaria, these findings were borne out by modelling evidence from the Brazilian tropical rainforest, where biodiversity (in this case, a high abundance of wild warm-blooded animals) correlated with a protective affect against malaria (Laporta et al. 2013).
The Role of Ecological Linkage Mechanisms
O’Sullivan et al. (2008) suggest that the anthropogenic impacts on the world’s forests and ecosystems can directly or indirectly result in observable human health outcomes, such as emerging infectious diseases, through ecological changes at the landscape scale. They refer to these changes as ecological linkage mechanisms (ELMs). Forests that have been logged, or converted to oil palm plantations, or restored from a degraded state, all show varying degrees of biodiversity loss resulting from reduced or altered habitat complexity and fragmentation. The result is a change in the community composition, behaviour and condition of animal hosts, vectors and ultimately pathogens, through disrupting predation and competition between species (Wilcox and Colwell 2005; Keesing et al. 2010; Estrada-Peña et al. 2014; Loh et al. 2016; Patz et al. 2008). In the case of zoonoses, the landscape may become pathogenic to humans through this disruption to the natural cycle of parasite transmission rates within reservoir hosts (Lambin et al. 2010; Murray and Daszak 2013).
The concept of ecological linkages between anthropogenic land-use change, biodiversity loss and human health is a well-researched field, and many studies provide support for the existence of such relationships. Findings linking deforestation and ecosystem disruption to emerging infectious diseases have been recorded in almost all parts of the world (Vittor et al. 2006, 2009; Morris et al. 2016; Kilpatrick 2011; McFarlane et al. 2013; Brock et al. 2016; Morand et al. 2014; Young et al. 2017; Tucker et al. 2017; Gottdenker et al. 2014; Jones et al. 2013; Kilpatrick and Randolph 2012). However, the ability to determine a general relationship between land-use change, biodiversity loss and disease risk remains elusive due to the complexity of factors involved (Loh et al. 2016) and the fact that not all findings demonstrate an increased risk of disease transmission (Tucker et al. 2017; Yasuoka and Levins 2007).
There is a paucity of data comparing different landscape assemblages and the health risk they pose to humans (Brock et al. 2016). Salkeld et al. (2013) analysed metadata and found only a weak and highly heterogeneous relationship between host biodiversity and zoonotic infectious disease risk. Oversampled pathogens such as Plasmodium may also skew the trend that anthropogenic changes drive disease transmission (Gottdenker et al. 2014). Furthermore, restoration of ecosystems back to more ecological functionality has not necessarily brought about improved human health conditions (Speldewinde et al. 2015).
To this end, O’Sullivan et al. (2008) stress the importance of examining in detail the linkage mechanisms in the role of emerging infectious diseases as the locally specific changes in the ecology that can be observed, understood and therefore potentially managed so as to reduce the negative outcomes in human health. In the case of zoonotic diseases such as P. knowlesi, numerous and complex factors have a role to play at various scales of influence (Estrada-Peña et al. 2014) and ultimately require a transdisciplinary approach (Loh et al. 2016). Studies such as these are urgently needed to inform land-use planning policies in tropical countries (Loh et al. 2016).
Ecological Linkage Mechanisms (I): Mosquito Vectors, Deforestation and Biodiversity Loss
Malaria in humans and non-human primates is a disease directly dependent on environmental conditions which influence the life cycle of the Anopheles mosquito vectors (Austin et al. 2017). These vectors are generally forest-dwelling species, so deforestation and changes to land use can alter the malaria transmission dynamics in critical ways (Austin et al. 2017; Tucker et al. 2017). Recent literature suggests an association between deforestation and increased malaria transmission (Vittor et al. 2006, 2009; Yasuoka and Levins 2007; Austin et al. 2017; Burkett-Cadena and Vittor 2018), or conversely between higher levels of biodiversity and low malaria transmission (Laporta et al. 2013).
For the Southeast Asian region, there is a paucity of information on the impact of deforestation and malaria (Guerra et al. 2006) often with contrasting findings occurring in relation to anthropogenic changes to land use. In Sri Lanka and South Korea, respectively, malaria vectors are associated with forest conversion to irrigated rice fields (Amerasinghe and Ariyasena 1990; Sithiprasasna et al. 2005). A recent study from Thailand examined mosquito vectors over a range of land uses and reported that all vector species were least abundant in the undisturbed forest sites compared to the altered habitats (Thongsripong et al. 2013). However, in Northern Thailand, forest fragmentation for agriculture increased landscape heterogeneity and resulted in a decreased density of two malaria species (An. maculatus s.s. and An. minimus s.l.) over at least one season in the agricultural area compared to undisturbed forest (Overgaard et al. 2003). Further recent research from this region has found wide spatial variation in the ecological factors influencing the prevalence of malaria in South Sumatra, Indonesia, with the researchers calling for a more in-depth understanding of the local ecological factors influencing disease transmission and prevalence of mosquito vectors (Hasyim et al. 2018).
Mosquitos require standing or slow-flowing water to oviposit their eggs, and habitat choice can range from sunlit pools, turbid water, to vegetation covered swamps full of organic matter (Patz et al. 2000). The suitability of the habitat influences the number of adult mosquito hatching from the pupae (Ramasamy and Surendran 2016). Biotic factors of predation and competition as well as abiotic factors of sunlight, rainfall, temperature, pH, turbidity, vegetation and nutrient availability all influence this outcome (Canelas et al. 2016). Any anthropogenic changes that improve habitat ecology for mosquitos may potentially create hot spots of transmission dynamics (Ramasamy and Surendran 2016).
Changes to canopy cover through the loss of large trees result in heavy rainfall reaching the ground. Nutrients are washed away through the erosion of the forest floor, the leaf litter, soil and plant roots. Streams and rivers silt up causing declining water quality and impermanent pools (Hecht and Cockburn 2010). Mosquito larvae are often found in higher abundances in ephemeral pools than nearby natural and permanent water bodies (Emidi et al. 2017). Predation and competition in natural larval habitats suppress mosquito population density (Mereta et al. 2013). However, predation on mosquito larvae may be low when ephemeral pools are used as breeding sites as predators will not have become established (Kweka et al. 2011). As some predators are chemically detectable by mosquitos (Saward-Arav et al. 2016), a lack of these predators may encourage ovipositing and increase mosquito abundance in these pools.
Deforestation and changes to landscapes alter the microclimate of aquatic breeding sites and suitability of outdoor resting places for adult mosquitos through changes to the vegetation, ambient temperature and level of humidity (Afrane et al. 2006, 2012; Patz and Olson 2006). Temperature is a particularly critical factor in malaria transmission, having a direct influence on both mosquito and parasite population (Pascual et al. 2006; Afrane et al. 2008). With the loss of canopy cover, more sunlight reaches the ground and the aquatic habitats, potentially creating ideal breeding conditions for Anopheline larvae (Barros and Honório 2015) through ecological changes outlined in the following paragraphs.
A highly significant correlation (P < 0.005) between malaria cases and forest patch size < 5 km2 was a major finding from recent research in the Brazilian Amazon (Chaves et al. 2018). The malaria vector in this region, Anopheles darlingi, favours the forest edge created around remnant patches for its larval stage development (Barros et al. 2011). Further research from the Amazon found An. darlingi larvae in increased abundance in water sources at the fringes of primary forest and an apparent preference for this habitat compared to both the forested and totally deforested zones (Barros and Honório 2015). Emerging macrophytes and algae in water sources receiving more light attract ovipositing females as algae are an important food source for mosquito larvae (Vittor et al. 2009; Brouard et al. 2011). Furthermore, aquatic habitats receiving more sunlight through loss of canopy cover have shown increased viability and survival of mosquito larvae as certain pathogenic fungi are inhibited under these conditions (Rueda Páramo et al. 2015).
Several studies from the western highlands of Kenya show warmer ambient and/or water temperatures linked to increased mosquito fecundity and survival (Kweka et al. 2016; Afrane et al. 2005, 2006; Munga et al. 2006), increased habitat range (Kulkarni et al. 2016; Afrane et al. 2012) and increased vectorial capacity (78% in this instance) through more rapid sporogonic development of Plasmodium falciparum, within the An. gambiae vector (Afrane et al. 2008). These changes can be quite marked. For example, comparisons between full forest canopy cover and sunlight-exposed deforested areas showed an increase in the survival of An. gambiae larvae from 1–2% to 55–57% (Tuno et al. 2005). On the China–Myanmar border, deforestation was found to increase the Anopheles pupation rate from 3.8% in the forested environment to 52.5% (Wang et al. 2016). Food source availability may be another factor involved in this process because of increased algae in sunlit aquatic habitats (Wang et al. 2016; Munga et al. 2006).
Other studies demonstrate that deforestation and/or conversion to oil palm can be highly detrimental to certain species of Anopheles mosquitos. Deforestation can result in certain populations of forest-dwelling mosquitos diminishing to be replaced by different species having a preference for the altered ecological conditions (Hii et al. 2018). The abundance of Anopheles donaldi in Sarawak, a forest-dependent human malaria vector which requires clean, shaded pools for breeding showed a decline in abundance of 64% over a 4-year period, most dramatically during the first 2 years upon conversion to oil palm (Chang et al. 1997). A study from the north-western region of India, where forest cover diminished by more than 50% over a decade (2000–2009), found Anopheles culicifacies s.l. increasing in abundance (as well as malaria parasites, based on ELISA analyses) in the deforested areas, replacing An. minimus s.l. as the dominant malaria vector in the region (Saxena et al. 2014).
How the loss of biodiversity from deforestation, reforestation or oil palm plantation establishment in Malaysian Borneo is affecting the mosquito vectors of P. knowlesi is still largely unknown (Vythilingam et al. 2016).
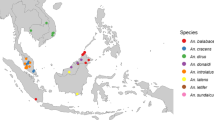
The vectors of P. knowlesi belong to the leucosphyrus group of Anopheles mosquitoes and are forest dwellers (Vythilingam et al. 2016; Collins 2012). The leucosphyrus group consists of two different complexes: the dirus complex of An. dirus and An. cracens, and the leucosphyrus complex of An. latens, An. balabacensis and An. introlatus (Moyes et al. 2016). The leucosphyrus complex is suggested to be the main P. knowlesi vectors in Malaysia, Indonesia, Singapore, Brunei and parts of the Philippines with the dirus complex vectors widespread in the northern countries of Myanmar, Thailand, Cambodia and Vietnam (Moyes et al. 2016). These complexes operate as geographically discrete groups (Shearer et al. 2016).
Research shows that An. balabacensis is one of the most important vectors for human malaria in Southeast Asia and appears to represent a widespread species complex of three or more species (Sallum et al. 2005a, b, 2007). An. balabacensis is also the primary mosquito species responsible for transmitting the P. knowlesi parasite in Sabah (Chua et al. 2017) where it displays a definite preference for logged forest over the primary forest (Brant et al. 2016). Furthermore, mitochondrial DNA analysis in subpopulations of An. balabacensis in Sabah was examined for genetic variation from 14 different study sites with previous recordings of P. knowlesi cases. Results showed that An. balabacensis is experiencing population expansion and growth (Manin et al. 2018).
An. latens, a vector of P. knowlesi in Kapit, Sarawak, bites both humans and monkeys on the ground, as well as monkeys in the canopy (Tan et al. 2008) The highest infection rates have been found to occur in the forest through forest-based activities as opposed to the peri-domestic area (Tan et al. 2008). An. latens is collected from both farming plots and forest locations. Its vectorial capacity was found to be highest in farming areas and lowest in the forest, highlighting how deforestation and a change in land use have been influential (Tan et al. 2008). All these mosquito species were rarely found outside of forested areas in the 1960s (Vythilingam et al. 2016). Nowadays, they appear to have adapted well to forest edges and farms (Vythilingam et al. 2016; Wong et al. 2015a). It is suggested that these vectors may be following the macaque hosts to the edges of the forest and into closer contact with human settlements as a result of the deforestation and landscape changes occurring in Malaysian Borneo (Vythilingam et al. 2016).
In summary, the ecological linkage mechanisms that result from deforestation and may be influencing P. knowlesi infection risk in Sabah are both biotic and abiotic. These include increased mosquito fecundity and survival, faster larval development and pupation rates, increased habitat range, increased vectorial capacity and overall increased abundance of larvae and adult vectors when conditions are suitable. Through deforestation, reforestation or conversion to oil palm plantations, species assemblages are disrupted for many different taxa including frogs, birds, fish, spiders and stream invertebrates (Luke et al. 2017b; Mercer et al. 2014; Faruk et al. 2013; Turner and Foster 2009; Cosset and Edwards 2017). Taxa such as these are known to predate upon adult mosquitos or larvae, or sometimes compete for food with mosquito larvae, as in the case of certain tadpoles (Shaalan and Canyon 2009; Mokany and Shine 2003).
Observations to date show that P. knowlesi vectors display preferences for logged forests and farms over unlogged forests. This may reflect habitat modifications resulting from biotic and abiotic ecological linkage mechanisms conducive to mosquito growth and survival.
Ecological Linkage Mechanisms (II): Simian Hosts, Deforestation and Biodiversity Loss
Primates are under threat in all tropical forest ecosystems, with estimates that around 75% of species are in decline (Estrada et al. 2017). It is well established that deforested areas contain less mammalian biodiversity than undisturbed habitats (Wilcove et al. 2013; Yue et al. 2015). Bernard et al. (2016) investigated habitat disturbance in northern Borneo where the authors found declining primate species richness associated with increasing levels of disturbance, with palm oil plantations harbouring the lowest level of species richness.
This displacement of primates by deforestation causes greater potential for contact with humans as they seek out alternative food sources, often near to human settlements (Brotcorne et al. 2014; Jarvis 2016; Peterson and Riley 2013; Gumert 2011). For example, the long-tailed macaque is known to be an opportunistic species and therefore already well adapted to the forest fringes that arise from deforestation (Gumert 2011). Increased macaque abundance in a smaller habitat may increase their risk of infection through crowding and stress from competition for food, compromising their immune systems (Loh et al. 2016).
Deforestation places certain forest species under a much greater threat of hunting, poaching and illegal trafficking of wildlife (Brodie et al. 2015b; Hambali et al. 2012, 2014). Deforestation, in drastically reducing biodiversity, results in a loss of the dilution effect where malaria mosquito vectors lose access to a range of mammals for blood meals, some of which may be dead-end hosts in which the parasite would be unable to replicate (Ostfeld and Keesing 2000b). This increases transmission rates of the parasite between available hosts. All these factors may lead to increased transmission of the parasite into humans.
Impacts on Human Health
Deforestation reduces the available habitat for the knowlesi vectors and macaque hosts, driving them to remaining forested areas and forest fringes. In Malaysian Borneo, village level data collected in the districts of Kudat and Kota Marudu in Sabah from 2008 to 2012 documented that P. knowlesi cases correlated positively with villages having high local remnant forest cover (bringing contact with macaques and mosquitos) together with large-scale deforestation in the surrounding areas (Fornace et al. 2016). Forest cover was found to have declined by close to 5% between 2008 and 2012 in both the study districts, with 51% of villages losing > 10% of forest cover within a 5 km radius (Fornace et al. 2016).
In Borneo, those individuals most at risk of contracting P. knowlesi infection have been found to be traditional farmers (most often male) working at the forest edge, particularly those who have been recently employed by the palm oil plantations or in jobs relating to agriculture and forestry (Vythilingam et al. 2014; Grigg et al. 2017). In the Kapit region of Sarawak, 87% of cases (n = 152) reported having been recently to the jungle or the forest fringes and were typically Iban (ethnic minority) farmers or forestry workers (Daneshvar et al. 2009).
In Sabah, plantation workers and farmers comprised 40% of those infected with the disease (n = 130) and 92% of those infected reported entering the forest or the fringes within the previous month (Barber et al. 2013). Macaque sightings have been reported by 50% of infected cases in Sabah (Barber et al. 2013) and 72% of cases in Kapit, Sarawak (Daneshvar et al. 2009). Data from 2016 for Sabah and Sarawak show P. knowlesi infection most common in adults (highest in adults > 55 years) and affecting more men (80%) than women (World Health Organization 2017). Infected children represent only 6–14% of cases (Daneshvar et al. 2018).
Integration
Zoonotic P. knowlesi malaria has emerged as an infection in humans in Southeast Asia, particularly in Malaysian Borneo (Ahmed and Cox-Singh 2015; World Health Organization 2017). Anthropogenic environmental change and the resulting loss of biodiversity appear to be increasing the risk of P. knowlesi malaria in humans through ecological linkage mechanisms as outlined in Fig. 1.
For P. knowlesi, the ecological linkage mechanisms proposed here include loss of the dilution effect through decreased biodiversity (Keesing et al. 2006; Civitello et al. 2015), displacement of macaque hosts from primary forest bringing increased contact with humans (Bernard et al. 2016; Brotcorne et al. 2014) and altered abundance, distribution and behaviour of the mosquito vectors for the disease as a result of the biotic and abiotic changes arising from deforestation (Tan et al. 2008; Brant et al. 2016; Wong et al. 2015b; Yakob et al. 2018).
Future Directions
-
With the continuing expansion of oil palm estates in Malaysia and Indonesia (Gaveau et al. 2016; Kassam 2017; Wilcove and Koh 2010), a revisiting of the sustainable oil palm certification scheme is essential to support small-scale farmers who achieve higher biodiversity from a more heterogeneous cropping landscape (Azhar et al. 2017).
-
Methods to increase biodiversity and ecosystem function (such as alley cropping) within oil palm estates need to become key management strategies (Ashraf et al. 2018). Research on critical thresholds for mammalian species richness within different scenarios of oil palm coverage is required in Malaysia and Indonesia to assist with mammal conservation in and around the plantations (Pardo et al. 2018).
-
Logging and agriculture still represent the biggest threats to biodiversity and threatened species around the planet (Sean et al. 2016). In Borneo, fragmented forest patches often undergo repeated logging for Dipterocarpaceae trees and as such can become highly degraded (Yeong et al. 2016). Selective logging of dipterocarp forests has been found to severely impact forest composition, structure and functioning with estimates of a 40% reduction in aboveground biomass even 22 years post-harvesting (Hector et al. 2011). Forest restoration is needed in these forest fragments and in underproductive oil palm plantations to increase available habitat and forest connectivity for Borneo’s wildlife (Hearn et al. 2018; Yeong et al. 2016). Wildlife corridors, where forests are allowed to regenerate, or are assisted with enrichment planting, are required to support movement of threatened wildlife (Brodie et al. 2015a).
-
There is a need to reverse the looming trend of non-human primate extinction in the tropical forests. The pig-tailed macaque (Macaca nemestrina) is already considered as Vulnerable under IUCN status (Brodie et al. 2015b). Unsustainable land use, poaching and hunting drive species to extinction and also exacerbate the risk of zoonotic diseases through increasing overlap of human and threatened primate habitats (Estrada et al. 2017). Primates such as the macaques are essential for maintaining tropical biodiversity and ecosystem health (Estrada et al. 2017).
-
Large-scale prospective studies using molecular methods in humans and monkeys are required to determine the geographic boundaries of P. knowlesi and its ability to infect other primate species (Fong 2017).
-
Most importantly, as this review paper highlights, a more complete understanding of the ecological processes and mechanisms that produce variations in P. knowlesi infection risk is needed (Brock et al. 2016; Loh et al. 2016). How land use influences parasite transmission by affecting vectorial capacity, behaviour and distribution is key to understanding the epidemiology of the disease (Benavente et al. 2017; Yakob et al. 2018).
-
In order to achieve these outcomes, transdisciplinary research initiatives are required to determine transmission hot spots and to develop rapid diagnostic testing, treatment options and prevention methods to break the transmission cycle (Estrada-Peña et al. 2014; Loh et al. 2016). The preservation of tropical biodiversity for both humans and animals (domestic and wild) is the best insurance policy against the rise of emerging infectious diseases such as P. knowlesi (Patz et al. 2000, 2004; Sandifer et al. 2015; Jones et al. 2013).
References
AFRANE, Y. A., GITHEKO, A. K. & YAN, G. 2012. The ecology of Anopheles mosquitoes under climate change: case studies from the effects of deforestation in East African highlands. Annals of the New York Academy of Sciences, 1249, 204-210.
AFRANE, Y. A., LAWSON, B. W., GITHEKO, A. K. & YAN, G. 2005. Effects of Microclimatic Changes Caused by Land Use and Land Cover on Duration of Gonotrophic Cycles of Anopheles gambiae (Diptera: Culicidae) in Western Kenya Highlands. Journal of Medical Entomology, 42, 974-980.
AFRANE, Y. A., LITTLE, T. J., LAWSON, B. W., GITHEKO, A. K. & YAN, G. 2008. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya.(RESEARCH). Emerging Infectious Diseases, 14, 1533.
AFRANE, Y. A., ZHOU, G., LAWSON, B. W., GITHEKO, A. K. & YAN, G. 2006. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. The American journal of tropical medicine and hygiene, 74, 772.
AHMED, M. & COX‐SINGH, J. 2015. Plasmodium knowlesi–an emerging pathogen. ISBT science series, 10, 134-140.
AMERASINGHE, F. & ARIYASENA, T. 1990. Larval survey of surface water-breeding mosquitoes during irrigation development in the Mahaweli Project, Sri Lanka. Journal of Medical Entomology, 27, 789-802.
ARATRAKORN, S., THUNHIKORN, S. & DONALD, P. F. 2006. Changes in bird communities following conversion of lowland forest to oil palm and rubber plantations in southern Thailand. Bird conservation international, 16, 71-82.
ASHRAF, M., ZULKIFLI, R., SANUSI, R., TOHIRAN, K. A., TERHEM, R., MOSLIM, R., NORHISHAM, A. R., ASHTON-BUTT, A. & AZHAR, B. 2018. Alley-cropping system can boost arthropod biodiversity and ecosystem functions in oil palm plantations. Agriculture, Ecosystems and Environment, 260, 19-26.
AUSTIN, K., F., MEGAN, O. B. & PRIYOKTI, R. 2017. Anthropogenic forest loss and malaria prevalence: a comparative examination of the causes and disease consequences of deforestation in developing nations. AIMS Environmental Science, 4, 217-231.
AZHAR, B., SAADUN, N., PRIDEAUX, M. & LINDENMAYER, D. B. 2017. The global palm oil sector must change to save biodiversity and improve food security in the tropics. Journal of Environmental Management, 203, 457-466.
AZHAR, B., SAADUN, N., PUAN, C. L., KAMARUDIN, N., AZIZ, N., NURHIDAYU, S. & FISCHER, J. 2015. Promoting landscape heterogeneity to improve the biodiversity benefits of certified palm oil production: Evidence from Peninsular Malaysia. Global Ecology and Conservation, 3, 553-561.
BARBER, B. E., WILLIAM, T., GRIGG, M. J., MENON, J., AUBURN, S., MARFURT, J., ANSTEY, N. M. & YEO, T. W. 2013a. A Prospective Comparative Study of Knowlesi, Falciparum, and Vivax Malaria in Sabah, Malaysia: High Proportion With Severe Disease From Plasmodium Knowlesi and Plasmodium Vivax But No Mortality With Early Referral and Artesunate Therapy. Clinical Infectious Diseases, 56, 383-397.
BARBIER, E. B. 2004. Explaining agricultural land expansion and deforestation in developing countries. American Journal of Agricultural Economics, 86, 1347-1353.
BARROS, F. S. M., ARRUDA, M. E., GURGEL, H. C. & HONÓRIO, N. A. 2011. Spatial clustering and longitudinal variation of Anopheles darlingi (Diptera: Culicidae) larvae in a river of the Amazon: the importance of the forest fringe and of obstructions to flow in frontier malaria. Bulletin of Entomological Research, 101, 643-658.
BARROS, F. S. M. & HONÓRIO, N. A. 2015. Deforestation and Malaria on the Amazon Frontier: Larval Clustering of Anopheles darlingi (Diptera: Culicidae) Determines Focal Distribution of Malaria. The American journal of tropical medicine and hygiene, 93, 939.
BELL, T. E. 2015. Changes in Ant Communities across a Tropical Rainforest Landscape which includes Old growth and Twice-logged areas in Sabah, Malaysia. Master of Research, Imperial College London.
BENAVENTE, E. D., DE SESSIONS, P. F., MOON, R. W., HOLDER, A. A., BLACKMAN, M. J., ROPER, C., DRAKELEY, C. J., PAIN, A., SUTHERLAND, C. J. & HIBBERD, M. L. 2017. Analysis of nuclear and organellar genomes of Plasmodium knowlesi in humans reveals ancient population structure and recent recombination among host-specific subpopulations. PLoS genetics, 13, e1007008.
BERNARD, H., BILI, R., MATSUDA, I., HANYA, G., WEARN, O. R., WONG, A. & AHMAD, A. H. 2016. Species Richness and Distribution of Primates in Disturbed and Converted Forest Landscapes in Northern Borneo. Tropical Conservation Science, 9, 1940082916680104.
BRADSHAW, C. J., SODHI, N. S. & BROOK, B. W. 2009. Tropical turmoil: a biodiversity tragedy in progress. Frontiers in Ecology and the Environment, 7, 79-87.
BRANT, H. L., EWERS, R. M., VYTHILINGAM, I., DRAKELEY, C., BENEDICK, S. & MUMFORD, J. D. 2016. Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malaria journal, 15, 370.
BROCK, P. M., FORNACE, K. M., PARMITER, M., COX, J., DRAKELEY, C. J., FERGUSON, H. M. & KAO, R. R. 2016. Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology, 143, 389-400.
BRODIE, J. F., GIORDANO, A. J., DICKSON, B., HEBBLEWHITE, M., BERNARD, H., MOHD‐AZLAN, J., ANDERSON, J. & AMBU, L. 2015a. Evaluating multispecies landscape connectivity in a threatened tropical mammal community. Conservation Biology, 29, 122-132.
BRODIE, J. F., GIORDANO, A. J., ZIPKIN, E. F., BERNARD, H., MOHD‐AZLAN, J. & AMBU, L. 2015b. Correlation and persistence of hunting and logging impacts on tropical rainforest mammals. Conservation Biology, 29, 110-121.
BROTCORNE, F., MASLAROV, C., WANDIA, I. N., FUENTES, A., BEUDELS‐JAMAR, R. C. & HUYNEN, M. C. 2014. The role of anthropic, ecological, and social factors in sleeping site choice by long‐tailed macaques (Macaca fascicularis). American journal of primatology, 76, 1140-1150.
Brouard O, Le Jeune AH, Leroy C, Cereghino R, Roux O, Pelozuelo L, Dejean A, Corbara B, Carrias JF, Evens T (2011) Are algae relevant to the detritus-based food web in tank-bromeliads? PLoS ONE 6: e20129.
BRÜHL, C., ELTZ, T. & LINSENMAIR, K. 2003. Size does matter – effects of tropical rainforest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodiversity & Conservation, 12, 1371-1389.
BRÜHL, C. A. & ELTZ, T. 2010. Fuelling the biodiversity crisis: species loss of ground-dwelling forest ants in oil palm plantations in Sabah, Malaysia (Borneo). Biodiversity and Conservation, 19, 519-529.
BRYAN, J. E., SHEARMAN, P. L., ASNER, G. P., KNAPP, D. E., AORO, G. & LOKES, B. 2013. Extreme differences in forest degradation in Borneo: comparing practices in Sarawak, Sabah, and Brunei. PloS one, 8, e69679.
BUDIHARTA, S., MEIJAARD, E., WELLS, J. A., ABRAM, N. K. & WILSON, K. A. 2016. Enhancing feasibility: Incorporating a socio-ecological systems framework into restoration planning. Environmental Science and Policy, 64, 83-92.
BURDON, F. J., MCINTOSH, A. R. & HARDING, J. S. 2013. Habitat loss drives threshold response of benthic invertebrate communities to deposited sediment in agricultural streams. Ecological Applications, 23, 1036-1047.
Burkett-Cadena ND, Vittor AY (2018) Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic and Applied Ecology 26:101–110.
CANELAS, T., CASTILLO-SALGADO, C. & RIBEIRO, H. 2016. Systematized Literature Review on Spatial Analysis of Environmental Risk Factors of Malaria Transmission. Advances in Infectious Diseases, 6, 52.
CARVALHO, K. S. & VASCONCELOS, H. L. 1999. Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biological Conservation, 91, 151-157.
CHANG, M., HII, J., BUTTNER, P. & MANSOOR, F. 1997. Changes in abundance and behaviour of vector mosquitoes induced by land use during the development of an oil palm plantation in Sarawak. Transactions of the Royal Society of Tropical Medicine and Hygiene, 91, 382-386.
CHAVES, L. S. M., CONN, J. E., LÓPEZ, R. V. M. & SALLUM, M. A. M. 2018. Abundance of impacted forest patches less than 5 km 2 is a key driver of the incidence of malaria in Amazonian Brazil. Scientific reports, 8, 7077.
CHELLAIAH, D. & YULE, C. M. 2018. Riparian buffers mitigate impacts of oil palm plantations on aquatic macroinvertebrate community structure in tropical streams of Borneo. Ecological Indicators, 95, 53-62.
CHUA, T. H., MANIN, B. O., DAIM, S., VYTHILINGAM, I. & DRAKELEY, C. 2017. Phylogenetic analysis of simian Plasmodium spp. infecting Anopheles balabacensis Baisas in Sabah, Malaysia. PLoS Neglected Tropical Diseases, 11, e0005991.
CHUNG, A. Y. C., EGGLETON, P., SPEIGHT, M. R., HAMMOND, P. M. & CHEY, V. K. 2000. The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bulletin of Entomological Research, 90, 475-496.
CIVITELLO, D. J., COHEN, J., FATIMA, H., HALSTEAD, N. T., LIRIANO, J., MCMAHON, T. A., ORTEGA, C. N., SAUER, E. L., SEHGAL, T., YOUNG, S. & ROHR, J. R. 2015. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proceedings of the National Academy of Sciences, 112, 8667-8671.
COLLINS, W. E. 2012. Plasmodium knowlesi: a malaria parasite of monkeys and humans. Annual review of entomology, 57, 107-121.
COSSET, C. C. P. & EDWARDS, D. P. 2017. The effects of restoring logged tropical forests on avian phylogenetic and functional diversity. Ecological Applications, 27, 1932-1945.
CROUZEILLES, R., CURRAN, M., FERREIRA, M. S., LINDENMAYER, D. B., GRELLE, C. E. & BENAYAS, J. M. R. 2016. A global meta-analysis on the ecological drivers of forest restoration success. Nature communications, 7, 11666.
CUNNINGHAM, A. A., DASZAK, P. & WOOD, J. L. N. 2017. One Health, emerging infectious diseases and wildlife: two decades of progress? Philosophical Transactions of the Royal Society B: Biological Sciences, 372.
CUSACK, J. 2011. Characterising small mammal responses to tropical forest loss and degradation in Northern Borneo using capture-mark-recapture methods. Department of Life Sciences, Silwood Park, Imperial College London.
Cushman SA, Macdonald EA, Landguth EL, Malhi Y, Macdonald DW (2017) Multiple-scale prediction of forest loss risk across Borneo. Landscape Ecology 32(8):1581–1598.
Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PC, Singh B (2009) Clinical and laboratory features of human Plasmodium knowlesi infection. Clinical Infectious Diseases 49(6):852-860.
Daneshvar C, William T, Davis TM (2018) Clinical features and management of Plasmodium knowlesi infections in humans. Parasitology 145(1):18-31
DEFRIES, R. S., RUDEL, T., URIARTE, M. & HANSEN, M. 2010. Deforestation driven by urban population growth and agricultural trade in the twenty-first century. Nature Geoscience, 3, 178.
DESPOMMIER, D., ELLIS, B. R. & WILCOX, B. A. 2006. The role of ecotones in emerging infectious diseases. EcoHealth, 3, 281-289.
EDWARDS, D., BACKHOUSE, A., WHEELER, C., KHEN, C. & HAMER, K. 2012. Impacts of logging and rehabilitation on invertebrate communities in tropical rainforests of northern Borneo. Journal of Insect Conservation, 16, 591-599.
EMIDI, B., KISINZA, W. N., MMBANDO, B. P., MALIMA, R. & MOSHA, F. W. 2017. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites & Vectors, 10, 304.
ESTRADA-PEÑA, A., OSTFELD, R. S., PETERSON, A. T., POULIN, R. & DE LA FUENTE, J. 2014. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends in Parasitology, 30, 205-214.
ESTRADA, A., GARBER, P. A., RYLANDS, A. B., ROOS, C., FERNANDEZ-DUQUE, E., DI FIORE, A., NEKARIS, K. A.-I., NIJMAN, V., HEYMANN, E. W. & LAMBERT, J. E. 2017. Impending extinction crisis of the world’s primates: Why primates matter. Science advances, 3, e1600946.
FARUK, A., BELABUT, D., AHMAD, N., KNELL, R. J. & GARNER, T. W. 2013. Effects of oil‐palm plantations on diversity of tropical anurans. Conservation biology, 27, 615-624.
FITZHERBERT, E. B., STRUEBIG, M. J., MOREL, A., DANIELSEN, F., BRÜHL, C. A., DONALD, P. F. & PHALAN, B. 2008. How will oil palm expansion affect biodiversity? Trends in ecology & evolution, 23, 538-545.
Fong, I (2017) Zoonotic malaria: Plasmodium knowlesi. In: Emerging Zoonoses, Berlin: Springer
FORNACE, K. M., ABIDIN, T. R., ALEXANDER, N., BROCK, P., GRIGG, M. J., MURPHY, A., WILLIAM, T., MENON, J., DRAKELEY, C. J. & COX, J. 2016. Association between Landscape Factors and Spatial Patterns of Plasmodium knowlesi Infections in Sabah, Malaysia. Emerging Infectious Diseases, 22, 201-209.
FUKUDA, D., TISEN, O. B., MOMOSE, K. & SAKAI, S. 2009. Bat diversity in the vegetation mosaic around a lowland dipterocarp forest of Borneo. Raffles Bulletin of Zoology, 57, 213-221.
GAVEAU, D. L., SHEIL, D., SALIM, M. A., ARJASAKUSUMA, S., ANCRENAZ, M., PACHECO, P. & MEIJAARD, E. 2016. Rapid conversions and avoided deforestation: examining four decades of industrial plantation expansion in Borneo. Scientific reports, 6, 32017.
GEIST, H. J. & LAMBIN, E. F. 2002. Proximate causes and underlying driving forces of tropical deforestation: Tropical forests are disappearing as the result of many pressures, both local and regional, acting in various combinations in different geographical locations. BioScience, 52, 143-150.
GLOR, R. E., FLECKER, A. S., BENARD, M. F. & POWER, A. G. 2001. Lizard diversity and agricultural disturbance in a Caribbean forest landscape. Biodiversity & Conservation, 10, 711-723.
GOTTDENKER, N. L., STREICKER, D. G., FAUST, C. L. & CARROLL, C. 2014. Anthropogenic land use change and infectious diseases: a review of the evidence. Ecohealth, 11, 619-632.
GRAHAM, L. L., GIESEN, W. & PAGE, S. E. 2017. A common‐sense approach to tropical peat swamp forest restoration in Southeast Asia. Restoration Ecology, 25, 312-321.
Gray C, Slade E, Mann D, Lewis O (2017) Designing oil palm landscapes to retain biodiversity using insights from a key ecological indicator group. bioRxiv 204347
GRAY, C. L., LEWIS, O. T., CHUNG, A. Y. & FAYLE, T. M. 2015. Riparian reserves within oil palm plantations conserve logged forest leaf litter ant communities and maintain associated scavenging rates. Journal of Applied Ecology, 52, 31-40.
GRIGG, M. J., COX, J., WILLIAM, T., JELIP, J., FORNACE, K. M., BROCK, P. M., VON SEIDLEIN, L., BARBER, B. E., ANSTEY, N. M., YEO, T. W. & DRAKELEY, C. J. 2017. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: a case-control study. The Lancet Planetary Health, 1, e97-e104.
GUERRA, C. A., SNOW, R. W. & HAY, S. I. 2006. A global assessment of closed forests, deforestation and malaria risk. Annals of tropical medicine and parasitology, 100, 189.
GUMERT, M. D. 2011. The common monkey of Southeast Asia: Longtailed macaque populations, ethnophoresy, and their occurrence in human environments. Monkeys on the edge: Ecology and management of long-tailed macaques and their interface with humans, 3-44.
HAMBALI, K., ISMAIL, A., MD-ZAIN, B. M., AMIR, A. & KARIM, F. A. 2014. Diet of Long-Tailed Macaques (Macaca fascicularis) at the entrance of Kuala Selangor Nature Park (Anthropogenic Habitat): Food selection that leads to human-macaque conflict. Acta Biologica Malaysiana, 3, 58-68.
HAMBALI, K., ISMAIL, A., ZULKIFLI, S. Z., MD-ZAIN, B. M. & AMIR, A. 2012. Human-macaque conflict and pest behaviors of long-tailed macaques (Macaca fascicularis) in Kuala Selangor Nature Park. Tropical Natural History, 12, 189-205.
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova SA, Tyukavina A, Thau D, Stehman SV, Goetz SJ, Loveland TR, Kommareddy A (2013) High-resolution global maps of 21st-century forest cover change. Science 342(6160):850-853
HASYIM, H., NURSAFINGI, A., HAQUE, U., MONTAG, D., GRONEBERG, D. A., DHIMAL, M., KUCH, U. & MÜLLER, R. 2018. Spatial modelling of malaria cases associated with environmental factors in South Sumatra, Indonesia. Malaria journal, 17, 87.
HEARN, A. J., CUSHMAN, S. A., GOOSSENS, B., MACDONALD, E., ROSS, J., HUNTER, L. T., ABRAM, N. K. & MACDONALD, D. W. 2018. Evaluating scenarios of landscape change for Sunda clouded leopard connectivity in a human dominated landscape. Biological Conservation, 222, 232-240.
Hecht SB, Cockburn A (2010) The fate of the forest: developers, destroyers, and defenders of the Amazon. University of Chicago Press, Chicago
HECTOR, A., PHILIPSON, C., SANER, P., CHAMAGNE, J., DZULKIFLI, D., O’BRIEN, M., SNADDON, J. L., ULOK, P., WEILENMANN, M. & REYNOLDS, G. 2011. The Sabah Biodiversity Experiment: a long-term test of the role of tree diversity in restoring tropical forest structure and functioning. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 3303-3315.
HII, J., VYTHILINGAM, I. & ROCA-FELTRER, A. 2018. Human and Simian Malaria in the Greater Mekong Subregion and Challenges for Elimination. Towards Malaria Elimination-A Leap Forward. IntechOpen.
HOBBS, R. J., ARICO, S., ARONSON, J., BARON, J. S., BRIDGEWATER, P., CRAMER, V. A., EPSTEIN, P. R., EWEL, J. J., KLINK, C. A. & LUGO, A. E. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global ecology and biogeography, 15, 1-7.
HOBBS, R. J. & CRAMER, V. A. 2008. Restoration ecology: interventionist approaches for restoring and maintaining ecosystem function in the face of rapid environmental change. Annual Review of Environment and Resources, 33, 39-61.
HOBBS, R. J., HIGGS, E. & HARRIS, J. A. 2009. Novel ecosystems: implications for conservation and restoration. Trends in ecology & evolution, 24, 599-605.
HOBBS, R. J. & NORTON, D. A. 1996. Towards a conceptual framework for restoration ecology. Restoration ecology, 4, 93-110.
HOLL, K. D. 2017. Research directions in tropical forest restoration. Annals of the Missouri Botanical Garden, 102, 237-250.
Hughes AC (2017) Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 8(1): 01624
ITIOKA, T., TAKANO, K., KISHIMOTO-YAMADA, K., TZUCHIYA, T., OHSHIMA, Y., KATSUYAMA, R.-I., YAGO, M., YATA, O., NAKAGAWA, M. & NAKASHIZUKA, T. 2015. Chronosequential changes in species richness of forest-edge-dwelling butterflies during forest restoration after swidden cultivation in a humid tropical rainforest region in Borneo. Journal of Forest Research, 20, 125-134.
James D, Phua M-H, Besar NA, Mokhtar M (2016) Aboveground carbon stock potential of teak (tectona grandis) under different land use system in Balung plantation, Tawau Sabah
Jarvis M (2016) The dietary impacts of deforestation on macaca fascicularis (long-tailed macaque) using metabarcoding. Imperial College, London
JOHNSON, P. & THIELTGES, D. 2010. Diversity, decoys and the dilution effect: how ecological communities affect disease risk. Journal of Experimental Biology, 213, 961-970.
JOHNSON, P. T., PRESTON, D. L., HOVERMAN, J. T. & RICHGELS, K. L. 2013. Biodiversity decreases disease through predictable changes in host community competence. Nature, 494, 230.
JONES, B. A., GRACE, D., KOCK, R., ALONSO, S., RUSHTON, J., SAID, M. Y., MCKEEVER, D., MUTUA, F., YOUNG, J. & MCDERMOTT, J. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proceedings of the National Academy of Sciences, 110, 8399-8404.
KASSAM, Z. 2017. Considerations of Development in Malaysian Borneo. EnviroLab Asia, 1, 5.
KEESING, F., BELDEN, L. K., DASZAK, P., DOBSON, A., HARVELL, C. D., HOLT, R. D., HUDSON, P., JOLLES, A., JONES, K. E. & MITCHELL, C. E. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468, 647-652.
KEESING, F., HOLT, R. D. & OSTFELD, R. S. 2006. Effects of species diversity on disease risk. Ecology Letters, 9, 485-498.
KEINATH, D. A., DOAK, D. F., HODGES, K. E., PRUGH, L. R., FAGAN, W., SEKERCIOGLU, C. H., BUCHART, S. H. & KAUFFMAN, M. 2017. A global analysis of traits predicting species sensitivity to habitat fragmentation. Global Ecology and Biogeography, 26, 115-127.
Kilpatrick AM (2011) Globalization, land use, and the invasion of West Nile virus. Science 334(6054):323-7
KILPATRICK, A. M. & RANDOLPH, S. E. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. The Lancet, 380, 1946-1955.
Koh LP (2008) The oil palm conundrum: how oil palm agriculture affects tropical biodiversity and what can we do about it. Princeton University, Princeton
KULKARNI, M. A., DESROCHERS, R. E., KAJEGUKA, D. C., KAAYA, R. D., TOMAYER, A., KWEKA, E. J., PROTOPOPOFF, N. & MOSHA, F. W. 2016. 10 Years of Environmental Change on the Slopes of Mount Kilimanjaro and Its Associated Shift in Malaria Vector Distributions. Frontiers in Public Health, 4, 281.
KWEKA, E. J., KIMARO, E. E. & MUNGA, S. 2016. Effect of Deforestation and Land Use Changes on Mosquito Productivity and Development in Western Kenya Highlands: Implication for Malaria Risk. Frontiers in public health, 4, 238.
KWEKA, E. J., ZHOU, G., GILBREATH, T. M., AFRANE, Y., NYINDO, M., GITHEKO, A. K. & YAN, G. 2011. Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasites & Vectors, 4, 128.
LAMBIN, E. F., TRAN, A., VANWAMBEKE, S. O., LINARD, C. & SOTI, V. 2010. Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts. International Journal of Health Geographics, 9, 54.
Laporta GZ, de Prado PI, Kraenkel RA, Coutinho RM, Sallum MA (2013) Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Neglected Tropical Diseases 7(3):2139
LAURANCE, W. F., CAMARGO, J. L., FEARNSIDE, P. M., LOVEJOY, T. E., WILLIAMSON, G. B., MESQUITA, R. C., MEYER, C. F., BOBROWIEC, P. E. & LAURANCE, S. G. 2018. An Amazonian rainforest and its fragments as a laboratory of global change. Biological Reviews, 93, 223-247.
LEBLOIS, A., DAMETTE, O. & WOLFERSBERGER, J. 2017. What has Driven Deforestation in Developing Countries Since the 2000 s? Evidence from New Remote-Sensing Data. World Development, 92, 82-102.
LEE, K.-S., COX-SINGH, J., BROOKE, G., MATUSOP, A. & SINGH, B. 2009. Plasmodium knowlesi from archival blood films: Further evidence that human infections are widely distributed and not newly emergent in Malaysian Borneo. International Journal for Parasitology, 39, 1125-1128.
LEE, K.-S., DIVIS, P. C., ZAKARIA, S. K., MATUSOP, A., JULIN, R. A., CONWAY, D. J., COX-SINGH, J. & SINGH, B. 2011. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS pathogens, 7, e1002015.
LEVI, T., KEESING, F., HOLT, R. D., BARFIELD, M. & OSTFELD, R. S. 2016. Quantifying dilution and amplification in a community of hosts for tick‐borne pathogens. Ecological Applications, 26, 484-498.
Loh E, Murray KA, Nava A, Aguirre A, Daszak P (2016) Evaluating the links between biodiversity, land-use change, and infectious disease emergence in tropical fragmented landscapes. Tropical Conservation 13:79-88.
LOVE, K., KURZ, D. J., VAUGHAN, I. P., KE, A., EVANS, L. J. & GOOSSENS, B. 2017. Bearded pig (Sus barbatus) utilisation of a fragmented forest–oil palm landscape in Sabah, Malaysian Borneo. Wildlife Research, 44, 603.
LUKE, S. H., BARCLAY, H., BIDIN, K., CHEY, V. K., EWERS, R. M., FOSTER, W. A., NAINAR, A., PFEIFER, M., REYNOLDS, G. & TURNER, E. C. 2017a. The effects of catchment and riparian forest quality on stream environmental conditions across a tropical rainforest and oil palm landscape in Malaysian Borneo. Ecohydrology, 10, e1827.
LUKE, S. H., DOW, R. A., BUTLER, S., VUN KHEN, C., ALDRIDGE, D. C., FOSTER, W. A. & TURNER, E. C. 2017b. The impacts of habitat disturbance on adult and larval dragonflies (Odonata) in rainforest streams in Sabah, Malaysian Borneo. Freshwater Biology, 62, 491-506.
MALHI, Y., GARDNER, T. A., GOLDSMITH, G. R., SILMAN, M. R. & ZELAZOWSKI, P. 2014. Tropical forests in the Anthropocene. Annual Review of Environment and Resources, 39, 125-159.
MANIN, B. O., DRAKELEY, C. J. & CHUA, T. H. 2018. Mitochondrial variation in subpopulations of Anopheles balabacensis Baisas in Sabah, Malaysia (Diptera: Culicidae). PloS one, 13, e0202905.
MANIN, B. O., FERGUSON, H. M., VYTHILINGAM, I., FORNACE, K., WILLIAM, T., TORR, S. J., DRAKELEY, C. & CHUA, T. H. 2016. Investigating the contribution of peri-domestic transmission to risk of zoonotic malaria infection in humans. PLoS neglected tropical diseases, 10, e0005064.
MARTIN, P. A., NEWTON, A. C., PFEIFER, M., KHOO, M. & BULLOCK, J. M. 2015. Impacts of tropical selective logging on carbon storage and tree species richness: A meta-analysis. Forest Ecology and Management, 356, 224-233.
MCCALLUM, H. I. 2015. Lose biodiversity, gain disease. Proceedings of the National Academy of Sciences, 112, 8523-8524.
MCFARLANE, R. A., SLEIGH, A. C. & MCMICHAEL, A. J. 2013. Land-use change and emerging infectious disease on an island continent. International journal of environmental research and public health, 10, 2699.
MERCER, E. V., MERCER, T. G. & SAYOK, A. K. 2014. Effects of forest conversions to oil palm plantations on freshwater macroinvertebrates: a case study from Sarawak, Malaysia. Journal of Land Use Science, 9, 260-277.
MERETA, S. T., YEWHALAW, D., BOETS, P., AHMED, A., DUCHATEAU, L., SPEYBROECK, N., VANWAMBEKE, S. O., LEGESSE, W., DE MEESTER, L. & GOETHALS, P. L. M. 2013. Physico-chemical and biological characterization of anopheline mosquito larval habitats (Diptera: Culicidae): implications for malaria control. Parasites & vectors, 6, 320.
MIETTINEN, J., SHI, C. & LIEW, S. C. 2011. Deforestation rates in insular Southeast Asia between 2000 and 2010. Global Change Biology, 17, 2261-2270.
MILLS, J. N. 2006. Biodiversity loss and emerging infectious disease: an example from the rodent-borne hemorrhagic fevers. Biodiversity, 7, 9-17.
MOKANY, A. & SHINE, R. 2003. Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Austral Ecology, 28, 33-37.
MORAND, S., JITTAPALAPONG, S., SUPUTTAMONGKOL, Y., ABDULLAH, M. T. & HUAN, T. B. 2014. Infectious diseases and their outbreaks in Asia-Pacific: biodiversity and its regulation loss matter. PLoS One, 9, e90032.
Morris AL, Guégan JF, Andreou D, Marsollier L, Carolan K, Le Croller M, Sanhueza D, Gozlan RE (2016) Deforestation-driven food-web collapse linked to emerging tropical infectious disease, Mycobacterium ulcerans. Science Advances 2(12):1600387
Moyes CL, Henry AJ, Golding N, Huang Z, Singh B, Baird JK, Newton PN, Huffman M, Duda KA, Drakeley CJ, Elyazar IR (2014) Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Neglected Tropical Diseases 8(3):2780
MOYES, C. L., SHEARER, F. M., HUANG, Z., WIEBE, A., GIBSON, H. S., NIJMAN, V., MOHD-AZLAN, J., BRODIE, J. F., MALAIVIJITNOND, S., LINKIE, M., SAMEJIMA, H., O’BRIEN, T. G., TRAINOR, C. R., HAMADA, Y., GIORDANO, A. J., KINNAIRD, M. F., ELYAZAR, I. R. F., SINKA, M. E., VYTHILINGAM, I., BANGS, M. J., PIGOTT, D. M., WEISS, D. J., GOLDING, N. & HAY, S. I. 2016. Predicting the geographical distributions of the macaque hosts and mosquito vectors of Plasmodium knowlesi malaria in forested and non-forested areas. Parasites & Vectors, 9, 242.
MUNGA, S., MINAKAWA, N., ZHOU, G., MUSHINZIMANA, E., BARRACK, O.-O. J., GITHEKO, A. K. & YAN, G. 2006. Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. The American journal of tropical medicine and hygiene, 74, 69.
MURRAY, K. A. & DASZAK, P. 2013. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Current opinion in virology, 3, 79-83.
MYERS, N., MITTERMEIER, R. A., MITTERMEIER, C. G., DA FONSECA, G. A. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403, 853.
O’SULLIVAN, L., JARDINE, A., COOK, A. & WEINSTEIN, P. 2008. Deforestation, Mosquitoes, and Ancient Rome: Lessons for Today. BioScience, 58, 756-760.
Ooi CH, Bujang MA, Bakar TM, Ngui R, Lim YA (2017) Over two decades of Plasmodium knowlesi infections in Sarawak: trend and forecast. Acta Tropica 176:83-90.
OSTFELD, R. S. 2009. Biodiversity loss and the rise of zoonotic pathogens. Clinical Microbiology and Infection, 15, 40-43.
OSTFELD, R. S. & KEESING, F. 2000a. Biodiversity and disease risk: the case of Lyme disease. Conservation Biology, 14, 722-728.
OSTFELD, R. S. & KEESING, F. 2000b. Biodiversity series: the function of biodiversity in the ecology of vector-borne zoonotic diseases. Canadian Journal of Zoology, 78, 2061-2078.
OSTFELD, R. S. & KEESING, F. 2017. Is biodiversity bad for your health? Ecosphere, 8, e01676-n/a
OVERGAARD, H. J., EKBOM, B., SUWONKERD, W. & TAKAGI, M. 2003. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landscape ecology, 18, 605.
PARDO, L. E., DE OLIVEIRA ROQUE, F., CAMPBELL, M. J., YOUNES, N., EDWARDS, W. & LAURANCE, W. F. 2018. Identifying critical limits in oil palm cover for the conservation of terrestrial mammals in Colombia. Biological Conservation, 227, 65-73.
PASCUAL, M., AHUMADA, J. A., CHAVES, L. F., RODÓ, X. & BOUMA, M. 2006. Malaria resurgence in the East African highlands: temperature trends revisited. Proceedings of the National Academy of Sciences of the United States of America, 103, 5829.
PATZ, J. & OLSON, S. 2006. Malaria risk and temperature: Influences from global climate change and local land use practices. Proceedings of the National Academy of Sciences of the United States of America, 103, 5635-5636.
PATZ, J. A., DASZAK, P., TABOR, G. M., AGUIRRE, A. A., PEARL, M., EPSTEIN, J., WOLFE, N. D., KILPATRICK, A. M., FOUFOPOULOS, J., MOLYNEUX, D., BRADLEY, D. J. & MEMBERS OF THE WORKING GROUP ON LAND USE CHANGE DISEASE, E. 2004. Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environmental Health Perspectives, 112, 1092-1098.
PATZ, J. A., GRACZYK, T. K., GELLER, N. & VITTOR, A. Y. 2000. Effects of environmental change on emerging parasitic diseases. International journal for parasitology, 30, 1395-1405.
PATZ, J. A., OLSON, S. H., UEJIO, C. K. & GIBBS, H. K. 2008. Disease Emergence from Global Climate and Land Use Change. Medical Clinics of North America, 92, 1473-1491.
Peterson JV, Riley EP (2013) Monyet yang dihargai, monyet yang dibenci: the human–macaque interface in Indonesia. In: The Macaque Connection, Springer
PRUGH, L. R., HODGES, K. E., SINCLAIR, A. R. & BRASHARES, J. S. 2008. Effect of habitat area and isolation on fragmented animal populations. Proceedings of the National Academy of Sciences, 105, 20770-20775.
RAJAHRAM, G. S., BARBER, B. E., WILLIAM, T., GRIGG, M. J., MENON, J., YEO, T. W. & ANSTEY, N. M. 2016. Falling Plasmodium knowlesi Malaria Death Rate among Adults despite Rising Incidence, Sabah, Malaysia, 2010–2014. Emerging Infectious Diseases, 22, 41-48.
Ramasamy R, Surendran SN (2016) Mosquito vectors developing in atypical anthropogenic habitats: global overview of recent observations, mechanisms and impact on disease transmission. Journal of Vector Borne Diseases 53(2):91.
Raven PH (1988) Our diminishing tropical forests. Biodiversity 15:119-122
ROMELL, E., HALLSBY, G., KARLSSON, A. & GARCIA, C. 2008. Artificial canopy gaps in a Macaranga spp. dominated secondary tropical rain forest—effects on survival and above ground increment of four under-planted dipterocarp species. Forest Ecology and Management, 255, 1452-1460.
RUDEL, T. K. 2017. The Dynamics of Deforestation in the Wet and Dry Tropics: A Comparison with Policy Implications. Forests, 8, 108.
Rueda Páramo ME, López Lastra CC, García JJ (2015) Persistence and pathogenicity of a native isolate of Leptolegnia chapmanii against Aedes aegypti larvae in different anthropic environments. Biocontrol Science and Technology 25(2):238-243.
SALKELD, D. J., PADGETT, K. A. & JONES, J. H. 2013. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecology Letters, 16, 679-686.
SALLUM, M., PEYTON, E. & WILKERSON, R. 2005b. Six new species of the Anopheles leucosphyrus group, reinterpretation of An. elegans and vector implications. Medical and veterinary entomology, 19, 158-199.
SALLUM, M. A. M., FOSTER, P. G., LI, C., SITHIPRASASNA, R. & WILKERSON, R. C. 2007. Phylogeny of the Leucosphyrus Group of Anopheles (Cellia)(Diptera: Culicidae) based on mitochondrial gene sequences. Annals of the Entomological Society of America, 100, 27-35.
SALLUM, M. A. M., PEYTON, E. L., HARRISON, B. A. & WILKERSON, R. C. 2005a. Revision of the Leucosphyrus group of Anopheles (Cellia) (Diptera, Culicidae). Revista Brasileira de Entomologia, 49, 01-152.
SANDIFER, P. A., SUTTON-GRIER, A. E. & WARD, B. P. 2015. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosystem Services, 12, 1-15.
SAVILAAKSO, S., GARCIA, C., GARCIA-ULLOA, J., GHAZOUL, J., GROOM, M., GUARIGUATA, M. R., LAUMONIER, Y., NASI, R., PETROKOFSKY, G., SNADDON, J. & ZRUST, M. 2014. Systematic review of effects on biodiversity from oil palm production. Environmental Evidence, 3, 4.
SAWARD-ARAV, D., SADEH, A., MANGEL, M., TEMPLETON, A. R. & BLAUSTEIN, L. 2016. Oviposition responses of two mosquito species to pool size and predator presence: varying trade-offs between desiccation and predation risks. Israel Journal of Ecology & Evolution, 62, 143-148.
SAXENA, R., NAGPAL, B., SINGH, V., SRIVASTAVA, A., DEV, V., SHARMA, M., GUPTA, H., TOMAR, A. S., SHARMA, S. & GUPTA, S. K. 2014. Impact of deforestation on known malaria vectors in Sonitpur district of Assam, India. Journal of vector borne diseases, 51, 211.
SCRIVEN, S. A., BEALE, C. M., BENEDICK, S. & HILL, J. K. 2017. Barriers to dispersal of rain forest butterflies in tropical agricultural landscapes. Biotropica, 49, 206-216.
SEAN, L. M., RICHARD, A. F., THOMAS, M. B. & JAMES, E. M. W. 2016. Biodiversity: The ravages of guns, nets and bulldozers. Nature, 536, 143.
SER 2004. The SER International Primer on Ecological Restoration. Tuscon Arizona: Society for Ecological Restoration International Science and Policy Working Group.
SHAALAN, E. A.-S. & CANYON, D. V. 2009. Aquatic insect predators and mosquito control. Tropical biomedicine, 26, 223-261.
SHEARER, F. M., HUANG, Z., WEISS, D. J., WIEBE, A., GIBSON, H. S., BATTLE, K. E., PIGOTT, D. M., BRADY, O. J., PUTAPORNTIP, C., JONGWUTIWES, S., LAU, Y. L., MANSKE, M., AMATO, R., ELYAZAR, I. R. F., VYTHILINGAM, I., BHATT, S., GETHING, P. W., SINGH, B., GOLDING, N., HAY, S. I. & MOYES, C. L. 2016. Estimating Geographical Variation in the Risk of Zoonotic Plasmodium knowlesi Infection in Countries Eliminating Malaria. PLOS Neglected Tropical Diseases, 10, e0004915.
SHUHADA, S. N., SALIM, S., NOBILLY, F., ZUBAID, A. & AZHAR, B. 2017. Logged peat swamp forest supports greater macrofungal biodiversity than large‐scale oil palm plantations and smallholdings. Ecology and evolution, 7, 7187-7200.
SINGH, B. & DANESHVAR, C. 2013. Human infections and detection of Plasmodium knowlesi. Clinical Microbiology Reviews 26 165-184
SITHIPRASASNA, R., LEE, W. J., UGSANG, D. M. & LINTHICUM, K. J. 2005. Identification and characterization of larval and adult anopheline mosquito habitats in the Republic of Korea: potential use of remotely sensed data to estimate mosquito distributions. International journal of health geographics, 4, 17.
SODHI, N. S., KOH, L. P., BROOK, B. W. & NG, P. K. 2004. Southeast Asian biodiversity: an impending disaster. Trends in Ecology & Evolution, 19, 654-660.
Sodhi NS, Posa MR, Peh KS, Koh LP, Soh MC, Lee TM, Lee JS, Wanger TC, Brook BW (2012) Land use changes imperil South-East Asian biodiversity. In: Land Use Intensification Effects on Agriculture, Biodiversity and Ecological Processes. Boca Raton, FL: CRC Press, pp 39–46
SPELDEWINDE, P., SLANEY, D. & WEINSTEIN, P. 2015. Is restoring an ecosystem good for your health? Science of The Total Environment, 502, 276-279.
SUARDI, H., BESAR, N. A., MUI-HOW, P. & MOKHTAR, M. 2016. Carbon stock estimation of agroforestry system in Tawau, Sabah. Transaction on Science and Technology, 3, 25-30.
SUZÁN, G., MARCÉ, E., GIERMAKOWSKI, J. T., MILLS, J. N., CEBALLOS, G., OSTFELD, R. S., ARMIÉN, B., PASCALE, J. M. & YATES, T. L. 2009. Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PloS one, 4, e5461.
Takano KT, Nakagawa M, Itioka T, Kishimoto-Yamada K, Yamashita S, Tanaka HO, Fukuda D, Nagamasu H, Ichikawa M, Kato Y, Momose K (2014) The extent of biodiversity recovery during reforestation after Swidden cultivation and the impacts of land-use changes on the biodiversity of a tropical rainforest region in Borneo. In: Social-Ecological Systems in Transition, Springer
TAN, C. H., VYTHILINGAM, I., MATUSOP, A., CHAN, S. T. & SINGH, B. 2008. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malaria Journal, 7, 52.
Tanner D, Kirk R (2008) Matrix to mosaic: habitat fragmentation from 1982–1999 in Sabah, Malaysian Borneo. Borneo Research Bulletin 39:255.
Taubert F, Fischer R, Groeneveld J, Lehmann S, Müller MS, Rödig E, Wiegand T, Huth A (2018) Global patterns of tropical forest fragmentation. Nature 554(7693):519
TEUSCHER, M., VORLAUFER, M., WOLLNI, M., BROSE, U., MULYANI, Y. & CLOUGH, Y. 2015. Trade-offs between bird diversity and abundance, yields and revenue in smallholder oil palm plantations in Sumatra, Indonesia. Biological Conservation, 186, 306-318.
THONGSRIPONG, P., GREEN, A., KITTAYAPONG, P., KAPAN, D., WILCOX, B. & BENNETT, S. 2013. Mosquito vector diversity across habitats in central Thailand endemic for dengue and other arthropod-borne diseases. PLoS neglected tropical diseases, 7, e2507.
Tuck SL, O’brien MJ, Philipson CD, Saner P, Tanadini M, Dzulkifli D, Godfray HCJ, Godoong E, Nilus R, Ong RC (2016) The value of biodiversity for the functioning of tropical forests: insurance effects during the first decade of the Sabah biodiversity experiment. In: Proceedings of Royal Society B, The Royal Society, 20161451
TUCKER LIMA, J. M., VITTOR, A., RIFAI, S. & VALLE, D. 2017. Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Philosophical Transactions of the Royal Society B: Biological Sciences, 372.
TUNO, N., OKEKA, W., MINAKAWA, N., TAKAGI, M. & YAN, G. 2005. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) Larvae in Western Kenya Highland Forest. Journal of Medical Entomology, 42, 270-277.
TURNER, E. C. & FOSTER, W. A. 2009. The impact of forest conversion to oil palm on arthropod abundance and biomass in Sabah, Malaysia. Journal of tropical ecology, 25, 23-30.
VITTOR, A. Y., GILMAN, R. H., TIELSCH, J., GLASS, G., SHIELDS, T., LOZANO, W. S., PINEDO-CANCINO, V. & PATZ, J. A. 2006. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. The American journal of tropical medicine and hygiene, 74, 3-11.
VITTOR, A. Y., PAN, W., GILMAN, R. H., TIELSCH, J., GLASS, G., SHIELDS, T., SÁNCHEZ-LOZANO, W., PINEDO, V. V., SALAS-COBOS, E. & FLORES, S. 2009. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. The American journal of tropical medicine and hygiene, 81, 5.
VYTHILINGAM, I., LIM, Y. A., VENUGOPALAN, B., NGUI, R., LEONG, C. S., WONG, M. L., KHAW, L., GOH, X., YAP, N., SULAIMAN, W. Y. W., JEFFERY, J., ZAWIAH, A. G. C., NOR ASZLINA, I., SHARMA, R. S., YEE LING, L. & MAHMUD, R. 2014. Plasmodium knowlesi malaria an emerging public health problem in Hulu Selangor, Selangor, Malaysia (2009–2013): epidemiologic and entomologic analysis. Parasites & Vectors, 7, 436.
Vythilingam I, Wong ML, Wan-Yussof WS (2016) Current status of Plasmodium knowlesi vectors: a public health concern? Parasitology 2016:1-9
Wang X, Zhou G, Zhong D, Wang X, Wang Y, Yang Z, Cui L, Yan G (2016) Life-table studies revealed significant effects of deforestation on the development and survivorship of Anopheles minimus larvae. Parasites & Vectors 9(1):323
WILCOVE, D. S., GIAM, X., EDWARDS, D. P., FISHER, B. & KOH, L. P. 2013. Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. Trends in ecology & evolution, 28, 531-540.
WILCOVE, D. S. & KOH, L. P. 2010. Addressing the threats to biodiversity from oil-palm agriculture. Biodiversity and Conservation, 19, 999-1007.
WILCOX, B. A. & COLWELL, R. R. 2005. Emerging and reemerging infectious diseases: biocomplexity as an interdisciplinary paradigm. EcoHealth, 2, 244.
William T, Jelip J, Menon J, Anderios F, Mohammad R, Mohammad TA, Grigg MJ, Yeo TW, Anstey NM, Barber BE (2014) Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malaria Journal 13(1):390
WILLIAM, T., JELIP, J., MENON, J., ANDERIOS, F., MOHAMMAD, R., AWANG MOHAMMAD, T. A., GRIGG, M. J., YEO, T. W., ANSTEY, N. M. & BARBER, B. E. 2014. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malaria Journal, 13, 390.
WONG, M. L., CHUA, T. H., LEONG, C. S., KHAW, L. T., FORNACE, K., WAN-SULAIMAN, W.-Y., WILLIAM, T., DRAKELEY, C., FERGUSON, H. M. & VYTHILINGAM, I. 2015a. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS neglected tropical diseases, 9, e0004135.
WONG, M. L., VYTHILINGAM, I., LEONG, C. S., KHAW, L. T., CHUA, T. H., OBRAIN, B., FERGUSON, H. & DRAKELY, C. 2015b. Incrimination of Anopheles balabacensis as the vector for simian malaria in Kudat Division, Sabah, Malaysia. Journal of Microbiology, Immunology and Infection, 48, S47-S48.
WOODRUFF, D. S. 2010. Biogeography and conservation in Southeast Asia: how 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodiversity and Conservation, 19, 919-941.
WORLD HEALTH ORGANIZATION 2017. Expert Consultation on Plasmodium knowlesi Malaria to Guide Malaria Elimination Strategies, Kota Kinabalu, Malaysia, 1-2 March 2017: meeting report. Manila: WHO Regional Office for the Western Pacific.
YAHYA, M. S., SYAFIQ, M., ASHTON‐BUTT, A., GHAZALI, A., ASMAH, S. & AZHAR, B. 2017. Switching from monoculture to polyculture farming benefits birds in oil palm production landscapes: Evidence from mist netting data. Ecology and evolution, 7, 6314-6325.
Yakob L, Lloyd AL, Kao RR, Ferguson HM, Brock PM, Drakeley C, Bonsall MB (2018) Plasmodium knowlesi invasion following spread by infected mosquitoes, macaques and humans. Parasitology 145(1):101–110
YASUOKA, J. & LEVINS, R. 2007. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. The American journal of tropical medicine and hygiene, 76, 450-460.
YEONG, K. L., REYNOLDS, G. & HILL, J. K. 2016. Enrichment planting to improve habitat quality and conservation value of tropical rainforest fragments. Biodiversity and conservation, 25, 957-973.
YOUNG, H. S., WOOD, C. L., KILPATRICK, A. M., LAFFERTY, K. D., NUNN, C. L. & VINCENT, J. R. 2017. Conservation, biodiversity and infectious disease: scientific evidence and policy implications. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160124.
YUE, S., BRODIE, J. F., ZIPKIN, E. F. & BERNARD, H. 2015. Oil palm plantations fail to support mammal diversity. Ecological Applications, 25, 2285-2292.
Acknowledgements
With gratitude, we would like to thank David Alloysius, the manager at the Inikea field site in Sabah, for his time and patience in showing us potential sampling sites last November. Also, thanks must go to the Swedish Research Council and the Swedish University of Agricultural Science for providing funding for this ongoing research within the wider project ‘Balancing production and ecosystem services from degraded tropical rainforests to aid the transition to a more sustainable economy’. The University of Western Australia has provided the opportunity to investigate the effects of forest restoration on human health as a PhD project for GD who also expresses her gratitude to all the supervisors overseeing this research for their time and efforts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidson, G., Chua, T.H., Cook, A. et al. The Role of Ecological Linkage Mechanisms in Plasmodium knowlesi Transmission and Spread. EcoHealth 16, 594–610 (2019). https://doi.org/10.1007/s10393-019-01395-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-019-01395-6




