Abstract
It has long been theorized that deer mice (Peromyscus maniculatus) are a primary reservoir of Yersinia pestis in California. However, recent research from other parts of the western USA has implicated deer mice as spillover hosts during epizootic plague transmission. This retrospective study analyzed deer mouse data collected for plague surveillance by public health agencies in California from 1971 to 2016 to help elucidate the role of deer mice in plague transmission. The fleas most commonly found on deer mice were poor vectors of Y. pestis and occurred in insufficient numbers to maintain transmission of the pathogen, while fleas whose natural hosts are deer mice were rarely observed and even more rarely found infected with Y. pestis on other rodent hosts. Seroprevalence of Y. pestis antibodies in deer mice was significantly lower than that of several chipmunk and squirrel species. These analyses suggest that it is unlikely that deer mice play an important role in maintaining plague transmission in California. While they may not be primary reservoirs, results supported the premise that deer mice are occasionally exposed to and infected by Y. pestis and instead may be spillover hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conventional view of sylvatic plague transmission in the western USA is that it consists of two cycles: an enzootic or maintenance cycle, in which Yersinia pestis, the causative agent, is transmitted between moderately resistant rodent reservoir hosts, and an epizootic cycle, which occurs when more susceptible hosts are infected with Y. pestis and amplify its transmission (Quan and Kartman 1956; Pollitzer and Meyer 1961). Deer mice (Peromyscus maniculatus) have been hypothesized to be an important enzootic reservoir for Y. pestis (Pollitzer and Meyer 1961; Quan and Kartman 1962; Nelson 1980; Stark et al. 1966). Deer mice with antibodies to Y. pestis have been found (Cavanaugh et al. 1965; Hudson and Kartman 1967), and Y. pestis-positive deer mouse carcasses and fleas have also been collected (Hudson et al. 1964; Smith et al. 2010). Plague-infected deer mice and their fleas have been found in association with other more susceptible rodent hosts during epizootic transmission (Cully and Williams 2001), including chipmunks (Tamias spp.) (Smith et al. 2010) and the California ground squirrel (Otospermophilus beecheyi) (Rutledge et al. 1979), the latter of which is often associated with plague transmission to humans (Craven et al. 1993). Positive fleas have also been found on deer mice during enzootic transmission (Thiagarajan et al. 2008).
Recent research has reexamined the role of deer mice in plague transmission (Gage and Kosoy 2005; Maher et al. 2010). Individual populations of deer mice had varying levels of susceptibility to Y. pestis infection (Gage and Kosoy 2005). A study of woodrats and plague concluded that the presence of Peromyscus in the vicinity of woodrat (Neotoma spp.) nests was insufficient evidence that Peromyscus was essential for plague maintenance (Kosoy et al. 2017). In prairie dog (Cynomys ludovicianus) plague ecology studies, researchers hypothesized that deer mice are more likely to be spillover hosts: infected during or after an epizootic event (Salkeld and Stapp 2008; Salkeld et al. 2016). While seropositive deer mice were found within colonies of the plague-susceptible prairie dog, they were detected in the year after the initial plague epizootic event (Salkeld and Stapp 2008; Salkeld et al. 2016). At that site, fleas whose natural hosts are deer mice (i.e., deer mouse-specific fleas) were never found infected with Y. pestis, while prairie dog fleas were found on deer mice during active plague transmission, indicating that they were feeding on deer mice in the absence of their primary hosts (Salkeld and Stapp 2008). Aetheca wagneri, one of the most common species of fleas on deer mice, has been found infected with Y. pestis, but is a very poor vector of the bacteria (Eisen et al. 2008). A predictive model estimated that at least 68 A. wagneri are required per deer mouse to maintain enzootic transmission of plague; however, most individual deer mice carry less than three A. wagneri (Eisen et al. 2008). Flea species that are competent for plague transmission are rarely found on deer mice (Maestas and Britten 2017). Therefore, it is more likely that deer mice act as short-term reservoirs of Y. pestis and are incapable of maintaining the level of transmission required for enzootic maintenance (Eisen and Gage 2009).
The role of deer mice in sylvatic plague transmission has long been studied in California (Quan et al. 1960; Nelson and Smith 1976; Nelson 1980; Davis et al. 2002; Smith et al. 2010), and its importance as an enzootic reservoir has not been refuted. Plague-positive deer mice and their fleas were found in the 1940s in coastal Monterey County (Kartman et al. 1958). Since then, plague has been detected in deer mice in other distinct bioregions of California, including the Transverse Ranges (Davis et al. 2002), the Cascades (Nelson and Smith 1976, 1980; Smith et al. 2010), and the Sierra Nevada (Holt et al. 2009; Smith et al. 2010; Danforth et al. 2016). In the Coastal region, plague outbreaks were never found in the absence of deer mice and California meadow voles (Microtus californicus) (Quan and Kartman 1962), another species that is considered an enzootic reservoir of plague (Gage and Kosoy 2005). At one site in the Transverse Ranges with evidence of ongoing epizootic transmission, deer mice were the second most prevalent rodent species and had the highest diversity of fleas in 18 years of surveillance (Davis et al. 2002). In the Cascades, deer mice were found in close association with plague-susceptible bushy-tailed woodrats (Neotoma cinerea), leading researchers to hypothesize that deer mice using abandoned woodrat dens enhanced the spread of a plague epizootic (Nelson and Smith 1976). In the Sierra Nevada, fleas collected from a deer mouse during an epizootic event tested positive for Y. pestis (Danforth et al. 2016).
In light of the recent deer mouse studies from other areas of the western USA, we analyzed 46 years of deer mouse samples collected during plague surveillance events in California for evidence of consistent and widespread exposure to Y. pestis, indicative of an enzootic plague reservoir. We hypothesized that deer mice are not a significant plague reservoir in California because (1) flea species found on deer mice have low vector competence for Y. pestis and occur in insufficient numbers to maintain Y. pestis transmission, (2) there is little evidence of flea exchange with other plague reservoirs or more susceptible amplifying hosts, and (3) deer mice show little evidence of exposure to Y. pestis, as demonstrated by serological testing. Instead, we hypothesized that deer mice are most commonly involved with plague transmission as a spillover host for Y. pestis, becoming infected during or after epizootic transmission events.
Methods
Data Collection
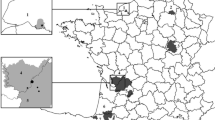
Data used in this analysis were collected by the California Department of Public Health, Vector-Borne Disease Section (CDPH-VBDS) and collaborating local agencies for plague surveillance from 1971 to 2016. Records from 1984 to 2016 were available in a searchable database, while those from 1971 to 1983 were in handwritten log books, and relevant data were extracted and entered into Excel spreadsheets. Rodent flea and carcass samples had been tested for the presence of Y. pestis via laboratory mouse inoculation, direct fluorescence antibody, culture, and/or PCR, while sera samples were tested for antibodies to Y. pestis by passive hemagglutination and passive hemagglutination inhibition (Kartman et al. 1958; Davis et al. 2002; Danforth et al. 2016). Rodent serum samples tested for antibodies to Y. pestis were considered positive if they had a titer of 1:16 or greater. For most flea samples, individuals were pooled into groups of 10 or fewer fleas of the same species collected off the same rodent host. To evaluate regional differences in deer mice exposure to Y. pestis, samples were assigned to one of four regions of the state with known plague transmission based on collection location: the Sierra Nevada, the Coastal region, the Cascade Range, and the Transverse Ranges (Fig. 1). Samples collected from the same county were placed in the same region, except for samples from Kern County, which were split between the Sierra Nevada and Transverse Ranges based on the specific location of the collection.
Variables Used
For the entire 46-year period, we evaluated the following data: deer mouse sera, deer mouse carcasses, fleas on deer mice, and deer mouse-specific flea species (Hubbard 1947; Lewis et al. 1988; Lewis and Haas 2001) found on other hosts. Data fields for all deer mouse samples (sera, flea pools, and carcasses) included: date, county, geographic region, specific location of collection, and samples collected from other rodent genera (seroprevalence as a continuous variable; carcasses and flea pools as presence/absence) from the same location within one calendar month of the deer mouse samples. Human cases with probable exposure from the same location and time period as deer mouse samples were also noted. A broad categorical variable we termed “epizootic conditions” was created to score whether each deer mouse sample was collected within 1 month of any indicator of epizootic plague activity at the same location (“1”) or without such indicators (“0”). Epizootic indicators were based on criteria in the California Compendium of Plague Control (CDPH 2016) and defined as: presence of a human plague case, Y. pestis-positive rodent carcass or flea pool (other than from deer mouse or other Peromyscus spp.), and/or elevated seroprevalence (≥ 25%) in rodents sampled (other than Peromyscus species). Collection events without one of these epizootic indicators were classified as enzootic transmission collections.
For deer mouse samples collected from 1985 to 2016, we used the database to match deer mouse samples collected in that time period with epizootic conditions and seroprevalence in other rodent genera at the same location from 6 months and 12 months prior. Additionally, for this time period, seroprevalence of Y. pestis antibodies for rodent species commonly involved with plague transmission in California was extracted from the database for comparison with deer mouse seroprevalence.
Data Analysis
Data were analyzed with R statistical software (R Core Team 2015). Fleas found on deer mice were evaluated for their Y. pestis-vector competence, derived from the published literature (Burroughs 1947; Kartman and Prince 1956; Pollitzer and Meyer 1961; Eisen et al. 2009), and by calculating the flea index (i.e., the total number of fleas collected on deer mice divided by the total number of deer mice sampled for fleas). To assess flea sharing between deer mice and other rodent hosts, fleas collected from deer mice were classified by their natural hosts (Hubbard 1947; Lewis et al. 1988; Lewis and Haas 2001) and deer mouse-specific fleas were analyzed to compare their occurrence on deer mice or other mammalian species. The extent of Y. pestis exposure in deer mice was analyzed by calculating the seroprevalence of Y. pestis antibodies in deer mice and then compared to seroprevalences of other rodent hosts. Finally, to determine whether deer mice are spillover hosts, the probability of detecting a positive deer mouse sample was analyzed with logistic regression, with candidate models ranked by Akaike’s information criterion (AIC). For each outcome, models were run using intercept only and with the following predictors: epizootic conditions (current, 6 months prior, and 12 months prior), seroprevalence in other rodents (current, 6 months prior, and 12 months prior), year, and region.
Results
Summary Statistics
From 1971 to 2016, 6808 samples were collected from deer mice, 5981 of which were tested for Y. pestis (flea pools and carcasses) or antibodies to Y. pestis (sera). In addition, 555 flea pools containing one or more deer mouse-specific flea species were collected on other rodents, 378 of which were tested for Y. pestis. Data were collected at 822 locations from 43 of California’s 58 counties, representing four broad regions where plague is endemic in California; 1317 samples were collected from the Coastal region, 1804 from the Cascades, 1721 from the Sierra Nevada, and 970 from the Transverse Ranges (Fig. 1).
The tested samples from deer mice included 1162 flea pools, 4588 sera, and 231 carcasses. Eighty (1.4%) samples tested positive. Five (0.4%) of the flea pools collected from deer mice and 9 (3.9%) of the deer mouse carcasses tested positive for Y. pestis bacteria, while 66 (1.4%) of the serum samples tested were positive for Y. pestis antibodies. Positive deer mouse sera were collected in all four regions, but no positive deer mouse fleas or deer mouse carcasses were detected on the Coast or Transverse Ranges. There was no significant difference in regional-level plague detection in deer mouse sera (P = 0.10), fleas (P = 0.76), or carcasses (P = 0.60). Deer mouse samples were collected in every year, with the number of samples collected each year ranging widely, from 7 in 1996 to 627 in 1977. There was a decline in the number of deer mice sampled after 1984. On average, deer mouse samples represented 16.8% of all rodent samples collected from 1971 to 1984, but only 3.4% from 1985 to 2016 (Fig. 2).
Fleas on Deer Mice
A total of 1989 flea pools were collected from deer mice, representing 3647 individual fleas of at least 36 species (Table 1). The three most commonly found flea species on deer mice were A. wagneri (647 pools, 32.5%), Opisodasys keeni (463 pools, 23.3%), and Malareus telchinus (224 pools, 11.3%). A review of published literature indicated that A. wagneri and M. telchinus are poor vectors of Y. pestis, while no vector competence information was found for O. keeni. The number of fleas of any species collected per deer mouse ranged from 1 to 27, with an overall flea index of 1.8. A maximum of 12 A. wagneri were collected per deer mouse with an index of 1.9. Of the 1162 flea pools tested for Y. pestis, just 5 (0.4%) were positive. Two of these flea pools consisted of O. keeni (1 pool of 1, 1 pool of 6), one contained one Catallagia chamberlini, one consisted of three Phalacropsylla allos, and the fifth positive pool consisted of three Peromyscopsylla hesperomys adelpha; there is no published vector competence data on the latter three species. Including deer mouse-specific fleas found on other hosts (Table 2, SI), only 0.4% (2/452) of A. wagneri flea pools, 1.3% (5/399) of O. keeni flea pools, and 0.3% (1/329) of M. telchinus flea pools tested positive for Y. pestis. For comparison, from 1985 to 2016, the three most common rodent flea species collected were Oropsylla montana, Ceratophyllus ciliatus, and Eumolpianus eumolpi, with ≥ 98.8% of those pools collected from sciurids, primarily chipmunks and ground squirrels. For these species, respectively, 2.9% (21/726), 2.5% (7/283), and 5.7% (9/157) of flea pools tested positive for Y. pestis bacteria.
Flea Exchange Between Deer Mice and Other Reservoirs
The majority (1466/1989 pools, 73.7%) of fleas from deer mice consisted of species whose natural host is the deer mouse and 409 (20.6%) came from flea species associated with Peromyscus species in general (Table 1). Only 70 flea pools (3.5%) came from flea species associated with sciurid rodents, none of which tested positive for Y. pestis. In comparison, over the 46-year time period 2021 flea pools consisting of species whose natural hosts are deer mice were identified on deer mice (Table 1) and other animals (Table 2, SI). Of those flea pools, 1466 (72.5%) were collected from deer mice. An additional 240 flea pools (11.9%) were collected from other Peromyscus hosts and 194 flea pools (9.6%) were collected from other rodents in the family Cricetidae, primarily Microtus and Neotoma species. Only 119 (5.9%) of the deer mouse flea pools were collected from other members of the order Rodentia, 74 of which were sciurids. Just 2 (< 0.1%) were collected from another mammal (short-tailed weasel, Mustela erminea). Of the deer mouse-specific flea pools found on other rodents, 5 (1.3%) of the 378 tested were positive for Y. pestis. These consisted of A. wagneri (2) collected from Neotoma cinerea and Tamias townsendii (now T. senex), O. keeni (2) from N. cinerea, and M. telchinus also from N. cinerea.
Deer Mouse Seroprevalence
Deer mouse sera samples were collected from 1024 trapping events over the 46-year period, with seropositive deer mice detected at 46 (4.5%) events. As stated previously, 66 (1.9%) of 4588 deer mouse sera samples were positive for Y. pestis antibodies. From 1985 to 2016, the seroprevalence of Y. pestis antibodies in deer mice was 1.4% (25/1730, 95% CI 0.9, 2.0). For comparison, over the same time period, several rodent species often associated with transmission in California had significantly higher seroprevalences, such as 13.2% in shadow chipmunks (Tamias senex, 193/1467, 95% CI 11.4, 14.9), 8.5% in long-eared chipmunks (Tamias quadrimaculatus, 21/247, 95% CI 5.0, 12.0), 16.2% in Douglas squirrels (Tamiasciurus douglasii, 51/315, 95% CI 12.1, 20.3), and 5.4% in California ground squirrels (697/13,037, 95% CI 5.0, 5.7).
Deer Mice as Spillover Hosts
Of the samples collected from deer mice, 1288 were collected at locations where other sampling provided indicators of contemporaneous epizootic plague activity. Deer mouse samples collected from 1985 to 2016 were more likely to be collected during an epizootic than those from 1971 to 1984 (χ2 = 9.14, P < 0.01), likely a result of unofficial changes in sampling priorities. However, there was no statistically significant difference in the average seroprevalence in other rodents during those time periods (P = 0.08). Therefore, models for predicting positive deer mouse samples were based on seroprevalence in other rodents.
The detection of any positive deer mouse sample was best predicted by the model using current seroprevalence in other rodents alone (Table 3). As there were no prior seroprevalence data from other rodent genera readily available for all samples collected before 1985, regressions involving seroprevalence from 6 or 12 months prior did not converge. In order to determine whether these relationships varied by type of deer mouse sample, the analysis was run for each sample type: flea pools, sera, and carcasses. The probability of detecting a positive deer mouse flea pool was not strongly associated with seroprevalence in other rodents. However, the model predicting detection of positive deer mouse sera was significantly associated with seroprevalence in other rodents, which varied by year and region of sample collection. All logistic regression models involving deer mouse carcasses and seroprevalence failed due to perfect separation of the variables; all nine positive rodent carcasses were collected when the seroprevalence in other rodents was zero or not tested.
Discussion
We found little evidence to suggest deer mice are significant enzootic plague reservoirs in California. The most commonly found flea species on deer mice was A. wagneri, a poor vector of Y. pestis (Salkeld and Stapp 2008; Eisen et al. 2008, 2009), with a lower pathogen acquisition efficiency and vector efficiency than O. montana and Xenopsylla cheopis, the two flea species most commonly associated with human plague cases (Eisen et al. 2009). The A. wagneri flea index and maximum load observed in this study are considerably lower than the predicted mean of 68 A. wagneri required per deer mouse to maintain transmission (Eisen et al. 2008). O. keeni, the second most frequently found flea species on deer mice, has been found to be naturally infected with Y. pestis (Pollitzer and Meyer 1961), but there are no data on its ability to transmit the bacteria. The third most commonly collected flea species, M. telchinus, is a poor vector, even less efficient than A. wagneri (Burroughs 1947). The Y. pestis-positive flea pools represented only 0.5% of the deer mice flea pools tested, considerably lower than the statewide average of 2.5% of all rodent flea pools tested (CDPH unpublished data 1984–2016). Those flea pools contained O. keeni, C. chamberlini, Ph. allos, and P. h. adelpha. As with O. keeni, there are records of Ph. allos and P. h. adelpha found to be naturally infected with Y. pestis (Stark and Kinney 1969; Danforth et al. 2016), but we found no published studies about their ability to transmit the bacteria. Similarly, we were unable to find information published on the vector capacity of C. chamberlini.
We found that most fleas collected on deer mice were deer mouse-specific fleas and that few deer mouse-specific fleas were found on species besides P. maniculatus or other Peromyscus species. These observations support the hypothesis that deer mice rarely share fleas with other rodent species, particularly those recognized as amplifying hosts for Y. pestis in California (Rutledge et al. 1979; Smith et al. 2010). California ground squirrels and chipmunks typically have larger flea loads, more Y. pestis-positive fleas, and host flea species known to be more efficient vectors of Y. pestis (Smith et al. 2010). Only five Y. pestis-positive pools of deer mouse-specific fleas were collected from other hosts, and none of those flea species are known to be efficient plague vectors. O. montana, the most commonly collected rodent flea species in California and a highly efficient vector (Eisen et al. 2009), was rarely found on deer mice and none of these tested positive for Y. pestis bacteria.
Furthermore, the low seroprevalence observed in deer mice in this analysis does not support their role as an important plague reservoir. Though deer mice are occasionally found to have antibodies to Y. pestis, their seroprevalence is often much lower than that of other species sampled in California, including chipmunks and ground squirrels. Between 1984 and 2004, the deer mouse was the fourth most common animal tested for plague serology in California (n = 1776), but its seroprevalence of 1.1% ranked only 13th highest (Holt et al. 2009), suggesting a more peripheral exposure to Y. pestis than other species tested. In the northern Sierra and Cascades, a higher prevalence of Y. pestis antibodies was found in chipmunks, Douglas squirrels, and California ground squirrels than in deer mice (Smith et al. 2010). The recognized plague endemic areas of California (CDPH 2016) generally coincide with the habitat ranges of one or more of the Tamias or other sciurid species (Jameson and Peeters 2004). In contrast, deer mice are widely distributed in California (Jameson and Peeters 2004), including lower elevations where plague transmission had been rarely detected in recent decades. Although plague occurrence is more likely limited by environmental factors (i.e., plague niche hypothesis) rather than host assemblages (Maher et al. 2010), there is little evidence to indicate that the deer mouse or its fleas play a significant role in maintaining Y. pestis transmission.
Prior evidence also suggested that deer mice were not an important enzootic reservoir of plague in California. In several investigations, seroprevalence in deer mice tested was 0% at most trapping events, regardless of region (Kartman et al. 1958; Hudson et al. 1964; Nelson and Smith 1976; Davis et al. 2002; Smith et al. 2010), though during epizootics in the Coastal region, seroprevalence was as high as 50% (n = 42) (Hudson et al. 1964). In the Cascades, seropositive deer mice were found in abandoned woodrat dens after the more susceptible species was extirpated by plague, not before (Nelson and Smith 1976). Researchers thought Microtus species were more likely to be the enzootic reservoir in the San Francisco Bay Area as they are more resistant than deer mice (Kartman et al. 1958). Despite its high flea diversity, other studies found deer mice in California only carry an average flea load of 0.1–1.5 (Stark and Miles 1962; Nelson and Smith 1976), again suggesting that maintaining plague transmission is highly unlikely (Eisen et al. 2008).
Though deer mice are not a primary reservoir of Y. pestis in California, they may be a spillover host. Regression analysis highlighted associations between the different indicators of plague transmission in deer mice and prevalence of Y. pestis antibodies in non-Peromyscus rodents collected at the same site within 1 month of deer mouse sample collection. Increasing seroprevalence in other rodents was positively associated with seropositive deer mice; however, that relationship was impacted by the year and region in which samples were collected. The effects of year and region may be due to the cyclical increases in plague transmission (Ben Ari et al. 2008) and regional variations in plague ecology (Holt et al. 2009; Smith et al. 2010).
There were several limitations to the analyses in this study that made it difficult to definitively determine whether deer mice are primarily spillover hosts for Y. pestis. Data were most often collected during public health responses when epizootic activity was suspected and less often from routine surveys during enzootic periods or from a designed study, particularly after 1984. After the early 1980s, a smaller proportion of plague samples collected in California came from deer mice. CDPH-VBDS staff during that time period observed that deer mice sera testing rarely found antibodies to Y. pestis, so they purposely reduced deer mice sampling (C. Smith, retired CDPH-VBDS, pers. comm. 2017 February 23) to focus on rodents demonstrating more consistent exposure to the pathogen. Inconsistent sampling of the same locations also made it difficult to ascertain when or if deer mouse seroprevalence rose in relationship to seroprevalence in other rodents, indicative of a spillover host. Evaluation of epizootic indicators prior to 1985 also would have provided a more robust data set to evaluate the influence of prior epizootic activity on deer mice samples. There is also evidence that flea infestations of deer mice occur more frequently and in higher numbers in the winter (Campos et al. 1985), but as most of our data were collected during the spring and summer, we did not include seasonality in flea-based models.
References
Ben Ari T, Gershunov A, Gage KL, Snall T, Ettestad P, Kausrud KL, Stenseth NC (2008) Human plague in the USA: the importance of regional and local climate. Biology Letters 4:737-40; https://doi.org/10.1098/rsbl.2008.0363
Burroughs AL (1947) Sylvatic plague studies. The vector efficiency of nine species of fleas compared with Xenopsylla cheopis. Journal of Hygiene 45(03):371-96
California Department of Public Health (2016) California compendium of plague control. Sacramento (CA)
Campos EG, Maupin GO, Barnes AM, Eads RB (1985) Seasonal occurrence of fleas (Siphonaptera) on rodents in a foothills habitat in Larimer County, Colorado, USA. Journal of Medical Entomology 22(3):266-270
Cavanaugh D, Thorpe B, Bushman J, Nicholes P, Rust Jr J (1965) Detection of an enzootic plague focus by serological methods. Bulletin of the World Health Organization 32(2):197
Craven RB, Maupin GO, Beard ML, Quan TJ, Barnes AM (1993) Reported cases of human plague infections in the United States, 1970–1991. Journal of Medical Entomology 30(4):758-61
Cully JF, Williams ES (2001) Interspecific comparisons of sylvatic plague in prairie dogs. Journal of Mammology 82(4):894-905
Danforth ME, Novak MG, Petersen J, Mead PS, Kingry LC, Weinburke M, Buttke D, Hacker G, Tucker J, Niemela M, Jackson B, Padgett K, Liebman K, Vugia D, Kramer V (2016) Investigation and response to 2 plague cases, Yosemite National Park, California, USA, 2015. Emerging Infectious Diseases 22(12):2045; https://doi.org/10.3201/eid2212.160560
Davis RM, Smith RT, Madon MB, Sitko-Cleugh E (2002) Flea, rodent, and plague ecology at Chuchupate campground, Ventura County, California. Journal of Vector Ecology 27:107-27
Eisen RJ, Eisen L, Gage KL (2009) Studies of vector competency and efficiency of North American fleas for Yersinia pestis: state of the field and future research needs. Journal of Medical Entomology 46(4):737-44
Eisen RJ, Gage KL (2009) Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Veterinary Research 40(2):1; https://doi.org/10.1051/vetres:2008039
Eisen RJ, Holmes JL, Schotthoefer AM, Vetter SM, Montenieri JA, Gage KL (2008) Demonstration of early-phase transmission of Yersinia pestis by the mouse flea, Aetheca wagneri (Siphonaptera:Ceratophylidae), and implications for the role of deer mice as enzootic reservoirs. Journal of Medical Entomology 45(6):1160-4
Gage KL, Kosoy MY (2005) Natural history of plague: perspectives from more than a century of research. Annual Review of Entomology 50:505-28; https://doi.org/10.1146/annurev.ento.50.071803.130337
Holt AC, Salkeld DJ, Fritz CL, Tucker JR, Gong P (2009) Spatial analysis of plague in California: niche modeling predictions of the current distribution and potential response to climate change. International Journal of Health Geographics 8:38; https://doi.org/10.1186/1476-072x-8-38
Hubbard CA (1947) Fleas of Western North America: Their Relation to the Public Health; Ames, Iowa: The Iowa State College Press
Hudson B, Kartman L (1967) The use of the passive hemagglutination test in epidemiologic investigations of sylvatic plague in the United States. Journal of Wildlife Diseases 3(2):50-9
Hudson B, Quan S, Goldenberg M (1964) Serum antibody responses in a population of Microtus californicus and associated rodent species during and after Pasteurella pestis epizootics in the San Francisco Bay area. Zoonoses Research 3(1):15-29
Jameson E, Peeters HJ (2004) Mammals of California. Berkeley and Los Angeles, CA: University of California Press
Kartman L, Miles VI, Prince FM (1958) Ecological studies of wild rodent plague in the San Francisco Bay area of California. I. Introduction. American Journal of Tropical Medicine and Hygiene 7(1):112-24
Kartman L, Prince FM (1956) Studies On Pasteurella pestis in fleas. V. The experimental plague-vector efficiency of wild rodent fleas compared with Xenopsylla cheopis, together with observations on the influence of temperature. American Journal of Tropical Medicine and Hygiene 5(6):1058-70
Kosoy M, Reynolds P, Bai Y, Sheff K, Enscore RE, Montenieri J, Ettestad P, Gage K (2017) Small-scale die-offs in woodrats support long-term maintenance of plague in the U.S. southwest. Vector Borne and Zoonotic Diseases 17(9):635-644; https://doi.org/10.1089/vbz.2017.2142
Lewis RE, Haas GE (2001) A review of the North American Catallagia Rothschild, 1915, with the description of a new species (Siphonaptera:Ctenophthalmidae:Neopsyllinae:Phalacropsyllini). Journal of Vector Ecology 26:51-69
Lewis RE, Lewis JH, Maser C (1988) The Fleas of the Pacific Northwest. Corvallis, Oregon: Oregon State University Press
Maestas LP, Britten HB (2017) Flea and small mammal species composition in mixed-grass prairies: implications for the maintenance of Yersinia pestis. Vector-Borne and Zoonotic Diseases 17(7)467-74
Maher SP, Ellis C, Gage KL, Enscore RE, Peterson AT (2010) Range-wide determinants of plague distribution in North America. American Journal of Tropical Medicine and Hygiene 83(4):736-42; https://doi.org/10.4269/ajtmh.2010.10-0042
Nelson BC (1980) Plague studies in California—the roles of various species of sylvatic rodents in plague ecology in California. Proceedings of the Ninth Vertebrate Pest Conference, pp 89–96
Nelson BC, Smith CR (1976) Ecological effects of a plague epizootic on the activities of rodents inhabiting caves at Lava Beds National Monument, California. Journal of Medical Entomology 13(1):51-61
Nelson B, Smith C (1980) Ecology of sylvatic plague in lava caves at Lava Beds National Monument, California. Traub R, H Starcke (editors), Fleas: Proceedings of the International Conference on Fleas, Ashton Wold, Peterborough, UK, 21–25 pp 273–275
Pollitzer R, Meyer KF (1961) The ecology of plague. In: Studies in Disease Ecology, May JM (editor) New York: Hafner Publishing Company, pp 433-590
Quan S, Kartman L (1956) The resistance of Microtus and Peromyscus to infection by Pasteurella pestis. Transactions of the Royal Society of Tropical Medicine and Hygiene 50(1):104-5
Quan S, Kartman L (1962) Ecological studies of wild rodent plague in the San Francisco Bay Area of California. VIII. Susceptibility of wild rodents to experimental plague infection. Zoonoses Research 1(7):121-44
Quan S, Miles V, Kartman L (1960) Ecological studies of wild rodent plague in the San Francisco Bay area of California. III. The natural infection rates with Pasteurella pestis in five flea species during an epizootic. American Journal of Tropical Medicine and Hygiene 9(1):85-90
R Core Team (2015) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
Rutledge L, Moussa M, Zeller B, Lawson M (1979) Field studies of reservoirs and vectors of sylvatic plague at Fort Hunter Liggett, California. Journal of Medical Entomology 15(5-6):452-8
Salkeld DJ, Stapp P (2008) No evidence of deer mouse involvement in plague (Yersinia pestis) epizootics in prairie dogs. Vector Borne and Zoonotic Diseases. 8(3):331-8
Salkeld DJ, Stapp P, Tripp DW, Gage KL, Lowell J, Webb CT, Brinkerhoff RJ, Antolin MF (2016) Ecological traits driving the outbreaks and emergence of zoonotic pathogens. Bioscience. 66(2)118-29
Smith CR, Tucker JR, Wilson BA, Clover JR (2010) Plague studies in California: a review of long‐term disease activity, flea‐host relationships and plague ecology in the coniferous forests of the Southern Cascades and northern Sierra Nevada mountains. Journal of Vector Ecology 35(1):1-12
Stark HE, Hudson BW, Pittman B (1966) Plague epidemiology, Atlanta, Georgia: US Department of Health, Education, and Welfare, Public Health Service
Stark HE, Kinney AR (1969) Abundance of rodents and fleas as related to plague in Lava Beds National Monument, California. Journal of Medical Entomology 6(3):287-94
Stark HE, Miles VI (1962) Ecological studies of wild rodent plague in the San Francisco Bay area of California. VI. The relative abundance of certain flea species and their host relationships on coexisting wild and domestic rodents. American Journal of Tropical Medicine and Hygiene 11(4):525-34
Thiagarajan B, Bai Y, Gage KL, Cully JF (2008) Prevalence of Yersinia pestis in rodents and fleas associated with black-tailed prairie dogs (Cynomys ludovicianus) at Thunder Basin National Grassland, Wyoming. Journal of Wildlife Diseases 44(3):731-6
Acknowledgements
We thank Dr. Anne Kjemtrup (CDPH-VBDS) and Dr. Ken Gage (Centers for Disease Control and Prevention) for reviewing this manuscript. We also thank all current and former CDPH-VBDS staff and employees of many local, state, and federal agencies who contributed to the collection of the plague surveillance data. Finally, we acknowledge the efforts of Robert Dugger who assisted us with digitizing historical data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interests to disclose.
Statement of Human and Animal Rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Danforth, M., Tucker, J. & Novak, M. The Deer Mouse (Peromyscus maniculatus) as an Enzootic Reservoir of Plague in California. EcoHealth 15, 566–576 (2018). https://doi.org/10.1007/s10393-018-1337-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-018-1337-2